Abstract
The risk of Coronavirus infection continues, and the fear of resurgence indicates the lack of a successful therapeutic strategy. In severe COVID-19 infection, many immune cells and their products are involved, making management difficult. The abundant release of cytokines and chemokines in severe COVID-19 patients leads to profound hyper inflammation and the mobilization of immune cells, triggering the cytokine storm. The complications associated with the cytokine storm include severe respiratory distress, intravascular coagulation, multi-organ failure, and death. The enormous formation of interleukin (IL)-6 and hemopoietic factors such as granulocyte–macrophage colony-stimulating factor (GM-CSF) are implicated in the severity of the infection. Moreover, these inflammatory cytokines and factors signal through the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway causing the activation of cytokine-related genes. The neutralization of these proteins could be of therapeutic help in COVID-19 patients and could mitigate the risk of mortality. IL-6 antagonist, IL-6 receptor antagonists, GM-CSF receptor inhibitors, and JAK-STAT inhibitors are being investigated to prevent intense lung injury in COVID-19 patients and increase the chances of survival. The review focuses the role of IL-6, GM-CSF, and JAK-STAT inhibitors in regulating the immune response in severely affected COVID-19 patients.
Keywords: COVID-19, Cytokine storm, IL-6 inhibitors, GM-CSF inhibitors, JAK-STAT inhibitors
1. Introduction
COVID-19 infection has been unstoppable so far, with over 78,604,532 confirmed cases and 1,744,235 deaths worldwide, as reported on the 26th of December 2020 (WHO 2020). In most of the infected COVID-19 patients, the symptoms are mild or moderate but could be deadly and life-threatening in a few. Clinical manifestations in severe cases are not restricted to the respiratory system but can inadvertently affect other organ systems (Singal et al., 2020). Accordingly, symptomatic manifestation in mild cases include cough, headache, and fever. In contrast, in severe cases, the occurrence of hyper inflammation, extensive lung involvement, multi-organ failure, acute respiratory distress syndrome (ARDS), and death have been reported (Geier and Geier, 2020, Song et al., 2020). In COVID-19 infected cases, the complications reported include thromboembolic stroke (Oxley et al., 2020), cardiac complications (Zhou et al., 2020), acute left ventricular disturbances (Zhou et al., 2020), dysrhythmia (Driggin et al., 2020), heart failure (Huang et al., 2019, Ruan et al., 2020), transient ischemic attack (Sharifian-Dorche et al., 2020), neurological complications affecting the central and peripheral nervous system (Shekhar et al., 2020). Healthcare providers are grappling to find the best alternative to combat the persistent spread of infection.
Although vaccines generally take more than a decade for development, the turnaround time for the coronavirus vaccine is relatively short. Despite this, the time to reach the masses is unpredictable. Also, the lack of specific drugs has made the situation intense and grim. Hence, more robust treatment strategies have been investigated to manage the COVID-19 crisis.
Moreover, in COVID-19 infected cases, exacerbation of the condition and the severity of the infection is seen due to an upregulated immune system. As there is a strong association between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and the immune system (Coperchini et al., 2020), biologics are used based on anecdotal evidence to block or antagonize specific immune pathways or cytokines or their receptors and blunt the immune response. Biologics are genetically engineered products used to manage rheumatoid arthritis, psoriatic arthritis (Megna et al., 2020), spondylitis, and Crohn’s disease (Becherer et al., 2020). IL-6, and GM-CSF (Huang et al., 2019, Zhou et al., 2020a) are elevated far beyond their threshold range in severe COVID-19 cases. These cytokines signal through the JAK/STAT pathway upregulating other signaling pathways, enhancing the expression of cytokines as well as chemokines. This narrative review deals with advocating repurposed biologics targeting IL-6, GM-CSF, and JAK-STAT pathways to manage severe SARS-CoV-2 infection.
2. Data sources
A literature search was conducted between September 1, 2020 and September 20, 2020 on PubMed, and Google Scholar to identify publications in English language related to biologics used in COVID-19. The search was conducted with the following keywords: “COVID-19, “severe acute respiratory syndrome coronavirus 2 infection”, “SARS‐CoV‐2 infection”, “cytokine storm”, “severe COVID-19”, “hyperinflammation”, “lung injury”, “biologics”, cytokine antagonists”, “Interleukin inhibitors”, “Granulocyte-Macrophage-Colony Stimulating Factor”, “JAK-STAT inhibitors”. Randomized clinical trials, case reports, articles containing information on the pharmacodynamics, pharmacokinetics and safety was introspected for pertinent information. The information on ongoing studies was retrieved from ClinicalTrials.gov., 2020, and the US Food and Drug Administration (FDA).
3. Hyperinflammation and the cytokine storm: A complex manifestation
3.1. COVID-19 infections: Activation of immune cells
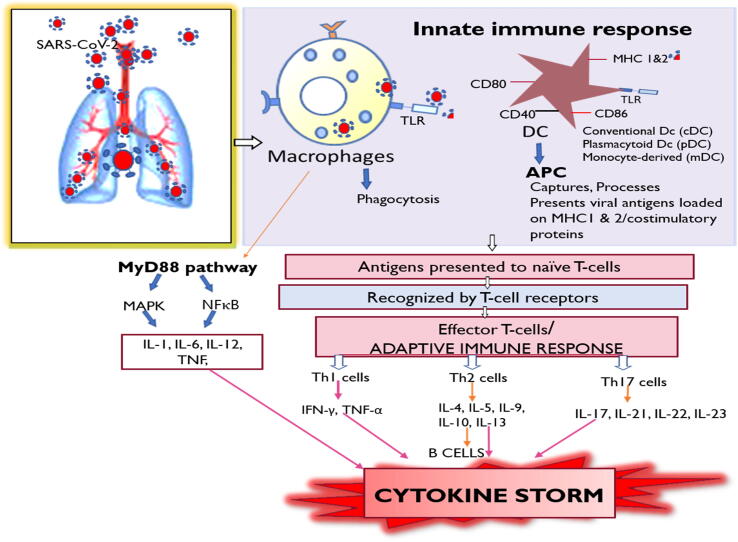
The human body contains a robust immune system to combat infections. The innate and adaptive immune system work in unison through an arsenal of cells that identify and destroy foreign intruders. As the respiratory tract is continuously exposed to pathogens and irritants, the resident and constantly patrolling immunologic sentinels are alarmed and sensitized. In the COVID-19 viral infection, as in other infections, an early immune response is mediated through dendritic cells (DC), monocyte-derived macrophages (Liao et al. 2020), and alveolar macrophages. DC has a prominent role in antigen presentation, while macrophages are responsible for endocytosis and viral digestion (Fig. 1). The release of cytokines facilitates the recruitment of polymorphonuclear leukocytes to the site to enhance viral clearance.
Fig. 1.
The entry of the virus leads to activation of the innate immune system which comprises of macrophages and dendritic cells. Coronavirus antigens are presented by the dendritic cells (DC) which serve as antigen presenting cells (APC) which load viral antigens on MHC-1 and 2 to the T cells leading to the activation of the adaptive immune system. Activation of cytotoxic T cells leads to the proliferation of Th1 cells, Th2 cells and Th17 cells. This leads to the elaboration of several cytokines and chemokines producing the cytokine storm. Also, the activated macrophages could stimulate the MyD88 pathway leading to the activation of downstream signaling pathways such as MAPK and NFκB resulting in the expression of cytokines. MHC: major histocompatibility complex; IL: Interleukins; TNF: tumor necrosis factor; IFNγ: Interferons; MyD88: Myeloid differentiation primary response 88; MAPK: Mitogen activated protein kinase; NFκB: Nuclear factor kappa B.
