ABSTRACT
Background
Human milk oligosaccharides (HMOs) are complex glycans that are highly abundant in human milk. While over 150 HMOs have been identified, it is unknown how individual HMOs change in concentration over 24 months of lactation.
Objectives
To understand how HMO concentrations change over 24 months of lactation.
Methods
Breast milk samples were collected from participants in a longitudinal cohort study of Hispanic mother-infant pairs at 1, 6, 12, 18, and 24 months postpartum. Concentrations of 19 of the most abundant HMOs were measured using HPLC. Because the parent study is ongoing and not all participants have finished all time points yet, the sample sizes ranged per time point (n = 207 at 1 month; n = 109 at 6 months; n = 83 at 12 months; n = 59 at 18 months; and n = 28 at 24 months). Approximately 88% of participants were classified as HMO secretors—a genetic factor that affects concentrations of HMOs such as 2’fucosyllactose (2’FL) and lacto-N-fucopentaose I—while the remaining 12% were classified as nonsecretors. Mixed models were used to examine changes in HMO concentrations and relative abundances over the course of lactation.
Results
The majority of HMOs significantly decreased in concentration over the course of lactation. The exceptions were 2’FL, sialyl-lacto-N-tetraose b, and disialyl-lacto-N-tetraose, which did not change with time, and 3-fucosyllactose (3FL) and 3′-sialyllactose (3’SL), which significantly increased. The concentration of 3FL increased 10-fold, from 195 (IQR 138–415) μg/mL at 1 month to 1930 (1100–2630) μg/mL at 24 months, while 3’SL increased 2-fold, from 277 (198–377) μg/mL to 568 (448–708) μg/mL over the same time period.
Conclusions
These results indicate that HMOs do not decrease in concentration uniformly across lactation. In particular, 3FL and 3’SL increased over the course of lactation in this cohort. Future studies are required to fully understand the functions of these HMOs.
Keywords: human milk oligosaccharides, breast milk, changes, lactation, milk
Introduction
Human milk oligosaccharides (HMOs) are complex glycans that are highly abundant in human milk, comprising 8% of the solid component, following lactose (53%) and fat (32%) (1). HMOs are made up of glucose, galactose, N-acetylglucosamine, fucose, and sialic acid in various linkages, resulting in a diverse pool of complex oligosaccharides. Over 150 structurally unique HMOs have been identified, and the HMO composition varies between individuals (2, 3). Approximately 70% of the breastfeeding population are classified as secretors, while the remaining 30% are classified as nonsecretors (4, 5). Secretors have an active secretor (Se) gene, which encodes the enzyme α1–2-fucosyltransferase (FUT2). As a result, they have high concentrations of 2’-fucosyllactose (2’FL), lacto-N-fucopentaose I (LNFPI), and other α1–2-fucosylated HMOs, while nonsecretors have very low concentrations of α1–2-fucosylated HMOs such as lacto-N-fucopentaose II (LNFPII) and lacto-N-fucopentaose III (LNFPIII) (1). As a consequence, the HMO composition in the milk of secretor and nonsecretors is distinct.
HMOs have been historically difficult to measure and synthesize (1, 6). As a result, studies characterizing their concentrations or investigating their individual functions are limited. However, HMOs are known to resist digestion and are thought to act as prebiotics in the infant gut, promoting the proliferation of “good” bacteria (7, 8). There is also evidence that HMOs modulate immune responses (9), act as nutrients for brain development (for example, as a source of sialic acid) (10), and protect against necrotizing enterocolitis (11). However, the functions of most HMOs remain unknown. In order to better understand HMOs and their biological functions, it is important to first understand how individual HMO concentrations change over the course of lactation. This information could provide clues as to which HMOs might be most important at critical windows of infant development. However, the few existing studies examining changes in HMOs over lactation have been conducted using short-term data [first 3–4 months postpartum (3, 12–16)]. Therefore, the aim of the current study was to characterize changes in HMOs in breast milk over the period in which breastfeeding is recommended [up to 24 months postpartum (17)], as a secondary analysis of an existing longitudinal cohort of Hispanic mother-infant pairs.
Methods
Study participants
Milk samples were collected as part of an ongoing longitudinal cohort study based at the University of Southern California and Children's Hospital Los Angeles (NIH R01 DK110793 01A1). The parent study follows Hispanic mothers and their full-term infants from 1–24 months postpartum. The primary aim of the parent study is to examine relationships between HMOs, the infant gut microbiome, and infant growth and cognitive development in the first 2 years of life. The content of the current paper was a secondary aim stated in the original protocol. The inclusion criteria for participation in the parent study were: 1) self-identified Hispanic ethnicity (maternal and paternal); 2) singleton birth; 3) intention to breastfeed for at least 3 months; 4) enrollment within 1 month of the infant's birth; 5) willingness/ability to understand the procedures of the study; and 6) ability to read English or Spanish at a fifth-grade level. The exclusion criteria were: 1) physician diagnosis of a major medical illness (including type 1/type 2 diabetes) or an eating disorder in mothers; 2) physical, mental, or cognitive issues that would prevent participation; 3) chronic use of any medication that affects body weight or body composition, insulin resistance, or lipid profiles; 4) current smoking (more than 1 cigarette in the past week) or use of other recreational drugs; 5) clinical diagnosis of gestational diabetes; 6) preterm(<37 weeks’ gestation)/low birth weight (<2.5 kg) infants, or diagnosis of any fetal abnormalities; and 7) maternal age less than 18 years old.