Cell-surface or endosomal receptors such as Toll-like receptors or cytosolic receptors such as NOD-like receptors present on DC and macrophages detect SARS-CoV-2 nucleic acids (Li et al., 2020). Apart from these, DCs and macrophages have a deluge of receptors expressed on their surface. Immunoglobulins receptors (fcR) complement receptors (CR), mannose receptors (MR), and scavenger receptors are abundantly expressed on the macrophage surface (Gordon and Read, 2002). Also, major histocompatibility class (MHC) 1 or 2 molecules present on the surface of these cells virtually recognize and take up the SARS-CoV-2 viral antigen. However, co-stimulatory molecules expressed on DC, such as CD40, CD80, CD86, and CCR2, facilitate recognition. Antigenic peptides loaded on MHC are presented to the T cells leading to their transformation into effector T lymphocytes. It promotes natural killer cell activity, and enhances the formation of antibodies, thereby initiating the adaptive immune response. Alternatively, activated DC and macrophages amplify the innate immune response by overproducing cytokines and chemokines (Costela-Ruiz et al., 2020). These products then partake in the pro-inflammatory or anti-inflammatory response.
3.2. SARS-CoV-2: Alteration in immune cells dynamics
The neutrophil count is enhanced, and monocyte-derived macrophages are elevated compared with alveolar macrophages in COVID-19 infected patients (Qin et al., 2020). Peripheral lymphopenia is evident in 80% of the severely infected patients (Jamilloux et al., 2020), and the lymphocyte to neutrophil ratio is significantly reduced (Copaescu et al., 2020). COVID-19 patients have shown stark exhaustion of cytotoxic T cells (Chen et al., 2020a, Chen et al., 2019, Chen et al., 2020b, Diao et al., 2019), B cells (Wang et al., 2020), and natural killer (NK) cells (Kuri-Cervantes et al., 2020). Reduction in the regulatory T cells (Kuri-Cervantes et al., 2020, Qin et al., 2020) leads to a decline in the secretion of anti-inflammatory cytokines such as IL-10 and TGF-β1. It also leads to an unabated proliferation of antigen-specific T-cells (Qin et al., 2020). In COVID-19 patients, phenotypic changes are characterized by the increased expression of markers such as CD38, CD69, and CD44 on CD8 + T-cells. Moreover, activation of 41BB, a co-stimulatory molecule, was significantly enhanced compared to healthy controls and intensive care unit (ICU) admitted COVID-19 patients. Substantially high CD14 + CD16 + inflammatory monocytes and more expression of Tim3 and PD-1 inhibitory proteins occur in both the T-cell subsets (Chen et al., 2020a, Chen et al., 2019, Chen et al., 2020b). COVID-19 patients with the severe pulmonary syndrome also had higher GM-CSF + IFNg + Th1 cells (Zhou et al., 2020). Besides, the involvement of T helper 17 (TH17) in the pathological manifestations of COVID-19 symptoms is also suggested (Chen and John Wherry, 2020).
3.3. Cytokines, chemokines, and the cytokine storm
In severe COVID-19 infected cases, intense inflammation occurs (Huang et al., 2019) due to the release of pro-inflammatory cytokines such as IL-1α, IL-1β, IL-6, IL-8, IL-12, IL-17, and TNF-α (tumor necrosis factor-α). Besides, chemokines such as IFN-β, RANTES (CCL5), macrophage inflammatory protein (MIP)‐1α (CCL3), MIP-1β (CCL4), IFN-γ-inducible protein 10 (IP-10, CXCL10), and MCP (monocyte chemoattractant protein; CCL2) promote the chemotaxis of immature DC, neutrophils and stimulate NK cells (Sallusto et al., 1999). DC and macrophages enhance the release of IFN-γ, which inhibits viral replication and improves the secretion of cytokines and chemokines. Neutrophils also increase the release of cytokines, chemokines, and TNF family cytokines. Airway inflammation can also be mediated by IL-17/IL-23 cytokine members, as they bolster the expression of pro-inflammatory mediators (Bordon et al., 2013).
Effector T-cells enhance the formation of IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, IL-23, and TNF-α (Fig. 1). One of the most prominent predictors of injury is the exponential rise in the levels of IL-6, mostly responsible for the acute phase response, and activation of TH17 cells (Cifaldi et al., 2015, Liao et al., 2020). In severely infected cases, granulocyte-colony stimulating factor (g-CSF), monocyte chemoattractant protein, interferon-γ inducible protein 10 (CXCL100), and MIP-1α trigger a severe systemic inflammatory response. A cytokine environment induces inflammatory CD14 + CD16 + monocytes, which expresses IL-6 and accelerates inflammation. SARS-CoV-2 virus causes aberrant immune activation and massive release of cytokines culminating in the cytokine storm or Cytokine Release Syndrome (CRS).
3.4. Manifestations of the cytokine storm
The unprecedented release of cytokines alters vascular permeability, increases extravasation of fluid, and activate the coagulation cascade—a severe life-threatening inflammatory lung injury called ARDS. In ARDS, fluid accumulates within the alveoli leading to labored breathing, hypoxemia, hypotension, ultimately causing respiratory and multi-organ failure (Cameron et al., 2008, Coperchini et al., 2020, Zhang et al., 2020a, Zhang et al., 2020b). In ADRS, inflammatory mediators promote the chemotaxis of neutrophils across the vascular endothelium and alveolar epithelial surfaces. As the immunological and clinical features of SARS-CoV-2 infection are identical to secondary hemophagocytic lymphohistiocytosis (sHLH), there is a perception that severe COVID infection could be a form of sHLH.
3.5. IL-6 and their inhibitors
IL-6 is a pro-inflammatory cytokine produced by Th1 cells (GM-CSF + IFN-γ + ) and inflammatory monocytes (CD14 + CD16 + lymphocytes, monocytes, fibroblasts, especially from bronchial epithelial cells. In severe COVID-19 patients, substantial inflation in the levels of IL-6 is observed (Atal and Fatima, 2020, Chen et al., 2020a, Chen et al., 2019, Chen et al., 2020b). IL-6 facilitates the differentiation of monocytes into macrophages, recruits immune cells to the site of injury, and enhances cytokine formation. IL-6 is a strong predictive marker for hyper inflammation and the cytokine storm. Elevation in the levels of IL-6 also results in lymphopenia (Chen et al., 2020a, Chen et al., 2019, Chen et al., 2020b). IL-6 elicits its response by binding to the transmembrane IL-6 receptor (IL-6R), widely distributed on monocytes, T cells, B cells, and vascular endothelial cells (Tanaka et al., 2016, Copaescu et al., 2020, Magro, 2020). In the classic signaling process, the interaction between IL and 6 to its receptors results in glycoprotein 130 dimerization, upregulation of the JAK-STAT, MAPK (mitogen activated protein kinase), and RAS (rat sarcoma virus)-RAF (rapidly accelerated fibrosarcoma) signaling pathways. Also, IL-6 enhances the expression of Suppressor of Cytokine Signaling 3 (SOCS3), a modulator of the IL-6 mediated immune response. However, cells that do not express IL-6R also respond to IL-6 through circulating soluble IL-6Rα (sIL-6R), a phenomenon termed as trans-signaling. Exaggerated release of IL-6 is mainly responsible for the acute severe systemic inflammatory response requiring support through mechanical ventilation. Also, elevated levels of IL-6 activate the coagulation cascade and increases the risk of mortality (Tanaka et al., 2016, Chen et al., 2020a, Chen et al., 2019, Chen et al., 2020b, Zhang et al., 2020a, Zhang et al., 2020b, Zhao, 2020). Recently three drugs have been repurposed for COVID-19 infections and are under clinical trials (Table 1).
Table 1.
Clinical trials of several cytokine inhibitors for sever COVID-19 infections.
| S.No. | Interleukin/ factors | Name of Drug | Title Acronym | NCT Number | Company | Clinical trials | Outcome measures | References |
|---|---|---|---|---|---|---|---|---|
| 1. | IL-6 | Tocilizumab (RoActemra) |
TOCIVID | NCT04322773 | Bispebjerg-Frederiksberg Hospital Copenhagen, Denmark. |
Phase 2 | Time to be free from supplementary oxygen therapy, Number of deaths. |
ClinicalTrials.gov Identifier NCT04322773: National Library of Medicine, 2020. |
| 2. | IL-6 | Sarilumab (SAR153191) |
– | NCT04327388 | Investigational Site Number 0,320,001 Caba, Argentina. |
Phase 3 | Time taken for improvement measured on a 7-point ordinal scale. | ClinicalTrials.gov Identifier: NCT04327388 National Library of Medicine, 2020. |
| 3. | IL-6 | Olokizumab | – | NCT04452474 | George Washington University Medical Center Washington, District of Columbia, United States. |
Phase 2 | Percentage of subjects with changed clinical status- with improvement for at least 2 categories of the 5-points clinical status scale relative to baseline or in the “Not hospitalized” category. | ClinicalTrials.gov Identifier: NCT04452474: National Library of Medicine, 2020. |
| 4. | IL-6 | Clazakizumab | – | NCT04363502 | Johns Hopkins Hospital Baltimore, Maryland, United States. |
Phase 2 | Changes in the level of C-reactive protein. | ClinicalTrials.gov Identifier: NCT04363502: National Library of Medicine, 2020 |
| 5. | IL-6 | Siltuximab | SISCO | NCT04322188 | ASST - Papa Giovanni XXIII Bergamo, Italy. |
– | The need of invasive ventilation in siltuximab patients and reduction of the need of time of ventilatory support. | ClinicalTrials.gov Identifier: NCT04322188: National Library of Medicine,2020. |
| 6. | Janus Kinase Inhibitor | TD-0903 | – | NCT04402866 | Theravance Biopharma, United Kingdom | Phase 2 | Ventilator-free days from the beginning to the 28th day. | ClinicalTrials.gov Identifier: NCT04402866: National Library of Medicine, 2020. |
| 7. | Janus Kinase Inhibitor | Ruxolitinib |
RUXO-COVID |
NCT04477993 | University of Sao Paulo General Hospital | Phase 2 | Death or ICU admission or mechanical ventilation at day 14. | ClinicalTrials.gov Identifier: NCT04477993: National Library of Medicine, 2020. |
| 8. | GM-CSF inhibitor | Gimsilumab (KIN-1901–2001) |
– | NCT04351243 | Kinevant Sciences GmbH | Phase 2 | Incidence of mortality. | ClinicalTrials.gov Identifier: NCT04351243: National Library of Medicine, 2020. |
| 9. | GM-CSF inhibitor | Mavrilimumab (KPL-301) |
– | NCT04463004 | UCLA Medical Center, California; Tulane Medical Center, Louisiana; Mercy Hospital,Missouri; University of Texas Health Sciences,Texas, United States. | Phase2/3 | The proportion of subjects alive and off oxygen at day 14. | ClinicalTrials.gov Identifier: NCT04463004: National Library of Medicine, 2020. |
| 10. | GM-CSF inhibitor | Mavrilimumab | – | NCT04399980 | The Cleveland Clinic | Phase 2 | Reduced dependency on oxygen supplementation at day 14 and proportion of subjects alive. | ClinicalTrials.gov Identifier: NCT04399980: National Library of Medicine, 2020. |
| 11. | GM-CSF inhibitor | Mavrilimumab | – | NCT04492514 | University of Cincinnati | Phase 2 | Proportion of subjects alive and off oxygen at day 14. | ClinicalTrials.gov Identifier: NCT04492514: National Library of Medicine, 2020. |
| 12. | GM-CSF inhibitor | Lenzilumab | – | NCT04351152 | Humanigen, Inc. | Phase 3 | Time taken to recover by day 28 considered as 3 categories on an 8-point ordinal scale. Hospitalized without the need of supplemental oxygen. Not hospitalized but requiring home oxygen. Non-hospitalized. |
ClinicalTrials.gov Identifier: NCT04351152: National Library of Medicine,2020. |
| 13. | GM-CSF inhibitor | Otilimab |
OSCAR | NCT04376684 | GlaxoSmithKline | Phase 2 | Proportion of patients alive with absence of respiratory failure at day 28. | ClinicalTrials.gov Identifier: NCT04376684: National Library of Medicine, 2020. |
| 14. | GM-CSF agent | Sargramostim | iLeukPulm |
NCT04411680 | Partner Therapeutics, Inc. | Phase 2 | Change in oxygenation parameter of P(A-a) O2 gradient by day 6 and percentage intubated by day 14. | ClinicalTrials.gov Identifier: NCT04411680: National Library of Medicine, 2020 |
| 15. | IL-17 | Secukinumab | COLORIT | NCT04403243 | Lomonosov Moscow State University Medical Research and Educational Center, Moscow, Moscow Region, Russian Federation. | Phase 2 | Time to death or mechanical ventilation and level of C reactive protein. | ClinicalTrials.gov Identifier: NCT04403243: National Library of Medicine, 2020. |
3.6. Tocilizumab
Tocilizumab is a humanized monoclonal antibody of IgG1 type which targets IL-6R or the soluble IL-6R (Zhang et al., 2020a, Zhang et al., 2020b). Tocilizumab is used to manage rheumatoid arthritis and has an off-label use in interstitial lung disease associated with rheumatological disorders (Khanna et al., 2018). Tocilizumab intercepts the binding of IL-6 with IL-6R and sIL-6R, thereby inhibiting signal transduction through the JAK-STAT pathway (Zhang et al., 2020a, Zhang et al., 2020b). In severe COVID-19 cases, tocilizumab (400 mg; IV) was administered to 21 patients, along with the standard of care, antivirals, and methylprednisolone. Tocilizumab resolved respiratory symptoms with improvement in the overall health (Xu et al., 2020). The National Health Commission of China has approved the use of Tocilizumab for COVID-19 in a dose of 4–8 mg/kg (Fu et al., 2020).
The half-life of these agents may vary from eight to thirty days. Toxicities observed in long term use are neutropenia, thrombocytopenia, elevated liver marker enzymes, and pruritis (Atal and Fatima, 2020, Di Giambenedetto et al., 2020). The common side effects include upper respiratory tract infections, headache, hypertension, and abnormal liver function tests.
3.7. Janus kinase (JAK) and their inhibitors in COVID-19 patients
There is a massive potential for repurposing JAK inhibitors in COVID-19 patients owing to their anti-inflammatory and immunomodulatory properties which has been harnessed in managing the cytokine storm. Also, coronavirus induces changes in leukocyte subsets, and lymphocytopenia is a significant marker in both COVID-19 and myelofibrosis patients (Wagner et al., 2020). Thus, immunomodulatory agents approved for myelofibrosis can be repurposed in SARS-CoV-2 patients.
SARS-CoV-2 invasion and entry into the host cells take place through the process of endocytosis with the help of S-protein on the viral envelope. This protein attaches to the ACE-2 (angiotensin converting enzyme-2) receptors expressed and distributed widely on different organs in the body, including lungs, heart, kidney, and blood vessels. Other receptors that help viral entry into the host cell include DPP4 or CD26 (cluster of differentiation) and clathrin-mediated endocytosis pathways (Raha et al., 2020). Although the virus enters the host cell mainly through ACE-2 receptors, they attack some cells which lack ACE-2 receptors such as lymphocytes, and the attack is mediated through the Janus kinase (JAKs), signal transducer, and activator of transcription proteins (STATs) pathway (Goker and Biray, 2020, Luo et al., 2020).
JAK and AP-2 (adaptor protein complex 2) associated protein kinase-1 (AAK1) are the central regulators of endocytosis. Targeting the primary regulators of endocytosis can be a promising approach for controlling infection (Richardson et al., 2020, Zhang et al., 2020a, Zhang et al., 2020b).
JAK/STAT pathway induces multiple molecular immune responses and mediates well-defined cytokine signaling pathways. There are mainly four proteins in the JAK non-receptor tyrosine kinase family, including JAK 1, JAK 2, JAK 3, and TyK2 (Tyrosine kinase 2). JAK1, JAK2, and TYK2 are expressed throughout the human body, and IL-6 transduces signaling via JAK1/JAK2/TYK2 complexes. JAK3 is primarily expressed by hematopoietic cells in the bone marrow. JAK activation is initiated by binding of cytokines such as IFN, IL-2, IL-6, IL-12, and IL-23 to the extracellular domain of the receptor, producing extracellular signals which are transmitted intracellularly through JAK non-receptor kinases. On receipt of extracellular signals, a conformational change occurs in specific JAK associated receptors leading to the phosphorylation of JAK tyrosine. As a result, docking sites for STATs are created, leading to their activation by dimerization. STATs are recruited to the nucleus, triggering proliferation, activation, and recruitment of immune cells (Convertino et al., 2020, Zhang et al., 2020a, Zhang et al., 2020b).