Ethics approval
Institutional review boards at the University of Southern California and Children's Hospital Los Angeles approved the study; written informed consent was obtained from all mothers, and financial compensation was provided.
Breast milk sample collection
Breast milk was collected at least 1.5 hours after the previous feeding and after the mother had fasted at least 1 hour. Participants provided a single full breast expression from the right breast in our clinical unit using an electric breast pump, ensuring the collection of fore-, mid-, and hind-milk as previously described (18). Breast milk was then mixed, aliquoted (into 10 × 500μL tubes, and the remainder into 5mL tubes), and stored at −80°C.
HMO analysis
One 500μL aliquot per participant per time point was sent to the Bode Lab at the University of California, San Diego, on dry ice. HMOs are extremely stable and, unlike other human milk components, do not degrade by repeated freeze/thaw cycles or pasteurization (19). HMO analysis was performed by HPLC after fluorescent derivatization (Vanquish Quaternary HPLC–fluorescent detection, Thermo Fisher Scientific). Solid phase extraction over C18 and Carbograph was used to remove lipids, proteins, salts, and so forth; then, we labeled the reducing end of oligosaccharides with 2-aminobenzamide, and removed excess label by sold phase extraction over silica. To ensure that analyte loss during the extraction procedure was accounted for, raffinose was added at the beginning of sample processing. The following HMOs were measured: 2′-fucosyllactose (2′FL), 3-fucosyllactose (3FL), 3′-sialyllactose (3’SL), 6′-sialyllactose, difucosyllactose (DFL), difucosyl-lacto-N-hexaose (DFLNH), difucosyl-lacto-N-tetraose (DFLNT), disialyl-lacto-N-hexaose (DSLNH), disialyl-lacto-N-tetraose (DSLNT), fucosyl-disialyl-lacto-N-hexaose (FDSLNH), fucosyl-lacto-N-hexaose (FLNH), LNFPI, LNFPII, LNFPIII, lactose-N-hexaose (LNH), lacto-N-neotetraose (LNnT), lacto-N-tetraose (LNT), sialyl-lacto-N-tetraose b (LSTb), and sialyl-lacto-N-tetraose c (LSTc). These are 19 of the most abundant HMOs, representing over 95% of the total HMO concentration and all structural features of HMOs, including chain elongation, branching, and all known types of fucosylation and sialylation. The HMO sum was determined based on the AUC, modeled against standard response curves for each of the HMOs, then converted into μg/mL based on the molecular weight of each HMO. Secretor status was determined by the presence or near absence of 2’FL (<100 nmol/mL) and LNFPI (20, 21). The full methodology is included in the Supplemental Methods.
Statistical analysis
Because the study is still ongoing and not all participants have finished all time points yet, the sample sizes ranged per time point; in this analysis there were data from 207 participants at 1 month; 109 at 6 months; 83 at 12 months; 59 at 18 months; and 28 at 24 months. Mixed-effects models were used because of their ability to handle unequal sample sizes within subjects (22). Time and secretor status were considered as fixed effects, while participant ID was considered a random effect. . Data are presented as medians (IQRs). All statistical analyses were performed in RStudio (version 1.2.5042) (23).
Results
At baseline (1 month postpartum), 88% of participants were classified as HMO secretors—defined by the presence of 2’FL and LNFPI at the 1-month time point—while the remaining 12% were classified as nonsecretors (Table 1). The median maternal BMI at baseline was 29.7 kg/m2 (IQR, 26.8–33.1 kg/m2) and the median maternal age was 28.5 years (IQR, 24.2–34.0 years). The median infant weight was 3.4 kg (IQR, 3.1–3.6 kg) at birth, and 54.1% were female (Table 1).