Inflammation related downstream targets are activated through IL-6/JAK/STAT3 pathway by binding IL-6 to its specific receptor. IL-6 is the most highly expressed cytokine in SARS-CoV-2 infection. JAK/STAT pathway and IL-6 inhibition can be employed to counteract the hyperinflammatory state. Signal transduction can be disturbed by inhibition of one or both JAK monomers (such as JAK1/JAK2, JAK1/JAK3, JAK1/TYK2, and JAK2/TYK2) recruited by cytokine receptors to the intracellular domain of the signaling complex (Jamilloux et al., 2020).
JAK1/JAK2/JAK3 inhibitors are biologic agents that modulate the immune response by inhibiting type I/II cytokine receptors. JAK inhibitors are approved to treat several immunological diseases, including rheumatoid arthritis, psoriasis, alopecia areata, ulcerative colitis, and myelofibrosis. Some agents of this class are specific inhibitors of JAK2, while others non-specifically inhibit JAK1, JAK3, or JAK2 (Choy, 2019). JAK2 inhibitors help control pulmonary edema by reducing IL-17, IL-22, and GM-CSF by blocking the differentiation of Th17 cells (Wu and Yang, 2020). JAK inhibitors include Ruxolitinib, Baricitinib, Fedratinib, Upadacitinib, Tofacitinib, and Filgotinib (Seif et al., 2017, Luo et al., 2020), and a few are under clinical trials (Table 1).
3.8. Tofacitinib
Tofacitinib, an inhibitor of JAK2/1/3, is an FDA approved drug for the treatment of rheumatoid arthritis. JAK3 inhibition results in blockade of IL-2, IL-4, IL-6, IL-7, IL-9, IL-15, IL-21; however, tofacitinib does not markedly inhibit AAK1. Side effects include neutropenia, a small decrease in platelets, increased levels of high-density lipoprotein, cellulitis, and herpes zoster infections (Mehta et al., 2020).
3.9. Ruxolitinib
Ruxolitinib is a potent inhibitor of JAK1/2 with moderate action against TYK2 and JAK3. Ruxolitinib is approved for polycythemia vera, myelofibrosis, and acute graft-versus-host disease. It also inhibits IL-6/JAK/STAT3 pathway, thereby reducing elevated IL-6 levels. In a multicenter, single-blind, randomized controlled trial, the use of Ruxolitinib in COVID-19 patients resulted in notable clinical improvement, and a favorable safety profile (Cao et al., 2019). Mild to moderate anemia and the risk of opportunistic infection are the adverse outcomes reported (Goker and Biray, 2020).
3.10. Baricitinib
Baricitinib is approved for the treatment of RA (Mogul et al., 2019), and the anti-inflammatory effect occurs through reversible JAK inhibition. It is a selective JAK1/JAK2 inhibitor but has less specificity for JAK3. The less specificity for JAK3 is considered beneficial as it could reduce pan-JAK associated immunosuppression (Seif et al., 2020, Weisberg et al., 2020). Baricitinib restrains viral endocytosis and assembly by inhibiting AAK1 and cyclin G-associated kinase (GAK). However, suppression of the JAK-STAT pathway can lead to the downregulation of interferon-controlled genes, thereby increasing the risk of other viral infections.
Baricitinib is rapidly absorbed on oral administration and has an absolute bioavailability is 80% (Mogul et al., 2019). The half-life in healthy volunteers is 6–9 h and extends up to 19 h in severe renal impairment. Only 6% is metabolized by CYP3A4 (Jorgensen et al., 2020).
Baricitinib has specific advantages over ruxolitinib and fedratinib. Baricitinib has an admissible safety profile (Stebbing et al., 2020), high affinity for AAKI, convenient once-daily oral dosing, higher potency, well-tolerated inhibitory doses in inflammation, and good pharmacokinetic profile (Stebbing et al., 2020); thus, baricitinib is the best among its class (Seif et al., 2020). Also, low protein binding, little interaction with metabolic enzymes, and drug transporters make it relatively safe to be administered along with antiviral agents compared to other JAK inhibitors (Stebbing et al., 2020). The co-administration of probenecid with baricitinib can decrease renal clearance (Jorgensen et al., 2020). The concomitant administration of omeprazole produces a two-fold increase in the prolongation of time to peak plasma concentration (Jorgensen et al., 2020).
The side effects observed are headache, nasopharyngitis, and upper respiratory tract infection. Contrary to its relative safety and efficacy profiles, studies showed a risk of anemia. The occurrence of neutropenia and lymphocytopenia increases the risk of coinfection and reactivate viral infections, especially herpes zoster (Jorgensen et al., 2020, Seif et al., 2020). Dose-related thrombocytosis occurs with a rapid increase after initiation and reaches a peak around the second week, then declines and stabilizes. The serious adverse effects include thrombosis, perforation in the gastrointestinal tract, and significant cardiovascular events (Seif et al., 2020).
3.11. Other JAK inhibitors
Memolitinib and oclacitinib inhibit both JAK1 and JAK2, blocking downstream signaling pathways. Fedratinib and pacritinib are other JAK2 inhibitors, with ongoing clinical trials for the treatment of myelofibrosis. Fedratinib can explicitly control the cytokine storm and could be useful in SARS-CoV-2 infected patients with a TH17-related cytokine storm.
3.12. JAK inhibitors: Monotherapy or combination therapy
Combination therapy with JAK1 or JAK 3 inhibitor and methotrexate results in better clinical outcomes compared to monotherapy (Gremese et al., 2019). Combination with tofacitinib shows synergistic effects, whereas combination with baricitinib does not exhibit superior clinical outcomes. Exploiting multi-targeted JAK inhibitory agents such as midostaurin, lestaurtinib, and sunitinib could help control the cytokine release syndrome, owing to their anti-inflammatory properties (Weisberg et al., 2020).
3.13. GM-CSF and COVID-19
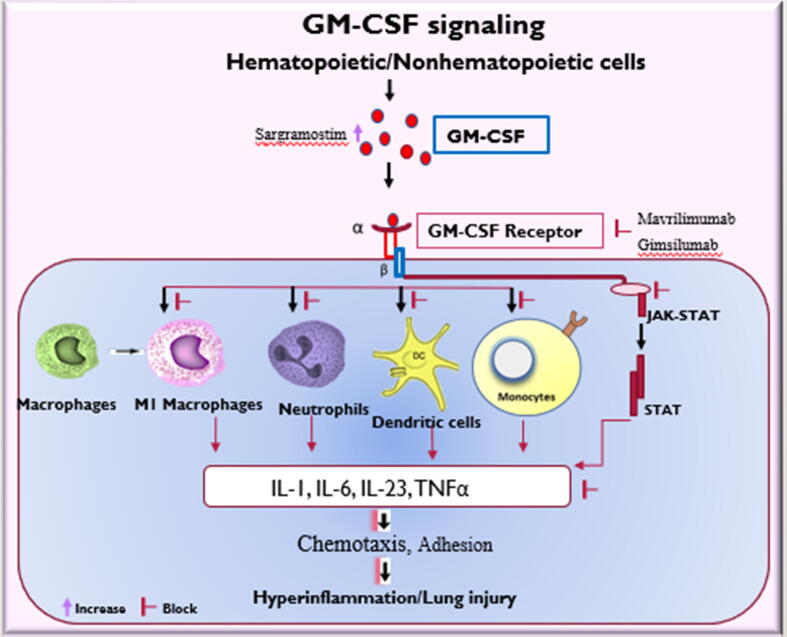
GM-CSF is a hematopoietic growth factor expressed in small quantities in the alveoli of the lungs. However, during an inflammatory state as in COVID-19 infections, the formation of GM-CSF is elevated by activated leukocytes (Zhou et al., 2020), DCs, Th17 cells, Th1, Th2, and CD8 + T cells (Shi et al., 2006), implicating its role in inflammation (Gasson, 1991). GM-CSF accentuates the infiltration of immune cells to the lungs causing significant damage in COVID-19 patients (Mangalmurti and Hunter, 2020). A report suggests that in ICU and non-ICU COVID-19 patients, CD4 + T-cells show elevated expression of GMCSF + and IL-6 + in relation to healthy controls. Also, in COVID-19 patients admitted to the ICU, CD8 + T-cells show a more significant expression of GM-CSF in relation to non-ICU patients and healthy controls (Zhou et al., 2020). GM-CSF + monocyte levels were elevated in COVID-19 patients inducing a formidable risk of lung injury due to the transformation to macrophages- or monocyte-derived dendritic cells (Bonaventura et al., 2020). Generally, the formation of GM-CSF is triggered by proinflammatory cytokines such as IL-6, IL-23, and TNF-α. In the presence of marked expression of pro-inflammatory cytokines, IL-6, IL-1β, TNF-α, IL-12p70, IL-23, and chemokines, such as CCL22, CCL24, CCL5, and CCL1 actuate GM-CSF-mediated macrophage proliferation and leukocyte recruitment in the lungs. Besides, GM-CSF plays a vital role in maintaining surfactant homeostasis in the lungs (Trapnell and Whitsett, 2002). The dysregulation of GM-CSF overtly activates myeloid cells resulting in the enormous release of pro-inflammatory mediators leading to hyper inflammation, damage to the pulmonary and extra-pulmonary sites (Fig. 2) (Lang et al., 2020). GM-CSF primes neutrophils and monocytes overexpressing cytokines and chemokines, leading to dysregulation of the innate immune pathway (Fig. 2) (Shi et al., 2006). Moreover, during inflammation, GM-CSF can promote the formation of reactive oxygen species, eicosanoids, and platelet-activating factor. The alveolar type II epithelial cells and multiple blood cells host the alpha subunit of the GM-CSF receptor (GM-CSFRα) to which GM-CSF binds. The β-chain subunit of the GM-CSF receptor is linked to the JAK2/STAT3/STAT5 signaling pathways (Hansen et al., 2008, Burmester et al., 2011). In addition to the release of pro-inflammatory cytokines, GM-CSF can activate several signaling cascades, which includes MAPK pathway, JAK2/STAT5, and the phosphoinositide 3 kinase (PI3K) pathway (Fig. 2) (Woodcock et al., 1999, Brown et al., 2012). Collectively, through all these mechanisms, GM-CSF adds fuel to the fire, resulting in the cytokine storm.
Fig. 2.
Granulocyte-Macrophage-Colony Stimulating Factor (GM-CSF) activates macrophages, monocytes, dendritic cells, neutrophils, and the JAK-STAT pathway. The leads to an enormous release of cytokines such as IL-1, Il-6, IL-23 and TNF- α which facilitate chemotaxis of other inflammatory cells resulting in hyperinflammation and lung injury. Sargramostim, a GM-CSF agent increases the activity of GM-CSF, which could be disrupted during SARS-CoV-2 infection. GM-CSF antagonists, mavrilimumab and gimsilumab block the GM-CSF receptor, thereby inhibiting the activation of hematopoietic cells and inhibiting the JAK-STAT pathway. This reduces the release of cytokines which prevent the occurrence of hyperinflammation and lung injury. IL: Interleukin; TNF-α: tumor necrosis factor alpha; JAK-STAT.
3.14. GM-CSF inducing agents and their inhibitors for COVID-19
In severe COVID-19 patients, lung injury is evident, which correlates with two courses of events. The first is the depletion of GM-CSF leading to lung injury and replenishment could have benefits. In accordance with this viewpoint, sargramostim is used in severe COVID-19 patients (Lang et al., 2020). On the other hand, disease severity in COVID-19 patients is exemplified by elevated levels of a pleiotropic cytokine, GM-CSF, and their receptor on mononuclear cells. Binding of GM-CSF to GM-CSFRα receptors can lead to an exorbitant expression of inflammatory cytokines (De Luca et al., 2020). The targeting of GM-CSF can reduce the risk of lung hyper inflammation and lung injury. Mavrilimumab, Gimsilumab, Lenzilumab, Otilimab, and Namilumab are some of the prototypes under this class that are under investigation for COVID-19 (Table 1).
3.15. Sargramostim
Sargramostim is a recombinant human GM-CSF under investigation for its ability to facilitate lung repair following injury by SARS-CoV-2 (Lang et al., 2020) (Table 1). Disruption of GM-CSF signaling affects the differentiation of alveolar macrophages affecting the breakdown and clearance of alveolar surfactant (Carey and Trapnell, 2010). Clinically, sargramostim is employed in chemotherapy-induced neutropenia to promote granulopoiesis (Mehta et al., 2015).
Sargramostim (125 μg twice daily for five days) is administered by inhalation or given intravenously (125 μg/m2 body surface) twice daily for five days, producing a 25% improvement in lung oxygenation parameters (Bosteels et al., 2020). In patients with ARDS or severe sepsis, the use of sargramostim improves oxygenation and lung compliance.
3.16. Mavrilimumab
Mavrilimumab, a humanized IgG4 monoclonal antibody, blocks GM-CSF receptor-α, thereby interfering with the binding of GM-CSF, a ligand for these receptors. Mavrilimumab blocks GM-CSF, thereby preventing the polarization of macrophages into the M1 phenotype and promoting their polarization into the M2-type. The latter produces IL-10 and chemokine CCL2 (chemokine (C-C motif) ligand 2), which possesses anti-inflammatory action (Fleetwood et al., 2007).
An open-label, prospective, interventional study in Italy studied the impact of a single dose of mavrilimumab to thirteen non-mechanically ventilated, severe COVID-19 patients with pneumonia and hyper inflammation (De Luca et al., 2020). The control group comprised of twenty-six non-mechanically ventilated severe COVID-19 patients with pneumonia and hyper inflammation. Besides, patients also received the standard of care, which included antiviral agents. By the 28th day of follow-up, mortality was not reported in the mavrilimumab treated arm, while a 27% death rate occurred in the control arm. Mavrilimumab reduced fever in 91% of the patients within the fourteenth day (De Luca et al., 2020). The dose of Mavrilimumab was 6 mg/kg as a single dose intravenously. The dose was deciphered based on the safety, efficacy, pharmacodynamic and pharmacokinetic profile of mavrilimumab (De Luca et al., 2020, Wang et al., 2012).
3.17. Gimsilumab
Gimsilumab (Roivant Sciences) is a human monoclonal antibody that blocks the GM-CSF receptor, thereby intercepting the interaction between GM-CSF and its receptor. GM-CSF-mediated signaling and cytokine release is pronounced in COVID-19 patients and is a warning sign of disease progression towards ARDS. Gimsilumab attenuates GM-CSF-mediated cytokine release and is under investigation for the management of COVID-19 with ARDS (ClinicalTrials.gov, Internet, ClinicalTrials.gov, Internet, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020).
3.18. Lenzilumab
Lenzilumab, a recombinant, non-fucosylated human IgG1 mAb acting against GM-CSF, is under investigation for chronic myelomonocytic leukemia. Lenzilumab has received FDA approval for emergency single-use in severe COVID-19 cases that progress to respiratory failure (Temesgen et al., 2020). Lenzilumab (600 mg; intravenously; three doses) was administered to twelve high-risk COVID-19 patients with severe pneumonia. Oxygenation capacity improved in 92% of the patients with a median time for discharge of 5 days (Temesgen et al., 2020). The FDA has approved the phase III studies of Lenzilumab (ClinicalTrials.gov, Internet, ClinicalTrials.gov, Internet, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020).
3.19. Otilimab and namilumab (IZN-101)
Otilimab (GlaxoSmithKline) and namilumab are human monoclonal antibodies used for rheumatoid arthritis and ankylosing spondylitis. In rheumatoid arthritis, Namilumab (Izana Bioscience, UK), an immunoglobulin G1 monoclonal antibody, neutralizes aberrantly produced GM-CSF. A clinical trial has been registered for Otilimab against COVID-19 (ClinicalTrials.gov, Internet, ClinicalTrials.gov, Internet, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020).