TABLE 1.
Participant descriptive statistics for the current analysis of Hispanic mother-infant pairs at each time point
| 1 month | 6 months | 12 months | 18 months | 24 months | ||
|---|---|---|---|---|---|---|
| n = 207 | n = 119 | n = 83 | n = 59 | n = 28 | P value | |
| Mother | ||||||
| Pre-pregnancy maternal BMI, kg/m2 | 28.2 (23.9–31.9) | 27.8 (24.3–31.1) | 27.3 (23.4–31.0) | 27.3 (22.2–30.9) | 27.4 (23.1–31.3) | 0.53 |
| Maternal BMI at 1 month postpartum, kg/m2 | 29.7 (26.8–33.1) | 29.9 (26.5–33.7) | 28.7 (25.1–32.8) | 29.1 (25.0–33.1) | 29.5 (26.6–33.4) | 0.33 |
| Maternal age at delivery, years | 28.5 (24.2–34.0) | 28.0 (25.0–34.0) | 28.0 (25.0–33.5) | 28.0 (24.5–35.0) | 31.5 (25.0–36.2) | 0.81 |
| Hollingshead index of socioeconomic status | 25.0 (18.0–33.0) | 25.0 (18.5–33.0) | 25.5 (18.2–33.0) | 27.0 (15.8–33.0) | 23.5 (15.0–33.0) | 0.99 |
| Nonsecretors, n (%) | 24 (11.6%) | 15 (12.6%) | 7 (8.4%) | 5 (8.5%) | 2 (7.1%) | 0.85 |
| Infant/child | ||||||
| Infant birthweight, kg | 3.37 (3.13–3.66) | 3.35 (3.12–3.66) | 3.32 (3.15–3.63) | 3.35 (3.18–3.66) | 3.23 (3.13–3.43) | 0.8 |
| Female infant, n (%) | 112 (54.1) | 65 (54.6) | 47 (56.6) | 30 (50.8) | 15 (53.6) | 0.98 |
| Breastfeedings per day | 10.0 (7.5–10.0) | 5.0 (1.0–10.0) | 4.0 (0.0–7.0) | 4.0 (0.0–5.0) | 4.0 (3.0–6.0) | <0.0001 |
| Child weight, kg | 4.60 (4.20–4.90) | 7.90 (7.50–8.50) | 9.60 (9.10–10.40) | 11.20 (10.50–11.95) | 12.30 (11.55–12.80) | <0.0001 |
| Child length/height, cm | 54.5 (53.3–55.8) | 67.3 (66.0–68.5) | 75.5 (73.5–77.0) | 81.0 (78.5–83.2) | 85.9 (83.9–87.6) | <0.0001 |
| Child weight-for-age z-score | 0.33 (−0.19 to 0.79) | 0.38 (−0.20 to 0.95) | 0.32 (−0.29 to 0.93) | 0.40 (−0.06 to 1.00) | 0.27 (−0.27 to 0.76) | 0.63 |
| Child weight-for-length z-score | 0.44 (−0.28 to 1.31) | 0.44 (−0.02 to 0.95) | 0.36 (−0.40 to 1.00) | 0.68 (0.04–1.30) | 0.42 (0.12–1.63) | 0.22 |
| Child length-for-age z-score | −0.01 (−0.61 to 0.56) | 0.20 (−0.62 to 0.79) | 0.37 (−0.43 to 0.85) | −0.28 (−1.12 to 0.69) | −0.27 (−1.18 to 0.34) | 0.084 |
| Child BMI z-score | 0.41 (−0.16 to 1.09) | 0.35 (−0.12 to 0.84) | 0.23 (−0.31 to 1.09) | 0.82 (0.25–1.50) | 0.56 (0.19–1.63) | 0.015 |
| Child BMI percentile | 66.0 (43.5–86.0) | 64.0 (45.0–80.0) | 59.0 (38.0–86.5) | 79.0 (60.0–93.0) | 71.0 (58.0–94.5) | 0.018 |
| Child BMI category1 | — | — | — | — | — | 0.2 |
| Underweight | 7 (3.4) | 3 (2.5) | 1 (1.2) | 0 (0.0) | 0 (0.0) | |
| Normal | 142 (68.6) | 89 (74.8) | 60 (72.3) | 35 (59.3) | 16 (57.1) | |
| Overweight | 32 (15.5) | 14 (11.8) | 16 (19.3) | 12 (20.3) | 5 (17.9) | |
| Obese | 26 (12.6) | 13 (10.9) | 6 (7.2) | 12 (20.3) | 7 (25.0) | |
Note that this is an ongoing prospective longitudinal cohort study, whereby each time point represents individuals from earlier time points that have reached and completed that time point. P values represent a 1-way ANOVA testing the effect of time. Data are shown as either median (IQR) or frequency (%)
Underweight was classified as a BMI percentile <5; normal weight as a BMI percentile <85; overweight as a BMI percentile <95; and obese as a BMI percentile 95–100.
Effect of time on absolute HMO concentrations
There was a significant main effect of time on concentrations of 3FL, 3’SL, LNFPI, LNFPII, LNFPIII, LSTc, DFLNT, LNH, FLNH, DFLNH, FDSLNH, and DSLNH, and on the total HMO sum (Figure 1B, D, H, I, J, L, M, N, P, Q, R, S, and T). All of these HMOs significantly decreased in concentration from 1 to 24 months, with the exception of 3FL and 3’SL, which increased 10-fold and 2-fold, respectively (Figure 1B and D). The 2’FL, LSTb, and DSLNT concentrations did not significantly change over time (Figure 1A, K, and O).
FIGURE 1.
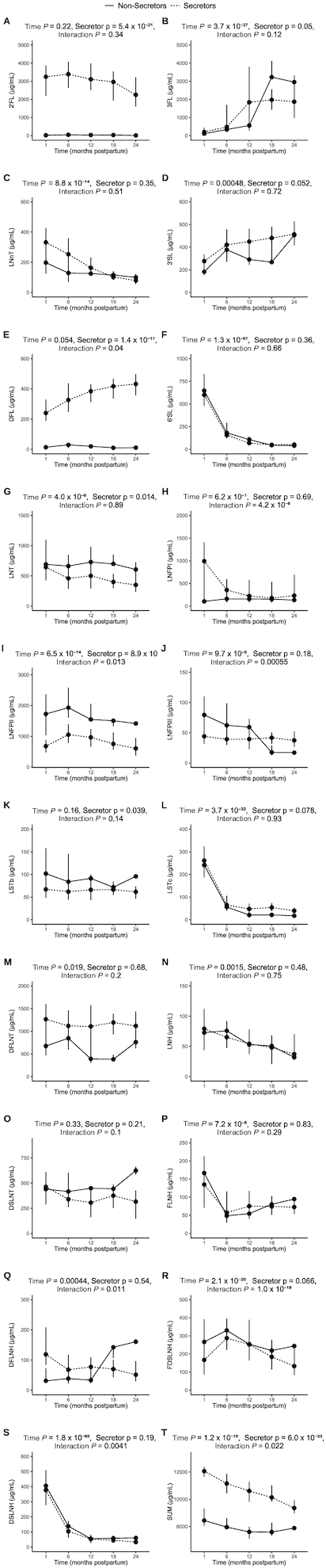
Concentrations of all measured HMOs from 1 to 24 months postpartum in breast milk from participants from the “Mother's Milk” study of Hispanic mother-infant pairs. (A) 2’FL; (B) 3FL; (C) LNnT; (D) 3’SL; (E) DFL; (F) 6’SL; (G) LNT; (H) LNFPI; (I) LNFPII; (J) LNFPIII; (K) LSTb; (L) LSTc; (M) DFLNT; (N) LNH; (O) DSLNT; (P) FLNH; (Q) DFLNH; (R) FDSLNH; (S) DSLNH; and (T) HMO sum. P values represent mixed-effects models where time and secretor status were considered fixed effects, while participant ID was considered a random effect. Data are shown as medians (IQRs). n = 207 (1 month); n = 119 (6 month); n = 83 (12 months); n = 59 (18 months); n = 28 (24 months). Abbreviations: 2’FL, 2′-fucosyllactose; 3FL, 3-fucosyllactose; 3’SL, 3′-sialyllactose; 6’SL, 6′-sialyllactose; DFL, difucosyllactose; DFLNH, difucosyl-lacto-N-hexaose; DFLNT, difucosyl-lacto-N-tetraose; DSLNH, disialyl-lacto-N-hexaose; DSLNT, disialyl-lactose-N-hexaose; FDSLNH, fucosyl-disialyl-lacto-N-hexaose; FLNH, fucosyl-lacto-N-hexaose; HMOs, human milk oligosaccharides; LNFPI, lacto-N-fucopentaose I; LNFPII, lacto-N-fucopentaose II; LNFPIII, lacto-N-fucopentaose III; LNH, lactose-N-hexaose; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetraose; LSTb, sialyl-lacto-N-tetraose b; LSTc, sialyl-lacto-N-tetraose c.
Effect of secretor status on absolute HMO concentrations
There was a significant main effect of secretor status on concentrations of 2’FL, 3FL, DFL, LNT, LNFPII, and LSTb, and on the total HMO sum (Figure 1A, B, E, G, I, K, and T). Of these HMOs, the 2’FL, 3FL, DFL, and LNFPI concentrations, as well as the total HMO sum, were higher in secretors versus nonsecretors, while the LNT, LNFPII, and LSTb concentrations were higher in nonsecretors versus secretors.
Effect of time x secretor status interaction on absolute HMO concentrations
There was a significant interaction between time and secretor status in concentrations of DFL, LNFPI, LNFPII, LNFPII, DFLNH, FDSLNH, and DSLNH, and in the total HMO sum (Figure 1E, H, I, J, Q, R, S, and T). DFL increased 2-fold from 1 to 24 months in secretors but not did not change over time in nonsecretors (Figure 1E). LNFPI decreased by 80% from 1 to 24 months in secretors but did not change in nonsecretors (Figure 1H). LNFPII remained relatively consistent in secretors but significantly decreased by 20% in nonsecretors (Figure 1I). LNFPIII stayed relatively constant in secretors but decreased almost 80% in nonsecretors (Figure 1J). DFLNH stayed constant in secretors but increased 4-fold from 1 to 24 months in nonsecretors (Figure 1Q). FDSLNH remained consistent in secretors but decreased 3-fold in nonsecretors (Figure 1R). Finally, the HMO sum decreased by 30% with time in secretors but remained relatively constant in nonsecretors (Figure 1T).
Supplemental Table 1 contains data on median (IQR) concentrations of each HMO depicted in Figure 1. Supplemental Table 2 contains the same data, except restricted to only those participants that had data available at all 5 time points. General findings were not affected by which data set was used.
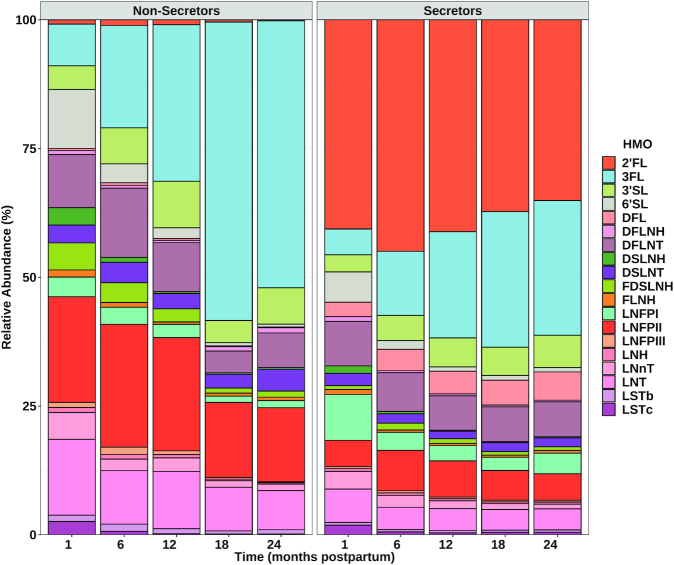
Effect of time on relative HMO concentrations
Figure 2 depicts how the relative abundances of individual HMOs changed over the course of lactation, split by secretor status. Supplemental Table 3 contains the P values to accompany Figure 2. There was a significant main effect of time on the relative abundance of all HMOs except 2’FL, DFL, and DSLNT. These trends generally followed the same patterns as absolute concentrations, whereby most HMOs decreased in relative abundance, with the exception of 3FL and 3’SL, which increased in relative abundance over the course of lactation. The concentration of 2’FL remained relatively consistent and was relatively abundant from 1 to 24 months, and was still the most abundant HMO overall at 24 months.