3.20. Possible adverse effects
Pulmonary alveolar proteinosis (PAP) is a life-threatening condition that occurs due to deficiency of GM-CSF in the lungs, characterized by the inability of alveolar macrophages to clear surfactant. Although rare, it is a risk associated with the use of GM-CSF inhibitors (Bonaventura et al., 2020).
3.21. IL-17 as pro-inflammatory cytokines in COVID-19 and their inhibitors
Th17 lymphocytes are unique subsets of T lymphocytes that overtly produce IL-17 under the influence of IL-6, IL-23, and TGF-β (Kuwabara et al., 2017). TH17 cells also produce chemokines such as CXCL-1, 2, 5, 8, CCL-2, and CCL-20. In addition to TH17 cells, a plethora of immune cells are stimulated by IL-1β and IL-23, leading to the formation of IL-17. On the other hand, TH17 cells can also produce IL-17, IL-21, IL-22, and GM-CSF. Unrestricted formation of IL-17 can drastically impact the alveolar architecture and the process of oxygenation (Mendoza, 2020). IL-6 can foster the hyperactivation of the JAK2/STAT3 pathway, increase the production of IL-17A, IL-17F, and IL-22. Therefore, modulation of IL-17 response is another possible approach in the treatment of COVID-19.
Secukinumab and Ixekizumab are human monoclonal antibodies selectively targeting IL-17A, neutralizing it, and blocking their interaction with the IL-17 receptor. Secukinumab is an approved drug for the management of psoriasis and psoriatic arthritis. However, their current use is investigational for COVID-19 (ClinicalTrials.gov, Internet, ClinicalTrials.gov, Internet, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020, ClinicalTrials.gov, Internet, 2020) (Table 1).
The adverse outcomes include nasopharyngitis, upper respiratory tract infection, mucocutaneous candidiasis (von Stebut et al., 2020), and diarrhea.
Brodalumab is a human monoclonal antibody that blocks the IL-17 receptor A (IL-17RA) interfering with the binding of IL-17 and other cytokines of the IL-17 family (von Stebut et al., 2020).
4. Conclusion
In serious COVID-19 patients, the release of enormous cytokines and chemokines causes the progression of symptoms resulting in a high fatality rate. The investigational use of biologics has been promising in managing hyper inflammation and the associated cytokine storm. However, their high cost and possible risks associated with suppressing immune cells need robust validation. The benefits of these approaches need to be weighed against their risks, as an unequivocal claim cannot be made until results of randomized clinical trials prove otherwise.
Footnotes
Peer review under responsibility of King Saud University.
References
- Atal S., Fatima Z. IL-6 Inhibitors in the treatment of serious COVID-19: A promising therapy? Pharmaceut Med. 2020;34:223–231. doi: 10.1007/s40290-020-00342-z. https://pubmed.ncbi.nlm.nih.gov/32535732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becherer J., Koller O., Zimmermann F. Externalizing behaviour, task-focused behaviour, and academic achievement: An indirect relation? Br. J. Educ. Psychol. 2020 doi: 10.1111/bjep.12347. https://www.ncbi.nlm.nih.gov/pubmed/32237146 [DOI] [PubMed] [Google Scholar]
- Bonaventura A., Vecchié A., Wang T.S., Lee E., Cremer P.C., Carey B. Targeting GM-CSF in COVID-19 Pneumonia: Rationale and Strategies. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.01625. https://pubmed.ncbi.nlm.nih.gov/32719685 1625-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordon J., Aliberti S., Fernandez-Botran R., Uriarte S.M., Rane M.J., Duvvuri P. Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. Int J Infect Dis. 2013;17:e76–e83. doi: 10.1016/j.ijid.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Bosteels C., Maes B., Van Damme K., De Leeuw E., Declercq J., Delporte A. Sargramostim to treat patients with acute hypoxic respiratory failure due to COVID-19 (SARPAC): A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:491. doi: 10.1186/s13063-020-04451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.L., Salerno D.G., Sadras T., Engler G.A., Kok C.H., Wilkinson C.R. The GM-CSF receptor utilizes β-catenin and Tcf4 to specify macrophage lineage differentiation. Differentiation. 2012;83:47–59. doi: 10.1016/j.diff.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester G., Feist E., Sleeman M., Wang B., White B., Magrini F. Mavrilimumab, a human monoclonal antibody targeting GM-CSF receptor-α, in subjects with rheumatoid arthritis: A randomised, double-blind, placebo-controlled, phase I, first-in-human study. Ann. Rheum. Dis. 2011;70:1542–1549. doi: 10.1136/ard.2010.146225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron M.J., Bermejo-Martin J.F., Danesh A., Muller M.P., Kelvin D.J. Human immunopathogenesis of severe acute respiratory syndrome (SARS) Virus Res. 2008;133:13–19. doi: 10.1016/j.virusres.2007.02.014. https://pubmed.ncbi.nlm.nih.gov/17374415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, Y., J. Wei, L. Zou, T. Jiang, G. Wang, L. Chen, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 146 (2020) 137-146.e133: http://www.sciencedirect.com/science/article/pii/S0091674920307387. [DOI] [PMC free article] [PubMed]
- Carey B., Trapnell B.C. The molecular basis of pulmonary alveolar proteinosis. Clin Immunol. (Orlando, Fla.) 2010;135:223–235. doi: 10.1016/j.clim.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.Y.C., Hoiland R.L., Stukas S., Wellington C.L., Sekhon M.S. Confronting the controversy: Interleukin-6 and the COVID-19 cytokine storm syndrome. Eur. Respir. J. 2020:2003006. doi: 10.1183/13993003.03006-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, N., M. Zhou, X. Dong, J. Qu, F. Gong, Y. Han, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet 395 (2020) 507-513. https://doi.org/10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed]
- Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin. Infect. Dis. 2020:ciaa449. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., John Wherry E. T cell responses in patients with COVID-19. Nat. Rev. Immunol. 2020;20:529–536. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy E.H. Clinical significance of Janus Kinase inhibitor selectivity. Rheumatology. 2019;58:953–962. doi: 10.1093/rheumatology/key339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifaldi L., Prencipe G., Caiello I., Bracaglia C., Locatelli F., De Benedetti F. Inhibition of natural killer cell cytotoxicity by interleukin-6: implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol. 2015;67:3037–3046. doi: 10.1002/art.39295. [DOI] [PubMed] [Google Scholar]
- Convertino I., Tuccori M., Ferraro S., Valdiserra G., Cappello E., Focosi D. Exploring pharmacological approaches for managing cytokine storm associated with pneumonia and acute respiratory distress syndrome in COVID-19 patients. Crit. Care. 2020;24:331. doi: 10.1186/s13054-020-03020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copaescu A., Smibert O., Gibson A., Phillips E.J., Trubiano J.A. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J. Allergy Clin. Immunol. 2020;146:518–534.e511. doi: 10.1016/j.jaci.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coperchini F., Chiovato L., Croce L., Magri F., Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. https://pubmed.ncbi.nlm.nih.gov/32513566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine. “Mavrilimumab to reduce progression of acute respiratory failure in COVID-19 pneumonia and systemic hyper-inflammation.” Retrieved 20th September, 2020, from https://clinicaltrials.gov/ct2/show/NCT04399980?cond=NCT04399980&draw=2&rank=1.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine. “Anti-il6 Treatment of Serious COVID-19 Disease With Threatening Respiratory Failure (TOCIVID).”Retrieved 20th September, 2020, from https://clinicaltrials.gov/ct2/show/NCT04322773?cond=NCT04322773&draw=2&rank=1.