FIGURE 2.
Relative abundance of all measured HMOs from 1 to 24 months postpartum in breast milk from participants from the “Mother's Milk” study of Hispanic mother-infant pairs. The order of HMOs in the legend represents the order of colors in the figure. P values for differences can be found in Supplemental Table 3. n = 207 (1 month); n = 119 (6 month); n = 83 (12 months); n = 59 (18 months); n = 28 (24 months). Abbreviations: 2’FL, 2′-fucosyllactose; 3FL, 3-fucosyllactose; 3’SL, 3′-sialyllactose; 6’SL, 6′-sialyllactose; DFL, difucosyllactose; DFLNH, difucosyl-lacto-N-hexaose; DFLNT, difucosyl-lacto-N-tetraose; DSLNH, disialyl-lacto-N-hexaose; DSLNT, disialyl-lactose-N-hexaose; FDSLNH, fucosyl-disialyl-lacto-N-hexaose; FLNH, fucosyl-lacto-N-hexaose; HMOs, human milk oligosaccharides; LNFPI, lacto-N-fucopentaose I; LNFPII, lacto-N-fucopentaose II; LNFPIII, lacto-N-fucopentaose III; LNH, lactose-N-hexaose; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetraose; LSTb, sialyl-lacto-N-tetraose b; LSTc, sialyl-lacto-N-tetraose c.
Effect of secretor status on relative HMO concentrations
There was a significant main effect of secretor status on the relative abundance of all HMOs except LNnT, 3SL, LNFPI, and LSTc. Only 2’FL and DFL were higher in relative abundance in secretors, while 3FL, 6SL, LNT, LNFPII, LNFPIII, LSTb, DSLNT, FDSLNH, and DSLNH were higher in relative abundance in nonsecretors.
Effect of time x secretor status interaction on relative HMO concentrations
There were also significant interactions between time and secretor status in the relative abundances of 3FL, LNnT, DFL, 6SL, LNFPI, LNFPII, LNFPIII, LSTb, LSTc, DFLNT, DFLNH, FDSLNH, and DSLNH. While the differences in changes over time between the 2 secretor groups were subtle for most of these HMOs, it is apparent that there was a greater increase in the relative abundance of 3FL in nonsecretors (20-fold) compared to in secretors [10-fold; from 2.4% (IQR 1.8–5.1%) to 24% (IQR 16–37%) in secretors vs. from 2.5% (IQR 1.1–6.1%) to 52% (IQR 47–57%) in nonsecretors].
There were no effects of any other factors (maternal age, BMI, or socioeconomic status) on changes in HMOs over time.
Discussion
The aim of this study was to characterize changes in individual HMOs spanning the entire period in which breastfeeding is recommended (up to 24 months postpartum) (17). Most of the HMOs examined decreased in both absolute concentration and relative abundance over the course of lactation. However, 2’FL, LSTb, and DSLNT remained relatively constant, while only 2 HMOs—3’SL and 3FL—increased in concentration with time. In addition to the previously documented differences in HMO concentrations between secretors and nonsecretors, a number of HMOs changed differently over the course of lactation in secretors and nonsecretors.
To our knowledge, all previous studies of HMO changes over time have been based on short-term data (3–5 months). Samuel et al. (12) examined HMO compositions over the first 4 months of lactation in a cohort of 370 European mothers. Coppa et al. (16) examined HMO changes over the first 3 months of lactation in 18 mothers from Italy. Ferreira et al. (14) examined HMO profiles in 101 Brazilian mothers from 0–4 months. Wu et al. (15) examined HMO compositions in a cohort of 222 mothers from 0–5 months postpartum. Sprenger et al. (13) examined HMO compositions over the first 4 months of lactation in a cohort of 50 Singaporean mothers. Finally, Thurl et al. (3) examined HMO compositions over the first 3 months of lactation in a cohort of 175 German women. In general, these previous studies reported that all HMOs decreased over the course of their respective study periods, with the exception of 3FL, which increased. Sprenger et al. (13), however, did not report on 3FL. Only Ferreira et al. (14) reported an increase in 3’SL in addition to 3FL. Similarly, both 3’SL and 3FL increased with time in the current study. Further, 2’FL, LSTb, and DSLNT concentrations did not change over the course of 24 months of lactation in the current study, which is in contrast to the majority of these previous, shorter-term studies, where concentrations of these HMOs decreased with time. However, Ferreira et al. (14) similarly reported no changes in 2’FL or LSTb concentrations, although they did see a significant decrease in the DSLNT concentration over time. It is possible that ethnic differences are responsible for the discrepancies across studies (predominately white European participants in Samuel et al. (12) and Thurl et al. (3), Chinese Singaporean participants in Sprenger at al. (13), Chinese participants in Wu et al. (15), Italian participants in Coppa et al. (16), Brazilian participants in Ferreira et al. (14), and Hispanic participants in the current study). It has been previously established that HMO concentrations differ by ethnicity and geographic location (24). For example, the mean concentration of 3FL is >4 times higher in milk collected in Sweden than in milk collected in rural Gambia, while DSLNT is twice as rich in milk from rural Gambia than in milk from Sweden, although the reasons for these ethnic differences are unknown (24). In any case, the results of the current study suggest that concentrations of most HMOs decrease over the course of lactation, with the exception of 3FL and 3’SL, which increase over the course of lactation, and 2’FL, LSTb, and DSLNT, which do not significantly change over the course of lactation.
The fact that these HMOs were stable or increased over the course of lactation suggests that they may have important biological functions that extend beyond the first few months of life. For example, 2’FL is among the most studied HMOs, and has been implicated in stimulating brain development (10, 25), improving cognitive outcomes (26), and rapidly increasing infant weight gain (27). DSLNT is most well known for preventing necrotizing enterocolitis, 1 of the most common and severe intestinal disorders in preterm infants (11, 28, 29). There are limited data on the function of LSTb, although it is similar in structure to DSLNT (30). Both 3FL and 2’FL have been found to bind norovirus, naturally acting as decoys to prevent norovirus infection (31–33). In addition, higher 3FL and 2’FL concentrations have been associated with reduced mortality in uninfected children born to mothers living with HIV (34). Also, 3’SL has been implicated in promoting intestinal epithelial cell maturation (35) and in preventing infections of influenza viruses (36, 37). It is therefore possible that increased concentrations of 3FL, 3’SL, and 2’FL provide ongoing immune support for the growing infant, although the functions of these HMOs cannot be determined with the current data and further investigation is required.
Strengths of this study include the numerous time points and the duration of time assessed (encompassing the entire 2-year period in which breastfeeding is recommended), the number of individual HMOs measured, and the mixed model design, which allowed for follow-up even with unequal sample sizes at each time point. Limitations include the diminishing sample size with time, including a small sample size of just 28 participants at 24 months. We also only captured 2 samples between birth and 6 months, which is the recommended period of exclusive breastfeeding. However, as mentioned, time points within this period have been explored in a number of previous studies, and the focus of the current study was to examine longer-term changes. Further, it should be acknowledged that this analysis does not take into account whether the degree of breastfeeding (exclusive breastfeeding, partial breastfeeding) affects concentrations of HMOs in breast milk. Indeed, this is a limitation of the field in general. A prior study did compare HMO concentrations with actual HMO intake by infants (measured by weighing infants before and after each breastfeeding over a 24-hour period), and found no significant difference in results between the 2 methods (27). However, whether the degree of breastfeeding affects human milk HMO concentrations needs to be further investigated. In addition, findings were limited to Hispanic mother-infant pairs in the Southwestern United States, and are not generalizable to other ethnicities or geographic locations. Future studies should examine these same changes over lactation in larger and more diverse cohorts, as well as examine any correlations with maternal and infant geno- and phenotypes. More research is also required to understand the biological functions of the specific HMOs that increased or were stable over time.
In conclusion, the results of this study suggest that while most HMOs diminish in concentration over 24 months of lactation, some (3FL and 3’SL) actually increase with time and others (2’FL, DSLNT, and LSTb) stay relatively consistent. Future research should aim to determine the functions of these HMOs.
Supplementary Material
Acknowledgments
We thank Carla Flores, Danielle Garcia, Elizabeth Campbell, Claudia Rios, Emily Leibovitch, Rosa Rangel, and the entire Goran Lab for their assistance in obtaining this data. We would also like to thank Dr. Jennifer Fogel for her editing assistance on the manuscript.
The authors’ responsibilities were as follows—JFP: performed the statistical analysis and primarily constructed the manuscript; PKB, RBJ, and TLA: assisted in the statistical analysis and interpretation of results; CY, JAN, and SK: quantified human milk oligosaccharides (HMOs); LB: supervised the HMO analysis and provided expertise in the interpretation of results; MIG: conceived of the presented idea and supervised the project; and all authors: discussed the results, contributed to the final manuscript, and read and approved the final manuscript.
Notes
Research reported in this publication was supported by the National Institute Diabetes and Digestive and Kidney Diseases (R01 DK110793). This work was also funded by the Gerber Foundation (15PN-013).
The funding source had no role in the design, execution, analyses, interpretation of data, writing of the report, or decision to submit the report for publication.
Author disclosures: PKB is funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (K99 HD098288). TLA is funded by the National Institute of Environmental Health Sciences (R00 ES027853). All the other authors report no conflicts of interest.
Supplemental Tables 1–3 and Supplemental Methods are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at http://jn.nutrition.org.
Abbreviations used: 2’FL, 2′-fucosyllactose; 3FL, 3-fucosyllactose; 3’SL, 3′-sialyllactose; DFL, difucosyllactose; DFLNH, difucosyl-lacto-N-hexaose; DFLNT, difucosyl-lacto-N-tetraose; DSLNH, disialyl-lacto-N-hexaose; DSLNT, disialyl-lacto-N-tetraose; FDSLNH, fucosyl-disialyl-lacto-N-hexaose; FLNH, fucosyl-lacto-N-hexaose; HMOs, human milk oligosaccharides; LNFPI, lacto-N-fucopentaose I; LNFPII, lacto-N-fucopentaose II; LNFPIII, lacto-N-fucopentaose III; LNH, lactose-N-hexaose; LNnT, lacto-N-neotetraose; LNT, lacto-N-tetraose; LSTb, sialyl-lacto-N-tetraose b; LSTc, sialyl-lacto-N-tetraose c.
Contributor Information
Jasmine F Plows, Children's Hospital Los Angeles, The Saban Research Institute, Los Angeles, CA, USA.