- ClinicalTrials.gov [Internet]. Bethesda (MD):National Library of Medicine. “Sarilumab COVID-19.” Retrieved 20th September, 2020, from https://clinicaltrials.gov/ct2/show/NCT04327388?cond=Sarilumab+COVID-19&draw=2&rank=1
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine. “Study of the efficacy and safety of a single administration of Olokizumab vs. placebo in addition to standard treatment in patients with severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) Infection (COVID-19). ”Retrieved 20th September, 2020, from https://clinicaltrials.gov/ct2/show/NCT04452474.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine. “Use of the interleukin-6 inhibitor Clazakizumab in patients with life-threatening COVID-19 infection.” Retrieved 20th September, 2020, from https://clinicaltrials.gov/ct2/show/NCT04363502.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine. “An observational study of the use of Siltuximab (SYLVANT) in patients diagnosed with COVID-19 infection who have developed serious respiratory complications (SISCO).”Retrieved 20th September, 2020, from https://clinicaltrials.gov/ct2/show/NCT04322188?cond=Siltuximab+for+covid-19&draw=2&rank=2.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine. “TD-0903 for ALI associated with COVID-19.” Retrieved 20th September, 2020, from https://clinicaltrials.gov/ct2/show/NCT04402866?cond=TD-0903+for+ALI+associated+with+COVID-19.&draw=2&rank=1.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine. “Ruxolitinib for Acute Respiratory Disorder Syndrome due to COVID-19 (RUXO-COVID).”Retrieved 20th September, 2020, from https://clinicaltrials.gov/ct2/show/NCT04477993?cond=NCT04477993&draw=2&rank=1.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine. “A Study to Assess the Efficacy and Safety of Gimsilumab in Subjects With Lung Injury or Acute Respiratory Distress Syndrome Secondary to COVID-19 (BREATHE).” Retrieved 20th September, 2020, from https://clinicaltrials.gov/ct2/show/NCT04351243?cond=NCT04351243&draw=2&rank=1.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine. “Mavrilimumab to Reduce Progression of Acute Respiratory Failure in COVID-19 Pneumonia and Systemic Hyper-inflammation.” Retrieved 20th September, 2020, from https://clinicaltrials.gov/ct2/show/NCT04463004?cond=NCT04463004&draw=2&rank=1.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine. “Mavrilimumab to reduce progression of acute respiratory failure in COVID-19 pneumonia and systemic hyper-inflammation.” Retrieved 20th September, 2020, from https://clinicaltrials.gov/ct2/show/NCT04399980?cond=NCT04399980&draw=2&rank=1.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine. “Mavrilimumab to Reduce Progression of Acute Respiratory Failure in COVID-19 Pneumonia and Systemic Hyper-inflation.” Retrieved 20th September, 2020, from https://clinicaltrials.gov/ct2/show/NCT04492514?cond=NCT04492514&draw=2&rank=1.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine. “Phase 3 Study to Evaluate Efficacy and Safety of Lenzilumab in Patients With COVID-19.” Retrieved 20th September, 2020, from https://www.clinicaltrials.gov/ct2/show/NCT04351152?term=Lenzilumab&cond=COVID-19&draw=2&rank=1.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine. “Investigating Otilimab in Patients With Severe Pulmonary COVID-19 Related Disease (OSCAR).”Retrieved 20th September, 2020, from https://clinicaltrials.gov/ct2/show/NCT04376684?cond=NCT04376684&draw=2&rank=1.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine. “Study of Sargramostim in Patients With COVID-19 (iLeukPulm).” Retrieved 20th September, 2020, from https://clinicaltrials.gov/ct2/show/NCT04411680?cond=NCT04411680&draw=2&rank=1.
- ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine. “COLchicine Versus Ruxolitinib and Secukinumab In Open Prospective Randomized Trial (COLORIT).”Retrieved 20th September, 2020, from https://clinicaltrials.gov/ct2/show/NCT04403243?cond=NCT04403243&draw=2&rank=1.
- Megna M., Napolitano M., Patruno C., Fabbrocini G. Biologics for psoriasis in COVID-19 era: What do we know? Dermatol. Ther. 2020 doi: 10.1111/dth.13467. e13467-e13467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca G., Cavalli G., Campochiaro C., Della-Torre E., Angelillo P., Tomelleri A. GM-CSF blockade with mavrilimumab in severe COVID-19 pneumonia and systemic hyperinflammation: a single-centre, prospective cohort study. The Lancet Rheumatology. 2020;2:E465–E473. doi: 10.1016/S2665-9913(20)30170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giambenedetto S., Ciccullo A., Borghetti A., Gambassi G., Landi F., Visconti E. Off-label use of tocilizumab in patients with SARS-CoV-2 infection. J. Med. Virol. 2020:1787–1788. doi: 10.1002/jmv.25897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao, B., C. Wang, Y. Tan, X. Chen, Y. Liu, L. Ning, et al. Reduction and functional exhaustion of T Cells in patients with Coronavirus Disease 2019 (COVID-19). Front Immunol. 11 (2020) 827-827. https://pubmed.ncbi.nlm.nih.gov/32425950. [DOI] [PMC free article] [PubMed]
- Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Biondi-Zoccai G. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J. Am. Coll. Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleetwood A.J., Lawrence T., Hamilton J.A., Cook A.D. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J. Immunol. 2007;178:5245–5252. doi: 10.4049/jimmunol.178.8.5245. [DOI] [PubMed] [Google Scholar]
- Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020;18:164. doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson J.C. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood. 1991;77:1131–1145. [PubMed] [Google Scholar]
- Geier M.R., Geier D.A. Respiratory conditions in coronavirus disease 2019 (COVID-19): Important considerations regarding novel treatment strategies to reduce mortality. Med. Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109760. https://www.ncbi.nlm.nih.gov/pubmed/32344310 109760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goker B.B., Biray A.C. The potential of JAK/STAT pathway inhibition by ruxolitinib in the treatment of COVID-19. Cytokine Growth Factor Rev. 2020:51–61. doi: 10.1016/j.cytogfr.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S.B., Read R.C. Macrophage defences against respiratory tract infections: The immunology of childhood respiratory infections. Br. Med. Bull. 2002;61:45–61. doi: 10.1093/bmb/61.1.45. [DOI] [PubMed] [Google Scholar]
- Gremese E., Alivernini S., Tolusso B., Zeidler M.P., Ferraccioli G. JAK inhibition by methotrexate (and csDMARDs) may explain clinical efficacy as monotherapy and combination therapy. J. Leukoc. Biol. 2019;106:1063–1068. doi: 10.1002/JLB.5RU0519-145R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen G., Hercus T.R., McClure B.J., Stomski F.C., Dottore M., Powell J. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell 134. 2008:496–507. doi: 10.1016/j.cell.2008.05.053. [DOI] [PubMed] [Google Scholar]
- Huang, C., Y. Wang, X. Li, L. Ren, J. Zhao, Y. Hu, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395 (2020) 497-506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed]
- Jamilloux Y., Henry T., Belot A., Viel S., Fauter M., El Jammal T. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmunity Rev. 2020;19 doi: 10.1016/j.autrev.2020.102567. 102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen S.C.J., Tse C.L.Y., Burry L., Dresser L.D. Baricitinib: A review of pharmacology, safety, and emerging clinical experience in COVID-19. Pharmacotherapy. 2020;40:843–856. doi: 10.1002/phar.2438. https://accpjournals.onlinelibrary.wiley.com/doi/abs/10.1002/phar.2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna, D., C. P. Denton, C. J. F. Lin, J. M. van Laar, T. M. Frech, M. E. Anderson, et al. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open-label period of a phase II randomised controlled trial (faSScinate). Ann Rheum Dis. 77 (2018) 212-220: 10.1136/annrheumdis-2017-211682. [DOI] [PMC free article] [PubMed]