Paige K Berger, Children's Hospital Los Angeles, The Saban Research Institute, Los Angeles, CA, USA.
Roshonda B Jones, Children's Hospital Los Angeles, The Saban Research Institute, Los Angeles, CA, USA.
Tanya L Alderete, University of Colorado Boulder, Boulder, CO, USA.
Chloe Yonemitsu, Department of Pediatrics and Mother-Milk-Infant Center of Research Excellence, University of California, San Diego, La Jolla, CA, USA.
Julia A Najera, Department of Pediatrics and Mother-Milk-Infant Center of Research Excellence, University of California, San Diego, La Jolla, CA, USA.
Sadaf Khwajazada, Department of Pediatrics and Mother-Milk-Infant Center of Research Excellence, University of California, San Diego, La Jolla, CA, USA.
Lars Bode, Department of Pediatrics and Mother-Milk-Infant Center of Research Excellence, University of California, San Diego, La Jolla, CA, USA.
Michael I Goran, Children's Hospital Los Angeles, The Saban Research Institute, Los Angeles, CA, USA.
References
- 1. Bode L. Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology. 2012;22:1147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chaturvedi P, Warren CD, Altaye M, Morrow AL, Ruiz-Palacios G, Pickering LK, Newburg DS. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology. 2001;11:365–72. [DOI] [PubMed] [Google Scholar]
- 3. Thurl S, Munzert M, Henker J, Boehm G, Müller-Werner B, Jelinek J, Stahl B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br J Nutr. 2010;104:1261–71. [DOI] [PubMed] [Google Scholar]
- 4. Azad MB, Vehling L, Chan D, Klopp A, Nickel NC, McGavock JM, Becker AB, Mandhane PJ, Turvey SE, Moraes TJet al. Infant feeding and weight gain: separating breast milk from breastfeeding and formula from food. Pediatrics. 2018;142:e20181092. [DOI] [PubMed] [Google Scholar]
- 5. Saboor M, Ullah A, Qamar K, Mir A, Mounaddin. Frequency of ABH secretors and non secretors: a cross sectional study in Karachi. Pak J Med Sci. 2014;30:189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Leeuwen SS. Challenges and pitfalls in human milk oligosaccharide analysis. Nutrients. 2019;11:2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asakuma S, Hatakeyama E, Urashima T, Yoshida E, Katayama T, Yamamoto K, Kumagai H, Ashida H, Hirose J, Kitaoka M.. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J Biol Chem. 2011;286:34583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marcobal A, Barboza M, Froehlich JW, Block DE, German JB, Lebrilla CB, Mills DA. Consumption of human milk oligosaccharides by gut-related microbes. J Agric Food Chem. 2010;58:5334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bode L, Kuhn L, Kim H-Y, Hsiao L, Nissan C, Sinkala M, Kankasa C, Mwiya M, Thea DM, Aldrovandi GM.. Human milk oligosaccharide concentration and risk of postnatal transmission of HIV through breastfeeding. Am J Clin Nutr. 2012;96:831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oliveros E, Ramirez M, Vazquez E, Barranco A, Gruart A, Delgado-Garcia JM, Buck R, Rueda R, Martin MJ. Oral supplementation of 2’-fucosyllactose during lactation improves memory and learning in rats. J Nutr Biochem. 2016;31:20–7. [DOI] [PubMed] [Google Scholar]
- 11. Bode L. Human milk oligosaccharides in the prevention of necrotizing enterocolitis: a journey from in vitro and in vivo models to mother-infant cohort studies. Front Pediatr. 2018;6:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Samuel TM, Binia A, de Castro CA, Thakkar SK, Billeaud C, Agosti M, Al-Jashi I, Costeira MJ, Marchini G, Martínez-Costa Cet al. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci Rep. 2019;9:11767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sprenger N, Lee LY, De Castro CA, Steenhout P, Thakkar SK.. Longitudinal change of selected human milk oligosaccharides and association to infants’ growth, an observatory, single center, longitudinal cohort study. PLoS One. 2017;12:e0171814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferreira AL, Alves R, Figueiredo A, Alves-Santos N, Freitas-Costa N, Batalha M, Yonemitsu C, Manivong N, Furst A, Bode Let al. Human milk oligosaccharide profile variation throughout postpartum in healthy women in a Brazilian cohort. Nutrients. 2020;12:790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu J, Wu S, Huo J, Ruan H, Xu X, Hao Z, Wei Y.. Systematic characterization and longitudinal study reveal distinguishing features of human milk oligosaccharides in China. Curr Dev Nutr. 2020;4:(8):nzaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coppa GV, Pierani P, Zampini L, Carloni I, Carlucci A, Gabrielli O.. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr Suppl. 1999;88:89–94. [DOI] [PubMed] [Google Scholar]
- 17. CDC . Breastfeeding report card, 2018. [Internet]. Atlanta (GA): 2018. [Accessed 2020 Sep 18].Available from: https://www.cdc.gov/breastfeeding/pdf/2018breastfeedingreportcard.pdf. [Google Scholar]
- 18. Fields DA, Demerath EW. Relationship of insulin, glucose, leptin, IL-6 and TNF-α in human breast milk with infant growth and body composition. Pediatr Obes. 2012;7:304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hahn W, Kim J, Song S, Park S, Kang NM.. The human milk oligosaccharides are not affected by pasteurization and freeze-drying. J Matern Fetal Neonatal Med. 2019;32:985–91. [DOI] [PubMed] [Google Scholar]
- 20. Azad MB, Robertson B, Atakora F, Becker AB, Subbarao P, Moraes TJ, Mandhane PJ, Turvey SE, Lefebvre DL, Sears MRet al. Human milk oligosaccharide concentrations are associated with multiple fixed and modifiable maternal characteristics, environmental factors, and feeding practices. J Nutr. 2018;148:1733–42. [DOI] [PubMed] [Google Scholar]
- 21. Cabrera-Rubio R, Kunz C, Rudloff S, García-Mantrana I, Crehuá-Gaudiza E, Martínez-Costa C, Collado MC. Association of maternal secretor status and human milk oligosaccharides with milk microbiota: an observational pilot study. J Pediatr Gastroenterol Nutr. 2019;68:256–63. [DOI] [PubMed] [Google Scholar]
- 22. Detry MA, Ma Y. Analyzing repeated measurements using mixed models. JAMA. 2016;315:407–8. [DOI] [PubMed] [Google Scholar]
- 23. R Core Team . R: a language and environment for statistical computing [Internet]. Vienna (Austria): R Foundation for Statistical Computing; 2019. Available from: https://www.R-project.org/. [Google Scholar]
- 24. McGuire MK, Meehan CL, McGuire MA, Williams JE, Foster J, Sellen DW, Kamau-Mbuthia EW, Kamundia EW, Mbugua S, Moore SEet al. What's normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am J Clin Nutr. 2017;105:1086–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vázquez E, Barranco A, Ramírez M, Gruart A, Delgado-García JM, Martínez-Lara E, Blanco S, Martín MJ, Castanys E, Buck Ret al. Effects of a human milk oligosaccharide, 2’-fucosyllactose, on hippocampal long-term potentiation and learning capabilities in rodents. J Nutr Biochem. 2015;26:455–65. [DOI] [PubMed] [Google Scholar]
- 26. Berger PK, Plows JF, Jones RB, Alderete TL, Yonemitsu C, Poulsen M, Ryoo JH, Peterson BS, Bode L, Goran MI. Human milk oligosaccharide 2’-fucosyllactose links feedings at 1 month to cognitive development at 24 months in infants of normal and overweight mothers. PLoS One. 2020;15:e0228323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Larsson MW, Lind MV, Laursen RP, Yonemitsu C, Larnkjær A, Mølgaard C, Michaelsen KF, Bode L. Human milk oligosaccharide composition is associated with excessive weight gain during exclusive breastfeeding–an explorative study. Front Pediatr. 2019;7:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Autran CA, Kellman BP, Kim JH, Asztalos E, Blood AB, Spence ECH, Patel AL, Hou J, Lewis NE, Bode L.. Human milk oligosaccharide composition predicts risk of necrotising enterocolitis in preterm infants. Gut. 2018;67:1064–70. [DOI] [PubMed] [Google Scholar]
- 29. Jantscher-Krenn E, Zherebtsov M, Nissan C, Goth K, Guner YS, Naidu N, Choudhury B, Grishin AV, Ford HR, Bode L.. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut. 2012;61:1417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moossavi S, Atakora F, Miliku K, Sepehri S, Robertson B, Duan QL, Becker AB, Mandhane PJ, Turvey SE, Moraes TJet al. Integrated analysis of human milk microbiota with oligosaccharides and fatty acids in the CHILD cohort. Front Nutr. 2019;6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jiang X, Huang P, Zhong W, Tan M, Farkas T, Morrow AL, Newburg DS, Ruiz-Palacios GM, Pickering LK.. Human milk contains elements that block binding of noroviruses to human histo-blood group antigens in saliva. J Infect Dis. 2004;190:1850–9. [DOI] [PubMed] [Google Scholar]
- 32. Shang J, Piskarev VE, Xia M, Huang P, Jiang X, Likhosherstov LM, Novikova OS, Newburg DS, Ratner DM.. Identifying human milk glycans that inhibit norovirus binding using surface plasmon resonance. Glycobiology. 2013;23:1491–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weichert S, Koromyslova A, Singh BK, Hansman S, Jennewein S, Schroten H, Hansman GS.. Structural basis for norovirus inhibition by human milk oligosaccharides. J Virol. 2016;90:4843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuhn L, Kim H-Y, Hsiao L, Nissan C, Kankasa C, Mwiya M, Thea DM, Aldrovandi GM, Bode L. Oligosaccharide composition of breast milk influences survival of uninfected children born to HIV-infected mothers in Lusaka, Zambia. J Nutr. 2015;145:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Holscher HD, Bode L, Tappenden KA.. Human milk oligosaccharides influence intestinal epithelial cell maturation in vitro. J Pediatr Gastroenterol Nutr. 2017;64:296–301. [DOI] [PubMed] [Google Scholar]
- 36. Kwon S-J, Na DH, Kwak JH, Douaisi M, Zhang F, Park EJ, Park J-H, Youn H, Song C-S, Kane RSet al. Nanostructured glycan architecture is important in the inhibition of influenza A virus infection. Nature Nanotech. 2017;12:48–54. [DOI] [PubMed] [Google Scholar]
- 37. Zevgiti S, Zabala JG, Darji A, Dietrich U, Panou-Pomonis E, Sakarellos-Daitsiotis M.. Sialic acid and sialyl-lactose glyco-conjugates: design, synthesis and binding assays to lectins and swine influenza H1N1 virus. J Pept Sci. 2012;18:52–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.