- Kuri-Cervantes L., Pampena M.B., Meng W., Rosenfeld A.M., Ittner C.A.G., Weisman A.R. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 2020;5:eabd7114. doi: 10.1126/sciimmunol.abd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara T., Ishikawa F., Kondo M., Kakiuchi T. The role of IL-17 and related cytokines in inflammatory autoimmune diseases. Mediators Inflamm. 2017;2017:3908061. doi: 10.1155/2017/3908061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F.M., Lee K.M.C., Teijaro J.R., Becher B., Hamilton J.A. GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat. Rev. Immunol. 2020;20:507–514. doi: 10.1038/s41577-020-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Fan Y., Lai Y., Han T., Li Z., Zhou P. Coronavirus infections and immune responses. J. Med. Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. https://pubmed.ncbi.nlm.nih.gov/31981224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- Luo W., Li Y.-X., Jiang L.-J., Chen Q., Wang T., Ye D.-W. Targeting JAK-STAT signaling to control cytokine release syndrome in COVID-19. Trends Pharmacol. Sci. 2020;41:531–543. doi: 10.1016/j.tips.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro, G. SARS-CoV-2 and COVID-19: Is interleukin-6 (IL-6) the ‘culprit lesion’ of ARDS onset? What is there besides Tocilizumab? SGP130Fc. Cytokine: X 2 (2020) 100029: http://www.sciencedirect.com/science/article/pii/S2590153220300094. [DOI] [PMC free article] [PubMed]
- Mangalmurti N., Hunter C.A. Cytokine Storms: Understanding COVID-19. Immunity. 2020;53:19–25. doi: 10.1016/j.immuni.2020.06.017. https://pubmed.ncbi.nlm.nih.gov/32610079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta H.M., Malandra M., Corey S.J. G-CSF and GM-CSF in Neutropenia. J. Immunol. 2015;195:1341–1349. doi: 10.4049/jimmunol.1500861. https://pubmed.ncbi.nlm.nih.gov/26254266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., Ciurtin C., Scully M., Levi M., Chambers R.C. JAK inhibitors in COVID-19: need for vigilance regarding increased inherent thrombotic risk. Eur. Respir. J. 2020:2001919. doi: 10.1183/13993003.01919-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza V.M.M. Interleukin-17: A potential therapeutic target in COVID-19. J. Infect. 2020;81 doi: 10.1016/j.jinf.2020.05.072. e136-e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogul A., Corsi K., McAuliffe L. Baricitinib: The second FDA-approved JAK inhibitor for the treatment of rheumatoid arthritis. Ann. Pharmacother. 2019;53:947–953. doi: 10.1177/1060028019839650. [DOI] [PubMed] [Google Scholar]
- Oxley, T. J., J. Mocco, S. Majidi, C. P. Kellner, H. Shoirah, I. P. Singh, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Eng J Med. 382 (2020) e60. https://www.nejm.org/doi/full/10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raha A.A., Chakraborty S., Henderson J., Mukaetova-Ladinska E., Zaman S., Trowsdale J. Investigation of CD26, a potential SARS-CoV-2 receptor, as a biomarker of age and pathology. Biosci. Rep. 2020;40 doi: 10.1042/BSR20203092. BSR20203092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. The Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Palermo B., Lenig D., Miettinen M., Matikainen S., Julkunen I. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur. J. Immunol. 1999;29:1617–1625. doi: 10.1002/(SICI)1521-4141(199905)29:05<1617::AID-IMMU1617>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Seif F., Aazami H., Khoshmirsafa M., Kamali M., Mohsenzadegan M., Pornour M. JAK Inhibition as a New Treatment Strategy for Patients with COVID-19. Int. Arch. Allergy Immunol. 2020:1–9. doi: 10.1159/000508247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif F., Khoshmirsafa M., Aazami H., Mohsenzadegan M., Sedighi G., Bahar M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23. doi: 10.1186/s12964-017-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifian-Dorche M., Huot P., Osherov M., Wen D., Saveriano A., Giacomini P.S. Neurological complications of coronavirus infection; a comparative review and lessons learned during the COVID-19 pandemic. J. Neurol. Sci. 2020;417 doi: 10.1016/j.jns.2020.117085. https://www.unboundmedicine.com/medline/citation/32871412/Neurological_complications_of_coronavirus_infection 117085-117085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar R., Sheikh A.B., Suriya S.S., Upadhyay S., Zafar A. Neurological complications among native Americans with COVID-19: Our experience at a tertiary care academic hospital in the U.S. J Stroke Cerebrovasc Dis. 2020;29 doi: 10.1016/j.jstrokecerebrovasdis.2020.105260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Liu C.H., Roberts A.I., Das J., Xu G., Ren G. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don't know. Cell Res. 2006;16:126–133. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- Singal C.M.S., Jaiswal P., Seth P. SARS-CoV-2, More than a Respiratory Virus: Its Potential Role in Neuropathogenesis. ACS Chem. Neurosci. 2020;11:1887–1899. doi: 10.1021/acschemneuro.0c00251. [DOI] [PubMed] [Google Scholar]
- Song J.-W., Zhang C., Fan X., Meng F.-P., Xu Z., Xia P. Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat. Commun. 2020;11:3410. doi: 10.1038/s41467-020-17240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet. Infect. Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8:959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- Temesgen Z., Assi M., Shweta F.N.U., Vergidis P., Rizza S.A., Bauer P.R. GM-CSF neutralization with Lenzilumab in severe COVID-19 pneumonia: A case-cohort study. Mayo Clin. Proc. 2020;95:2382–2394. doi: 10.1016/j.mayocp.2020.08.038. https://pubmed.ncbi.nlm.nih.gov/33153629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell B.C., Whitsett J.A. Gm-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu. Rev. Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- von Stebut E., Boehncke W.-H., Ghoreschi K., Gori T., Kaya Z., Thaci D. IL-17A in psoriasis and beyond: Cardiovascular and metabolic implications. Front. Immunol. 2020;10 doi: 10.3389/fimmu.2019.03096. https://www.frontiersin.org/article/10.3389/fimmu.2019.03096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J., DuPont A., Larson S., Cash B., Farooq A. Absolute lymphocyte count is a prognostic marker in Covid-19: A retrospective cohort review. Int J Lab Hematol. 2020;42:761–765. doi: 10.1111/ijlh.13288. https://pubmed.ncbi.nlm.nih.gov/32779838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Lau Y.Y., Liang M., Vainshtein I., Zusmanovich M., Lu H. Mechanistic modeling of antigen sink effect for mavrilimumab following intravenous administration in patients with rheumatoid arthritis. J. Clin. Pharmacol. 2012;52:1150–1161. doi: 10.1177/0091270011412964. [DOI] [PubMed] [Google Scholar]
- Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L. Characteristics of peripheral lymphocyte subset alteration in COVID-19 pneumonia. J. Infect. Dis. 2020;221:1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg E., Parent A., Yang P.L., Sattler M., Liu Q., Liu Q. Repurposing of kinase inhibitors for treatment of COVID-19. Pharmaceutical Res. 2020;37 doi: 10.1007/s11095-020-02851-7. https://pubmed.ncbi.nlm.nih.gov/32778962 167-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2020). “WHO Coronavirus Disease (COVID-19) Dashboard.” Retrieved 27/12, 2020, from https://covid19.who.int/.
- Woodcock J.M., Bagley C.J., Lopez A.F. The functional basis of granulocyte-macrophage colony stimulating factor, interleukin-3 and interleukin-5 receptor activation, basic and clinical implications. Int. J. Biochem. Cell Biol. 1999;31:1017–1025. doi: 10.1016/s1357-2725(99)00084-9. [DOI] [PubMed] [Google Scholar]
- Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020;53:368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han M., Li T., Sun W., Wang D., Fu B. Effective treatment of severe COVID-19 patients with tocilizumab. PNAS. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. https://pubmed.ncbi.nlm.nih.gov/32350134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. 105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhang Y., Qiao W., Zhang J., Qi Z. Baricitinib, a drug with potential effect to prevent SARS-COV-2 from entering target cells and control cytokine storm induced by COVID-19. Int. Immunopharmacol. 2020;86 doi: 10.1016/j.intimp.2020.106749. https://pubmed.ncbi.nlm.nih.gov/32645632 106749-106749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: Role of chloroquine and anti-IL-6 monoclonal antibodies. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105982. 105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y., B. Fu, X. Zheng, D. Wang, C. Zhao, Y. qi, et al. (2020). Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. Natl Sci Rev. 7 (2020) 998–1002. https://doi.org/10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed]