Abstract
Plasmacytoid dendritic cell (pDC) represents a distinct lineage of bone-marrow–derived cells that reside mainly in blood and lymphoid organs in the steady state but are also present in sites of infection, inflammation, and cancer. The Basic Protocol in this unit describes 1) detection and quantitation of human pDC in peripheral blood; 2) isolation of human pDC by magnetic-activated cell (MACS) sorting and fluorescent-activated cell (FACS) sorting; 3) evaluation of human pDC function by stimulation with TLR7/9 agonists; 4) detection of human pDC in lymphoid tissues of humanized mice by flow cytometry; 5) functional study of human pDC in hu-mice in vivo; and 6) specific depletion of human pDC in vivo in hu-mice using monoclonal antibody targeting human pDC. These assays, therefore, provide comprehensive methods to the phenotypic and functional study in vitro, and the investigation of human plasmacytoid dendritic cells in hu-mice in vivo.
Keywords: plasmacytoid dendritic cell, pDC, TLR, magnetic-activated cell sorting, humanized mouse
INTRODUCTION
Plasmacytoid dendritic cells (pDC) represent a distinct lineage of bone-marrow–derived cells that reside mainly in blood and lymphoid organs in the steady state but can also be present in sites of infection, inflammation, and cancer. Human pDCs exert their activity in immune response mainly through their rapid production of large amounts of type I interferons (IFN-I). After activation, pDCs rapidly lose the ability to produce IFN-I and become differentiated dendritic cells, being able to prime adaptive response to antigens (Colonna et al., 2004; Liu, 2005). In addition, pDCs can interact with other immune cells, such as NK cells, conventional DCs, T cells, and B cells through their secretion of cytokines and chemokines including IFN-I, as well as through their expression of various antigen-presenting and costimulatory receptors. pDCs, therefore, play a central role in regulating both innate and adaptive immunity.
In human peripheral blood, pDCs consist of 0.2%–0.8% of total mononuclear cells (Liu, 2005; Ronnblom and Alm, 2001). pDCs are a unique lineage of cells that is evidenced by both their phenotypic cell surface markers and functional characteristics. They do not express the other lineage-specific markers, including CD3 (T cells), CD19 (B cells), CD14 (monocytes), CD16 and CD56 (NK cells), and CD11c (conventional DCs), but do express several signature gene products, such as blood dendritic cell antigen-2 (BDCA-2), dendritic cell antigen-4 (BDCA-4), immunoglobulin-like transcript7 (ILT7), CD123 and CD4 (Summers et al., 2001). Resting pDCs express low levels of MHC class I, class II, and low to undetectable level of CD80 and CD86. After stimulation, activated pDCs express IFN-I and other cytokines/chemokines, and undergo differentiation into mature DCs, which express high levels of MHC class I and class II and costimulatory molecules CD80 and CD86, obtaining the capacity to prime and activate T cells(Liu, 2005).
Human pDCs selectively express Toll-like receptor (TLR)-7 and TLR-9 and are specialized in rapidly producing high levels of IFN-I following viral stimulation, as compared to the other blood cell types(Hornung et al., 2002; Krug et al., 2001b). In addition to IFN-I, human pDCs also produce moderate amounts of other pro-inflammatory cytokines such as TNF-α and IL-6 following viral stimulation(Liu, 2005). Notably, surface markers BDCA-2, BDCA-4 and ILT7, selectively expressed by pDCs, appear to be inhibitory receptors for TLR7/9-induced IFN-I production in pDCs, when binding to their natural ligand or specific antibody (Grage-Griebenow et al., 2007).
This manuscript has two parts. Part I describes the definition of pDC by flow cytometry; isolation of pDCs from human peripheral blood using either MACS beads-based or flow cytometry/FACS-based assays; pDC stimulation using TLR9 agonists; measurement of IFN-I production in pDCs using flow cytometry-based assay. Part II describes the method to analyze pDCs in blood, lymph node, spleen, bone marrow and liver in humanized mice by flow cytometry; pDC function test in humanized mice using spleen and bone marrow cells. Part II also includes methods for depleting human pDC in humanized mice using anti-BDCA2 antibody and the determination of pDC depletion during HIV-1 infection. The unit, therefore, primarily discusses and focuses on the definition and isolation, functional test and manipulation of human pDC in blood and lymphoid tissues.
BASIC PROTOCOL 1: ANALYSIS OF HUMAN pDCs IN PERIFERAL BLOOD MONONUCLEAR CELLS
This protocol describes the preparation of human peripheral blood mononuclear cells (PBMC), cell staining for flow cytometry and the identification of CD4+CD123highLin− (lineage markers: CD3, CD14, CD16, CD19, CD11c) pDCs in PBMC(Grouard et al., 1997). pDCs in frozen cells PBMC can aslo be analyzed for the percentage of pDCs using this protocol,
Materials
Human peripheral blood cells from buffy coats (Gulf Coast Regional Blood Center)
Trypan blue solution (SIGMA, cat. NO. T8154)
TC20™ Automated Cell Counter (BIO-RAD)
Counting Slides (BIO-RAD, cat. NO. 145-0011)
ACK lysis buffer (Gibico, cat. NO. A1049201)
Ficoll-Paque™PLUS (GE Healthcare, cat. NO. GE17-1440-02)
Phosphate-buffered saline (PBS) without Ca or Mg (Gibco, cat. NO. 14190144)
Bovine serum albumin (BSA) (Sigma-Aldrich, cat. NO. A7906)
Fetal Bovine Serum (FBS) (Gibco, cat. NO. 10100147)
Fluorochrome-labeled antibodies: mouse anti-human CD45 (Clone 2D1), CD3 (Clone HIT3a), CD4 (Clone RPA-T4), CD19 (Clone HIB19), CD14 (Clone HCD14), CD11c (Clone 3.9), CD123 (Clone 6H6)
UltraComp eBeads™Compensation Beads (Invitrogen, cat. NO. 01-2222-42)
Live/dead cell dye (7-AAD, (Invitrogen, cat. NO. A1310)
Human TruStain FcX™ (Fc Receptor Blocking Solution) (Biolegend, cat. NO. 422302)
Formalin, Buffered, 10% (Phosphate Buffer/Certified) (Fisher Scientific, cat. NO. SF100-20 (4-fold diluted solution in PBS)
12 × 75 mm 5mL Polystyrene Round-Bottom Tube with Cell-Strainer Cap (35 μm nylon mesh) (FALCON, cat. NO. 352235)
96 well PP 1.2 mL cluster tubes (Corning, cat. NO. CLS4401-960EA)
Refrigerated Centrifuge
CyAn™ ADP Analyzer (Beckman Coulter)
Flow cytometry analysis of human pDCs in human peripheral blood mononuclear cells
-
1
In a class II biological safety cabinet, dilute 15 ml blood 1:1 with 15 ml PBS in 50ml conical tubes.
-
2
In a separate 50 mL conical tube, add 15 mL ficoll. Then add 30 ml PBS and Blood mixture from step 1 gently to the top of the ficoll. Centrifuge 30 min at 2,000 rpm at 23°C with brake off.
-
3
Remove most of the top serum layer and pipet lymphocyte layer into a 50 ml conical tube.
-
4
Add 30 ml PBS with 2% FBS, 2 mM EDTA to the lymphocytes and centrifuge at 1,500 rpm, 23°C for 10 min.
-
5
Pour off supernatant and add 30 ml PBS with 2% FBS, 2 mM EDTA, centrifuge one more time at 1500 rpm, 23°C for 10 min.
-
6
Resuspend cells in 20 ml pre-chilled PBS at 4°C, and count cells (roughly 3–5×108 cells can be isolated from a buffy coat).
-
7
Stain 1 × 106 cells PBMC in 50 μl FACS staining buffer with 0.2 μg human Fc blocking Ab for 10 min on ice to minimize non-specific staining.
-
8
Add 50 μl staining buffer, containing 5 μl human Fc blocker, gently mix the cells and incubate for 10 min on ice.
-
9
Add 50 μl staining buffer, containing 0.5–1 μg of each fluorochrome-labeled antibody (as determined by titration) and cell viability dye, gently mix to resuspend cells.
For compensation controls, 1–2 drops of compensation beads should be stained at the same time. Be sure to use the same antibodies in the single-color controls as you use in your mix.
-
10
Incubate 30 min on ice in the dark.
-
11
Wash cells by adding 1 ml staining buffer, centrifuging at 300 × g, 4°C for 5 min then remove the supernatant.
-
12
Resuspend the cells in 300 μl 4-fold diluted fixation buffer in PBS and filter cells using strainer.
-
13
Perform flow cytometry within 48 hours after fixation.
Flow cytometry data analysis
-
14
Set a forward scatter (FSC-Lin)/side scatter (SSC-Lin) gate on leukocytes populations
-
15
Set an FSC-Area/Pulse Width (or FSC-Height) gate on the singlets
-
16
Create gate on live human CD45+ cells.
-
17
Create gates on lineage negative cells (CD3-CD19-CD11c-CD14-)
-
18
Create gate on CD4+CD123high cells for pDCs.
-
19
Calculate pDC as % of total CD45+ cells or % of Lin− populations.
BASIC PROTOCOL 2: pDC SEPARATION USING MACS BEADS
This protocol describes the method for high purity pDCs separation using a MACS beads-based assay. The Diamond Plasmacytoid Dendritic Cell Isolation Kit II was used for the isolation of extremely pure (almost 100%) pDCs from PBMCs. The isolation of pDCs is performed in a two-step procedure. First, the non-pDCs are magnetically labeled with a cocktail of biotin-conjugated antibodies to lineage-specific antigens and Anti-Biotin MicroBeads. Additionally, non-pDCs are directly magnetically labeled with a cocktail of antibodies conjugated with MicroBead against antigens that are not expressed on pDCs. The labeled cells are subsequently depleted by separation over a MACS Column. In the second step, the pre-enriched pDCs are labeled with pDC-specific CD304 MicroBeads and isolated by positive selection.
Materials:
Fresh human PBMCs (3–5×108 cells isolated from a buffy coat as described in Basic protocol 1).
Ice
CD304 (BDCA-4/Neuropilin-1) MicroBead Kit (Miltenyi Biotech, cat. NO. 130-090-532)
Phosphate-buffered saline (PBS) without Ca or Mg (Gibco, cat. NO. 14190144)
Bovine serum albumin (BSA) (Sigma-Aldrich, cat. NO. A7906)
UltraPure™ 0.5 M EDTA (Invitrogen, cat. NO. 15575020)
MACS Buffer: PBS with 0.5% bovine serum albumin (BSA), and 2 mM EDTA. Keep buffer cold (2–8°C).
Degas buffer before use, as air bubbles could block the column.BSA can be replaced by bovine serum (FBS). Buffers or media containing Ca2+ or Mg2+ are not recommended for use.
MACS LD Columns (Miltenyi Biotec, cat. NO. 130-042-901) or MS Columns (Miltenyi Biotec, cat. NO. 130-042-201)
autoMACS Separator or autoMACS® Pro Separator (Miltenyi Biotec)
RPMI 1640 medium (Gibco, cat. NO. 11875093)
Fetal Bovine Serum (FBS) (Gibco, cat. NO. 10100147)
Fluorochrome-labeled antibodies: CD123 (Clone 6H6) and CD303 (Clone 201A)
UltraComp eBeads-Compensation Beads (Invitrogen, cat. NO. 01-2222-42)
Fluorochrome-labeled antibodies: CD123 (Clone 6H6) or BDCA2 antibody (Clone 201A)
LIVE/DEAD™ Fixable Aqua Dead Cell Stain Kit (Invitrogen, cat. NO. L34957)
Human TruStain FcX™ (Fc Receptor Blocking Solution) (Biolegend, cat. NO. 422302)
Formalin, Buffered, 10% (Phosphate Buffer/Certified) (Fisher Scientific, cat. NO. SF100-20 (4-fold diluted solution in PBS)
12 × 75 mm 5mL Polystyrene Round-Bottom Tube with Cell-Strainer Cap (35 μm nylon mesh) (FALCON, cat. NO. 352235)
96 well PP 1.2 mL cluster tubes (Corning, cat. NO. CLS4401-960EA)
Refrigerated Centrifuge
Flow cytometer (BD LSRFortessa™ F)
Protocol:
Prepare PBMCs (3–5×108 cells) in final volume of 40 mL PBS 2 mM EDTA on ice (preparation as described in Basic Protocol 1). Aliquot 50 ul into 1.5 mL EP tube, put on ice for a control in the flow cytometry used in step 29.
Centrifuge cell suspension at 300 × g, 4°C for 10 minutes. Remove supernatant completely.
Resuspend cells in 300 μL of MACS buffer per 10⁸ total cells.
Add 100 μL of FcR blocking Reagent in MACS buffer per 10⁸ total cells
Add 100 μl of CD304 MicroBeads per 10⁸ PBMCs.
Mix thoroughly and incubate for 15 minutes (min) at 4 °C in refrigerator.
Wash cells by adding 10 mL of buffer per 10⁸ cells and centrifuge cell suspension at 300 × g, 4°C for 10 min. Remove supernatant completely.
Resuspend up to 108 cells in 500 μL of MACS buffer.
Apply cell suspension onto the column. Collect about 2 mL flow-through containing unlabeled cells.
Wash column with 2×1 mL of MACS buffer. Collect the flow-through.
Remove column from the separator and place it on a 15 mL collection tube.
Pipette 1 mL of buffer onto the column. Immediately flush out the magnetically labeled cells (purified pDC) by firmly pushing the plunger into the column.
Centrifuge at 300 × g, 4°C for 5 min. Remove supernatant completely.
Resuspend cells in 1ml RPMI-1640 10% FBS. Aliquot 10 μL into 1.5 mL EP tube, put on ice for later use.
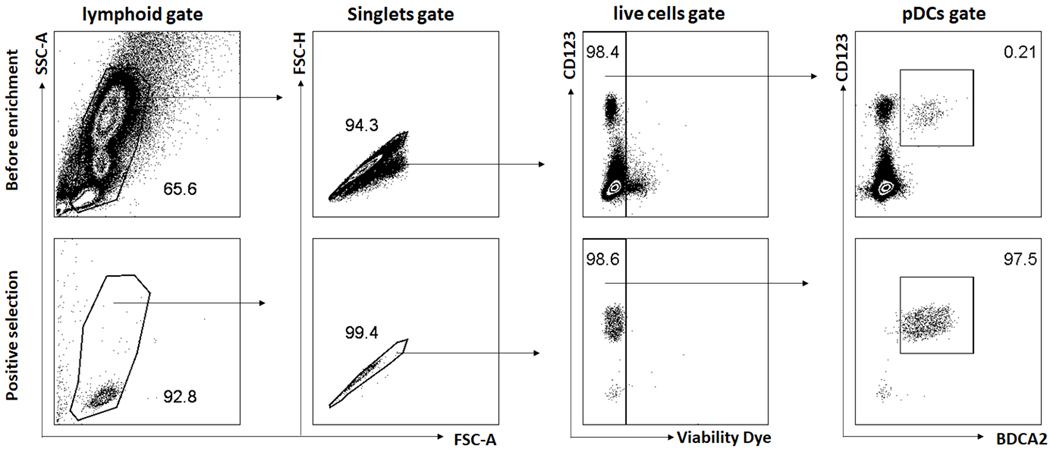
Stain cells in the aliquot from each step with flow antibodies (CD123, CD303 and viability dye) and analyze flow data as described in Basic protocol 1. Representative results are shown in Figure 2.
Figure 2.
Enrichment of human pDCs using MACS beads. Human pDCs in PBMCs were isolated using CD304 (BDCA-4/Neuropilin-1) MicroBead Kit. Representative plots show (CD123+CD303+) pDC percentages in total PBMCs before enrichment and after BDCA4 positive selection.
ALTERNATE PROTOCOL 2: pDC SORTING USING FLOW CYTOMETER
This protocol describes the basic protocol for preparation of cells and sorting of CD4+CD123highLin− (lineage markers: CD3, CD14, CD16, CD19, CD11c) pDCs in human PBMC using flow cytometer(Grouard et al., 1997). Cells can be either freshly prepared or frozen in liquid nitrogen. For the low percentage of pDCs in human PBMC, it is critical to pre-check of pDCs percentage by flow cytometry as described above to determine the appropriate input PBMC in order to sort out targeted numbers of pDCs with high purity. Notably, antibodies specific for the strong inhibitory molecule like BDCA-2 or ILT7 should not be used for sorting to avoid unexpected effects on the cells’ function or gene expression.
Materials
Human peripheral blood from buffy coats (Gulf Coast Regional Blood Center)
Trypan blue solution (SIGMA, cat. NO. T8154)
TC20™ Automated Cell Counter (BIO-RAD)
Counting Slides (BIO-RAD, cat. NO. 145-0011)
ACK lysis buffer (Gibico, cat. NO. A1049201)
Ficoll-Paque™PLUS (GE Healthcare, cat. NO. GE17-1440-02)
Phosphate-buffered saline (PBS) without Ca or Mg (Gibco, cat. NO. 14190144)
Bovine serum albumin (BSA) (Sigma-Aldrich, cat. NO. A7906)
Fluorochrome-labeled antibodies: mouse anti-human CD45 (Clone 2D1), CD3 (Clone HIT3a), CD4 (Clone RPA-T4), CD19 (Clone HIB19), CD16 (Clone 3G8), CD14 (Clone HCD14), CD11c (Clone 3.9), CD123 (Clone 6H6) or BDCA2 antibody (Clone 201A)
UltraComp eBeads™Compensation Beads (Invitrogen, cat. NO. 01-2222-42)
Live/dead cell dye (7-AAD, (Invitrogen, cat. NO. A1310)
Human TruStain FcX™ (Fc Receptor Blocking Solution) (Biolegend, cat. NO. 422302)
Formalin, Buffered, 10% (Phosphate Buffer/Certified) (Fisher Scientific, cat. NO. SF100-20 (4-fold diluted solution in PBS)
12 × 75 mm 5mL Polystyrene Round-Bottom Tube with Cell-Strainer Cap (35 μm nylon mesh) (FALCON, cat. NO. 352235)
Refrigerated Centrifuge
UltraPure™ 0.5M EDTA (Invitrogen, cat. NO. 15575020)
RPMI 1640 medium (Gibco, cat. NO. 11875093)
Fetal Bovine Serum (FBS) (Gibco, cat. NO. 10100147)
RNeasy Plus lysis buffer (QIAGEN, cat. NO. 1053393)
Flow sorter (BD FACSAria II)
For sorting 1 × 105 pDCs, prepare 100 million PBMCs by density gradient centrifugation as described in Basic Protocol 1.
Incubate PBMCs in 200 μL PBS (containing 2 mM EDTA, 10 μL Fc Receptor Blocking Solution) for 15 min on ice.
Wash with 1 mL 2 mM EDTA pre-chilled PBS at 4°C, centrifuge at 300×g, 4°C for 5 min.
Discard supernatant and resuspend cells in 200 μL 2 mM EDTA PBS (containing 2–4 μg of each fluorochrome-labeled antibodies and 50 μg/mL 7-AAD) for 20 min.
Repeat step 3.
Discard supernatant and resuspend cells in 1 ml 2 mM EDTA PBS to make 1 ×107/mL solution.
Filter through 5 mL Polystyrene Round-Bottom Tube with Cell-Strainer.
Setup flow sorter machine and gating for strategy for pDCs (refer to Basic protocol 1)
Place 15 mL collection tube containing 1 mL RPMI 1640 %FBS to harvest sorted pDCs.
Centrifuge at 300×g, 4°C for 5 min.
Resuspend cells in 1 mL 10% FBS RPMI-1640, leave on ice and use cell freshly.
To assess the purity of the cells, take10 μL from the 1mL sample, stain and run flow cytometry outlined as per Basic protocol 2 steps 29
BASIC PROTOCOL 3: EVALUATION OF HUMAN pDC FUNCTION BY STIMULATION WITH TLR AGONISTS IN VITRO
Human pDC preferentially express TLR7 and 9, which recognize single strand RNA and DNA, respectively. Upon activation, pDCs rapidly secret IFN-I. Thus, specific pDCs function can be evaluated by stimulating through either the TLR7 or TLR9 pathway, measuring IFN-I production or gene expression 4-to-24 hours following different stimuli (Lo et al., 2012; O’Brien et al., 2011). TLR7 recognizes RNA viruses and the commonly used synthetic TLR7 agonists for pDCs stimulation including imiquimod (R837) and resiquimod (R848). Unlike R848, R837 activates only TLR7 but not TLR8(Lee et al., 2003). TRL9 recognizes CpG rich viral DNA. The commonly used synthetic CpG oligonucleotides can be classified into A, B and C(Krug et al., 2001a; Marshall et al., 2005). Each of these CpG classes induces distinct cytokine gene expression patterns in peripheral blood mononuclear cells and distinct alpha interferon responses in plasmacytoid dendritic cells(Abel et al., 2005; Kerkmann et al., 2003; O’Brien et al., 2011). Therefore, it is important to understand the property and kinetics of different stimulus in terms of pDCs activation, in order to design experiment and do timely detection.
Materials
Fresh isolated pDCs (1× 103 cells × 3 replicates) (Basic protocol 2) or PBMC (5×105 cells × 3 replicates) (Basic protocol 1)
15B antibody conjugated with saporin toxin (15B-sap) and IgG-sap control (conjugated by Advanced Targeting Systems. Inc. c/o CytoLogistics)
TLR9 agonist CpG A (InvivoGen, cat. NO. tlrl-2216
IL-3 (Gibco, cat. NO. PHC0034)
RPMI 1640 medium (Gibco, cat. NO. 11875093)
Fetal bovine serum (Gibco, cat. NO. 10100147),
96 well cell culture round bottom plateCostar, cat. NO. 3799)
Human IFN-α pan ELISA development kit (HRP) (MABTECH, cat. NO. 3425-1H-6)
Freshly make 50 mL RPMI-1640 complete medium containing 10% FBS and 10 ng/mL IL-3.
-
For testing pDC-specific type I interferon response, culture purified pDC (1×103 cell) in 0.2 mL RPMI-1640 complete medium containing 1 μM CpG (set PBS as control) in 96-well round bottom tissue culture plates in triplicates. All cultures are incubated in a 37°C, 5% CO2 humidified incubator.
Work under sterile conditions in a class II biological safety cabinet. Selection of purified pDCs or PBMCs in this test is dependent on reagent availability.
12 hours after stimulation, harvest the cell culture supernatant and store at −20°C 。
Measurement of type I interferon in the supernatant using Human IFN-α pan ELISA development kit.
The ELISA protocol, including plate coating (2 hours at 37°C or overnight at 4°C) and detection steps (incubation, wash, final HRP-substrate reaction and measurement), takes at least 8 hours to complete depending on the plate coating time.
ALTERNATE PROTOCOL 3: INTRACELLULAR STAINING OF CYTOKINES IN pDC
This protocol describes the detection of type I interferon expression in pDCs using flow cytometry-based method. This protocol is optimized for staining type I interferon in pDCs present in PMBC cell cultures and is used to prove the specific type I interferon response in pDCs and quantify type I interferon expression in the cells.
Additional Materials
PBMC (1×106 cells × 3 replicates)
Brefeldin A (Biolegend, cat. NO. 420601)
Fixation/Permeabilization Solution Kit (BD Bioscience, cat. NO. 554714)
Fluorochrome-labeled antibodies: mouse anti-human CD45 (Clone 2D1), CD3 (Clone HIT3a), CD4 (Clone RPA-T4), CD19 (Clone HIB19), CD16 (Clone 3G8), CD14 (Clone HCD14), CD11c (Clone 3.9), and CD123 (Clone 6H6).
Fluorochrome-labeled antibodies specific to human IFN-α (LT27:295), TNF-α (MAb11)
UltraComp eBeads™Compensation Beads (Invitrogen, cat. NO. 01-2222-42)
Live/dead cell dye (7-AAD, (Invitrogen, cat. NO. A1310)
Human TruStain FcX™ (Fc Receptor Blocking Solution) (Biolegend, cat. NO. 422302)
Phosphate-buffered saline (PBS) without Ca or Mg (Gibco, cat. NO. 14190144)
Formalin Buffer, 10% (Phosphate Buffer/Certified) (Fisher Scientific, cat. NO. SF100-20 (4-fold diluted solution in PBS)
12 × 75 mm 5mL Polystyrene Round-Bottom Tube with Cell-Strainer Cap (35 μm nylon mesh) (FALCON, cat. NO. 352235)
96 well PP 1.2 mL cluster tubes (Corning, cat. NO. CLS4401-960EA)
Refrigerated Centrifuge
Flow cytometer (BD LSRFortessa™)
-
1
Make RPMI-1640 medium containing 10% FBS (complete medium) (10 ng/mL IL-3 is optional).
-
2
Culture resting PBMCs (1×106) in 0.15 mL RPMI-1640 complete medium containing 1 μM CpG ODN (set PBS as control) in 96-well tissue culture plates in triplicates.
-
3
Incubate in a 37°C, 5% CO2 humidified incubator for 3–4 hours.
-
4
Add 50 μL complete medium containing Brefeldin A (4×), mix even and incubate for another 5–6 hours.
-
5
Transfer the cell culture into 1.7 mL EP tubes, spin down cells at 300 ×g, 4°C for 5 min.
-
6
Discard supernatants and resuspend cells in 50 μL PBS containing 5 μL Of Fc Receptor Blocking Solution and incubate at 23°C for 5–10 min.
-
7
Wash cells by adding 1ml PBS and centrifuge at 300 ×g 4°C for 5 min.
-
8
Discard supernatants and resuspend cells with 50μl PBS containing 0.5–1μg Fluorochrome-labeled antibodies for cell surface markers staining (human CD45, CD3, CD4, CD19, CD16, CD14, CD11c, CD123) and incubate on ice for 30 min.
-
9
Wash cell by adding 1ml PBS and centrifuge at 300 ×g 4°C for 5 min.
-
10
Discard supernatants and thoroughly resuspend cells with 100 μL BD Fixation/Permeabilization and incubate on ice for 20 min.
-
11
Wash cells by adding 1 mL 1× BD Perm/Wash™ buffer, centrifuge at 400 ×g, 4°C for 5 min. Discard supernatants.
-
12
Repeat step 11.
-
13
Resuspend cells with 50 μL 1× BD Perm/Wash™ buffer containing 0.5–1 μg Fluorochrome-labeled antibodies for intracellular staining (human IFN-α and TNF-α) and incubate on ice for 30 min.
For compensation controls, 1–2 drops of UltraComp eBeads™Compensation Beads should be stained at the same time. Be sure to use the same antibodies in the single-color controls as you use in your mix.
-
14
Wash cells by adding 1 mL 1× BD Perm/Wash™ buffer, centrifuge at 400 ×g, 4°C for 5min.
-
15
Discard supernatants and resuspend cells in 200 μL 4-fold Formalin Buffer in PBS.
-
16
Perform flow cytometry within 48 hours after fixation.
Flow cytometry data analysis
-
17
Set a forward scatter (FSC-A)/side scatter (SSC-A) gate on leukocytes populations
-
18
Set an FSC-Area/ FSC-H gate on the singlets
-
19
Create gate on live human CD45+ cells.
-
20
Create gates on lineage negative cells (CD3-CD19-CD11c-CD14-)
-
21
Create gate on CD4+CD123high cells for pDCs.
-
22
Create gate on IFN-α positive cells.
BASIC PROTOCOL 4: PHENOTYPIC ANALYSIS OF HUMAN pDCs IN LYMPHOID ORGANS IN HUMANIZED MICE
Human blood cells are derived from hematopoietic stem cells (HSC). We have reported that functional human pDCs can develop in the central and peripheral lymphoid organs in immune deficient mice transplanted with human HSC(Zhang et al., 2011). In addition, we have shown that pDCs can be infected, activated, and functionally impaired by HIV-1, and that persistent pDC activation contributes to HIV-1 immune pathogenesis(Reszka-Blanco et al., 2015; Sivaraman et al., 2011; Zhang et al., 2011; Zhang et al., 2015; Zhao et al., 2018). This protocol describes how to dissociate leukocytes from lymphoid tissues in humanized mice and identify human pDCs using a flow cytometry method.
Materials
Male and female adult NSG (NOD-scid IL2Rgnull) mice (The Jackson Laboratory, stock. NO. 005557) prepare five 2–5 days old neonatal mice, no sex preference.
Human CD34+ hematopoietic stem cell-transplanted NSG (NOD-scid IL2Rgnull) neonatal mice (hu-mice): pick 1 mouse at the age of 12 weeks after transplantation and with human CD45+ reconstitution over 20% in blood.
Avertin (See reagents and solutions)
26G × 5/8 (0.45mm × 16 mm) Syringe (BD, ref. NO. 309587)
scissors (F.S.T, cat. NO. 14084-09) and forceps (F.S.T, cat. NO. 11001-13)
RPMI 1640 medium (Gibco, cat. NO. 11875093) containing 2% FBS (Gibco, cat. NO. 10100147)
Centrifuge Tube: 15ml (Corning, ref, NO. 430790), 50 mL (Corning, ref, NO. 430828)
Tissue cells dissociator: gentleMACSTM Octo Dissociator (Miltenyi Biotec, cat. NO. 130-095-937)
gentleMACSTM C Tubes (Miltenyi Biotec, cat. NO. 130-093-237)
Frosted microscope slides (Fisher scientific, cat. NO. 12-550-343)
Cell strainer: Falcon® 5 mL Round Bottom Polystyrene Test Tube, with Cell Strainer Snap Cap (FALCON ref. NO. 352235); 40μm Nylon strainer (FALCON ref352340);
Centrifuge
ACK lysis buffer (Gibco, cat. NO. A1049201)
Guava easyCyte 8HT (Millipore)
RNase-Free Disposable Pellet Pestles (Fisher Scientific, cat. NO. 12-141-364)
Forceps (Dumont, cat. NO. 11271-30(or #7))
10ml syringe (BD, ref. NO. Ref. 303134)
Percoll density gradient media (Cytiva, cat. NO. 17089101)
Heparin Solution (STEMCELL, cat. NO. 07980)
Phosphate-buffered saline (PBS) without Ca or Mg (Gibco, cat. NO. 14190144)
Fluorochrome-labeled antibodies: rat anti-mouse CD45 (30-F11), mouse anti-human CD45 (Clone 2D1), CD3 (Clone HIT3a), CD4 (Clone RPA-T4), CD14 (Clone HCD14), CD11c (Clone 3.9), and CD123 (Clone 6H6).
UltraComp eBeads™Compensation Beads (Invitrogen, cat. NO. 01-2222-42)
Live/dead cell dye (7-AAD, (Invitrogen, cat. NO. A1310)
Human TruStain FcX™ (Fc Receptor Blocking Solution) (Biolegend, cat. NO. 422302)
Formalin, Buffered, 10% (Phosphate Buffer/Certified) (Fisher Scientific, cat. NO. SF100-20 (4-fold diluted solution in PBS)
Live/dead cell dye (7-AAD, (Invitrogen, cat. NO. A1310)
Immune deficient mice are housing/breeding/humanized in a BSL2 animal room.
Anesthetize hu-mice with 400–500 μL avertin (200–250 mg/ml Tribromoethanol) through i.p injection.
After 2 min, check pain reflex by pinching toes using Dumont forceps #7, wait until no response.
Pull out eyeball to bleed animal to death, harvest blood into EP tube containing 100 μL 0.25 mM EDTA, mix evenly and put tubes on ice (lyse the red blood cells before antibody staining)
Open the abdominal cavity, harvest the spleen and place into tubes containing enough 2% FBS RPMI 1640 to cover the tissue and put tube on ice.
Cut off the legs at the hip joint, cut off the calves and remove the skin and muscle, put femurs into tubes containing enough 2% FBS RPMI 1640 to cover the tissue and place the tubes on ice.
For dissociation of leukocytes in spleen
Cut spleen into 3 to 5 pieces, put into C tubes containing 5 mL 2% FBS RPMI 1640.
Load C tubes onto the dissociator and select default program for spleen to dissociate cells in the tissue.
Filter the through the cell strainer and wash the strainer with 5 mL 2% FBS RPMI 1640.
Spin down cells at 300 × g, 4°C 5 min. Discard the supernatant.
Resuspend cells with 3 to 5 mL ACK buffer, at 23°C for 5 min.
Add equal volume of 2% FBS RPMI 1640, spin down cells at 300 × g, 4°C 5 min, discard the supernatant.
Resuspend cells in 5 mL 2% FBS RPMI 1640, filter through cell strainer. Spin down cells at 300 × g, 4°C 5 min.
Discard the supernatant, resuspend cells in 10 mL 2% FBS RPMI 1640, leave tubes on ice.
For dissociation of leukocytes in bone marrow
Expose bone tissue on both ends of the femur by cutting the bone layer by layer carefully with scissors until the 26G needle can penetrate each end by using a little force
Wash out bone marrow tissue and cells with 5 mL 2% FBS RPMI 1640 from one end and wash with another 5ml medium for the other end.
Spin down cell at 300 × g, 4°C for 5 min, discard the supernatant.
Resuspend cells with 3 mL ACK buffer, room temperature 5min.
Add equal volume of 2%FBS RPMI 1640, spin down cells at 300 × g, 4°C for 5 min, discard the supernatant.
Resuspend cells in 5 mL 2% FBS RPMI 1640, filter through cell strainer. Spin down cells at 300 × g, 4°C for 5 min.
Discard the supernatant, resuspend cells in 10 mL 2%cFBS RPMI 1640, leave tubes on ice.
For dissociation of leukocytes lymph nodes
Excise the mesenteric lymph nodes using forceps (Dumont #7), put in to 1.5 mL EP tubes containing 1 mL 2% FBS RPMI 1640.
Discard 800 μL of the medium, disassociate the tissue with a pestle.
Wash the pestle with 800 μL 2% FBS RPMI 1640.
Filter the suspension through a cell strainer, and wash the cell strainer using 1ml medium.
Spin down the cells at 300 × g, 4°C for 5min, discard the supernatant.
Resuspend cells in 1 mL 2% FBS RPMI 1640, leave tubes on ice.
For dissociating intrahepatic leukocytes in liver
Cut the liver into several pieces
Disassociate each tissue piece with a plunger from a 10ml syringe using the rubber end on a cell strainer.
Wash the smashed tissues with 1-to-2ml 2%FBS RPMI 1640 medium and harvest the flow through
Repeat step 2–3 until all tissue is disassociated, only fiber tissues are left on the cell strainer.
Spin the above cell suspension at 300 x g RT for 5min.
Resuspend the pellet in 40% Percoll solution containing 100 U/mL heparin
Load onto the layer of 70% Percoll solution
Spin at 300 × g, 23°C for 30min.
Aspirate cells from the Percoll interface
Harvest cells by centrifugation at 300 × g, 4°C for 5min
Discard supernatant and washed cells with 5 mL PBS
Spin down cells at 300 × g, 4°C for 5min.
Resuspend cells in 1 mL 2% FBS RPMI 1640, leave tubes on ice.
Human leukocytes quantification
Mix cells evenly and pipet 20 μL cells for counting using the Guava easyCyte
Add 50 μL 2% FBS PBS containing 0.5–1 μg of each antibody (anti-human CD45, anti-mouse CD45) and 50 μg/ml 7-AAD.
Count cells use Guava ExpressPlus program.
Flow cytometry analysis for pDCs
For staining pDCs in leukocytes from humanized mice lymphoid tissues, anti-mouse CD45 antibody should be included to exclude mouse leukocytes. We use a cell viability dye that falls in the same channel as the fluorescent anti-mouse CD45 antibody, which allows exclusion of both mouse cells and dead cells at the same time.
-
1
Aliquot 2 × 105 cells from lymph node, spleen, bone marrow or liver.
-
2
Centrifuge at 300 ×g, 4°C for 5 min and discard supernatants.
-
3
Resuspend cells in 50 μL PBS containing 5 μL Of Fc Receptor Blocking Solution and incubate at room temperature for 5–10 min.
-
4
Wash cells by adding 1 mL PBS and centrifuge at 300 ×g, 4°C for 5min.
-
5
Discard supernatants and resuspend cells with 50 μL PBS containing 0.5–1 μg Fluorochrome-labeled antibodies for cell surface markers staining (mouse CD45, human CD45, CD3, CD4, CD14, CD11c, CD123 and cell viability dye) and incubate on ice for 30 min.
-
6
Wash cells by adding 1 mL PBS and centrifuge at 300 ×g, 4°C for 5 min.
-
7
Discard supernatants and resuspend cells in 200 μL 4-fold Formalin Buffer in PBS.
-
8
Perform flow cytometry within 48 hours after fixation.
Flow cytometry data analysis
-
9
Set a forward scatter (FSC-A)/side scatter (SSC-A) gate on lymphocytes populations
-
10
Set an FSC-Area/ FSC-H gate on the singlets
-
11
Create gate on live human CD45+ and mouse CD45− cells
-
12
Create gates on lineage negative cells
-
13
Create gate on CD4+CD123high cells for pDCs.
Basic Protocol 5: Functional study of human pDCs in humanized mice during HIV-1 infection
We have reported that functional pDCs are developed in all lymphoid organs in CD34+HSC transplanted -hu-mice(Zhang et al., 2011). During HIV infection in hu-mice, pDCs from both spleen and BM can be productively infected and activated by acute HIV infection. However, pDCs are functionally impaired in a HIV-infected animal model, when cells were stimulated ex vivo by TLR7 or 9 pathways(Zhang et al., 2011), as reported with pDCs from HIV-infected patients (refs). This section describes the protocol to test pDC function in the context of HIV infection in humanized mice in vivo. All steps in the presence of live HIV should be done in either BSL2 plus or BSL3 lab under BSL3 regulation.
Materials
Male and female adult NSG (NOD-scid IL2Rgnull) mice (The Jackson Laboratory, stock. NO. 005557) prepare five 2–5 days old neonatal mice, no sex preference.
Human CD34+ hematopoietic stem cell-transplanted NSG (NOD-scid IL2Rgnull) neonatal mice (hu-mice): Use a mouse 12 weeks after transplantation that has over 20% human CD45+ reconstitution in blood.
HIV-1 JRCSF strain (NIH AIDS Reagent Program, cat. NO. 2708)
Avertin (See reagents and solutions)
26G × 5/8 (0.45mm x 16 mm) Syringe (BD, ref. NO. 309587)
scissors (F.S.T, cat. NO. 14084-09) and forceps (F.S.T, cat. NO. 11001-13)
RPMI 1640 medium (Gibco, cat. NO. 11875093) containing 2% FBS (Gibco, cat. NO. 10100147)
Centrifuge Tube: 15ml (Corning, ref, NO. 430790), 50ml (Corning, ref, NO. 430828)
Tissue cells dissociator: gentleMACSTM Octo Dissociator (Miltenyi Biotec, cat. NO. 130-095-937)
gentleMACSTM C Tubes (Miltenyi Biotec, cat. NO. 130-093-237)
Frosted microscope slides (Fisher scientific, cat. NO. 12-550-343)
Cell strainer: Falcon® 5 mL Round Bottom Polystyrene Test Tube, with Cell Strainer Snap Cap (FALCON ref. NO. 352235); 40 μm Nylon strainer (FALCON ref352340);
Centrifuge
ACK lysis buffer (Gibco, cat. NO. A1049201)
Guava easyCyte 8HT (Millipore)
RNase-Free Disposable Pellet Pestles (Fisher Scientific, cat. NO. 12-141-364)
Forceps (Dumont, cat. NO. 11271-30(or #7))
10 mL syringe (BD, ref. NO. Ref. 303134)
Percoll density gradient media (Cytiva, cat. NO. 17089101)
Heparin Solution (STEMCELL, cat. NO. 07980)
Phosphate-buffered saline (PBS) without Ca or Mg (Gibco, cat. NO. 14190144)
Fluorochrome-labeled antibodies: rat anti-mouse CD45 (30-F11), mouse anti-human CD45 (Clone 2D1), CD3 (Clone HIT3a), CD4 (Clone RPA-T4), CD14 (Clone HCD14), CD11c (Clone 3.9), and CD123 (Clone 6H6).
UltraComp eBeads™Compensation Beads (Invitrogen, cat. NO. 01-2222-42)
Live/dead cell dye (7-AAD, (Invitrogen, cat. NO. A1310)
Human TruStain FcX™ (Fc Receptor Blocking Solution) (Biolegend, cat. NO. 422302)
Formalin, Buffered, 10% (Phosphate Buffer/Certified) (Fisher Scientific, cat. NO. SF100-20 (4-fold diluted solution in PBS)
Live/dead cell dye (7-AAD, (Invitrogen, cat. NO. A1310)
Scalpel (Bard-Parker, ref. NO. 371610)
UltraPure™ 0.5M EDTA (Invitrogen, cat. NO. 15575020)
QIAamp Viral RNA Mini Kit (50) (QIAGEN, cat. NO. 52904) TaqMan™ Fast Virus 1-Step Master Mix (Applied Biosystems, cat. NO. 4444432)
1.7 mL eppendorf tubes (Denville, cat. NO. C2170)
eppendorf tube centrifuge
Denville Barrier Tips: 10 μL (cat. NO. P1096-FR), 200 μL (cat. NO. P1122) or 1 mL (cat. NO. P1126)
Applied Biosystems QuantStudio 12K Flex Real-Time PCR System, with 384 well plate adaptor
TLR9 agonist (CpG A (InvivoGen, cat. NO. tlrl-2216)
Human IFN-α pan ELISA development kit (HRP) (MABTECH, cat. NO. 3425-1H-6)
-
1
Anesthetize mice with avertin (i.p. injection, 125–250 mg/kg body weight, may vary with different preparations)
-
2
Infect animals with 50 μL (100 ng p24/mL, diluted in PBS) of virus containing 5 ng p24, through retro-orbital injection.
-
3
At one week after virus challenge, harvest 50 μL mouse blood by tail vein bleeding and mix with 50 μL 20 μM EDTA (diluted in PBS)
-
4
isolate 50 μL plasma into 1.5 mL EP tubes.
-
5
Extract viral RNA from plasma using QIAamp Viral RNA Mini Kit according to the manufacturer’s protocol.
-
6
Perform real-time PCR for detecting HIV viral load in plasma to confirm infection (Cheng et al., 2017a; Cheng et al., 2017b; Li et al., 2014; Li et al., 2017a)
-
7
Terminate animals at two weeks after infection and harvest spleen and bone marrow cells
-
8
Count human leukocytes using Guava 8HT and ExpressPlus program
Ex vivo stimulation and analysis of type I interferon response in pDCs
-
9
Make RPMI-1640 medium containing 10% FBS (complete medium) (10 ng/mL IL-3 is optional).
-
10
Culture resting spleen or bone marrow cells (5×105 human CD45+ cells) in 0.15 mL RPMI-1640 complete medium containing 1 μM CpG A (set PBS as control) in 96-well tissue culture plates in triplicates.
-
11
Incubate in a 37°C, 5% CO2 humidified incubator for 3–4 hours.
-
12
Add 50 μL complete medium containing Brefeldin A (4×), mix and incubate for another 5–6 hours.
-
13
Transfer the cell culture into 1.7 mL EP tubes, spin down cells at 300 ×g, 4°C for 5 min.
-
14
Discard supernatants and resuspend cells in 50 μL PBS containing 5 μL Of Fc Receptor Blocking Solution and incubate at room temperature for 5–10 min.
-
15
Wash cell by adding 1 mL PBS and centrifuge at 300 ×g, 4°C for 5 min.
-
16
Discard supernatants and resuspend cells with 50 μL PBS containing 0.5–1 μg Fluorochrome-labeled antibodies for cell surface markers staining (mouse CD45, human CD45, CD3, CD4, CD14, CD11c, CD123) and incubate on ice for 30 min.
-
17
Wash cells by adding 1 mL PBS and centrifuge at 300 ×g, 4°C for 5 min.
-
18
Discard supernatants and thoroughly resuspend cells with 200 μL BD Fixation/Permeabilization solution and incubate on ice for 20 min.
-
19
Wash cells by adding 1 mL 1× BD Perm/Wash™ buffer, centrifuge at 400 ×g, 4°C for 5 min. Discard supernatants.
-
20
Repeat step 11.
-
21
Resuspend cells with 50 μL 1× BD Perm/Wash™ buffer containing 0.5–1 μg Fluorochrome-labeled antibodies for intracellular staining (human IFN-α and TNF-α) and incubate on ice for 30 min.
For compensation controls, 1–2 drops of UltraComp eBeads™Compensation Beads should be stained at the same time. Be sure to use the same antibodies in the single-color controls as you use in your mix.
-
22
Wash cells by adding 1ml 1× BD Perm/Wash™ buffer, centrifuge at 400 ×g, 4°C for 5 min.
-
23
Discard supernatants and resuspend cells in 200 μL 4-fold Formalin Buffer in PBS.
-
24
Perform flow cytometry within 48 hours after fixation.
Flow cytometry data analysis
Gating strategy for pDCs is referred to Basic protocol 4 with a final step to create gate on IFN-α or TNF-α positive pDCs.
Basic Protocol 6: pDCs depletion in humanized mice
We have employed a specific monoclonal antibody to deplete human pDCs in humanized mice. An efficient depletion was achieved in peripheral blood, lymphoid nodes and spleen in humanize mice treated with the antibody. However, only partial depletion was observed in the bone marrow(Li et al., 2014; Li et al., 2017b; Zhang et al., 2015). To achieve complete pDC depletion in the BM, we conjugated the BDCA-2 antibody with saporin toxin, which efficiently depletes pDCs in the bone marrow. This basic protocol describes the procedures for depleting human pDCs using specific antibody or immunotoxin in humanized mice after antibody or immunotoxin treatment.
Materials
Male or female adult NSG (NOD-scid IL2Rgnull) mice (The Jackson Laboratory, stock. NO. 005557) prepare five 2–5 days old neonatal mice, no sex preference.
Human CD34+ hematopoietic stem cell-transplanted NSG neonatal mice (hu-mice): pick 9 mice at the age of 12 weeks after transplantation and with human CD45+ reconstitution over 20% in blood.
Purified mouse anti-human BDCA-2 antibody (15B, IgG2a, hold in lab)
Phosphate-buffered saline (PBS) without Ca or Mg (Gibco, cat. NO. 14190144)
0.35mm(28G) x 12.7mm syringe (BD, ref. NO. 329461)
Scalpel (Bard-Parker, ref. NO. 371610)
UltraPure™ 0.5M EDTA (Invitrogen, cat. NO. 15575020)
Denville Barrier Tips: 200μl (cat. NO. P1122) or 1ml (cat. NO. P1126)
1.7 mL eppendorf tubes (Denville, cat. NO. C2170)
Ice
Fluorochrome-labeled antibodies: rat anti-mouse CD45 (30-F11), mouse anti-human CD45 (Clone 2D1), CD3 (Clone HIT3a), CD4 (Clone RPA-T4), CD19 (Clone HIB19), CD11c (Clone 3.9), and CD123 (Clone 6H6).
ACK lysis buffer (Gibico, cat. NO. A1049201)
Centrifuge
Cell strainer: Falcon® 5 mL Round Bottom Polystyrene Test Tube, with Cell Strainer Snap Cap (FALCON ref. NO. 352235); 40μm Nylon strainer (FALCON ref352340);
Formalin, Buffered, 10% (Phosphate Buffer/Certified) (Fisher Scientific, cat. NO. SF100-20 (4-fold diluted solution in PBS)
Dilute purified pDCs depletion antibody to 1 mg/mL (purified antibody) or 0.025 mg/mL (15B-sap) using PBS.
On day 0, inject 200 μL PBS or diluted antibody i.p (assume 20g mouse, 10 mg/kg for purified antibody or 0.25 mg/kg 15B-sap).
On day 3, do the second treatment with the same dose of antibody i.p.
On day 7, bleed animals from the tail vein by cutting a light wound using a scalpel, harvest 100 μL blood using pipet and put into Eppendorf tube containing 50 μL 0.25mM EDTA, mix evenly and put tubes on ice. (alternatively, terminate mice and recover lymphoid tissues for analysis).
Spin down blood cells at 500 × g, 23°C for 5min
Remove plasma and add in 50μl PBS containing 0.5μg-1μg of fluorochrome-labeled antibodies, mix evenly and incubate on ice for 30min.
Add 1ml ACK, mix evenly, 23°C for 5min.
Spin down cells at 300 × g, 23°C for 5min.
Remove supernatant and add 500μl ACK, mix evenly, 23°C for 5min.
Spin down cells at 300 × g, 23°C for 5min.
Remove supernatant and resuspend cells with 200 μL fixation buffer.
Perform flow cytometry within 48 hours after fixation.
Flow cytometry data analysis.
Flow cytometry data analysis
The gating strategy for pDCs is described in Basic protocol 4 with a final step that gates on IFN-α or TNF-α positive pDCs. The anticipated results are referred to our previous report (Li et al., 2014; Li et al., 2017b; Zhang et al., 2015).
Determination of pDCs depletion in lymphoid tissue by flow cytometry
After determination of pDCs depletion in blood as described above, terminate the mice within 2 days after the last pDC-depletion antibody treatment. Cell dissociation from lymphoid tissue, flow cytometry staining and analysis for pDCs is referred to Basic Protocol 4. Representative flow cytometry plots are presented our previous report (Li et al., 2014; Li et al., 2017b; Zhang et al., 2015).
Specific depletion analyzed by flow cytometry
Since pDCs are of low percentage in each lymphoid tissue, specific depletion of pDCs can be determined by comparing the percentage of T cells (huCD45+CD3+), B cells (huCD45+CD3-CD19+), dendritic cells (huCD45+CD3-CD19-CD56-CD14-CD11c+), monocytes (huCD45+CD3-CD19-CD56-CD11c-CD14+) and NK cells (huCD45+CD3-CD19-CD11c-CD14-CD56+) in huCD45+ cells between control and pDCs-depleted animals(Li et al., 2014), using the flow cytometry data from the above steps in Basic Protocol 6. Representative flow cytometry plots are presented our previous report (Li et al., 2014; Li et al., 2017b; Zhang et al., 2015).
Determination of a functional depletion of pDCs in vivo in acute HIV infection Additional materials
HIV virus stock
TLR9 agonist CpG B (InvivoGen, cat. NO. tlrl-2006
MILLIPLEX® Analyte Kit (Select IFN-α2 as well as other cytokines) (MilliporeSiGMA)
After determination of pDCs depletion in blood as described above, proceed to the following steps within 48 hours.
Infect animals with HIV (5ng p24/mouse, diluted in PBS) through retro-orbital injection.
Bleed mice at 7 days after HIV infection, separate serum and freeze at −80°C untilanalysis.
Thaw serum samples and detect the IFN-α production in serum using Luminex kit.
REAGENTS AND SOLUTIONS:
Avertin
(2.5 g of 2,2,2-tribromoethanol (Sigma, cas. NO. T48402) with
5 ml of t-amyl alcohol (Sigma, cas. NO. A1685),
100 ml of distilled water, filter solution through 0.22 μM strainer)
COMMENTARY
BACKGROUND INFORMATION:
The type I interferon (IFN-I) producing cells with plasmacytoid morphology in the blood were first reported in the late 1950s (Lennert and Remmele, 1958). These interferon producing cells (IPCs) were identified in human first in the late 1990s, and named plasmacytoid dendritic cells (pDCs) (Grouard et al. 1997;(Cella et al., 1999; Siegal et al., 1999). The characterization of pDCs has been previously reviewed (Liu, 2005; Reizis et al., 2011a; Reizis et al., 2011b; Swiecki and Colonna, 2015). Their ability to rapidly and efficiently produce IFN-I has been extensively investigated, regarding how they promote antiviral immunity and their adjuvant properties via activation of other innate and adaptive immune cells. It is reported that human pDCs identified with current markers in human blood are of heterogeneity (Alcantara-Hernandez et al., 2017; Reizis, 2019). Although pDCs can be activated through different cell surface receptors and cytosolic nucleic acid sensors (Kim et al., 2010), the activation of TLR7 and TLR9 by nucleic acids (RNA and DNA, respectively) appears to be the major activation leading to IFN production (Guiducci et al., 2009; Liu, 2005; Reizis, 2019; Swiecki and Colonna, 2015). Signaling through these two TLRs leads to the rapid and massive production of all type I and type III IFNs(Duramad et al., 2003; Ito et al., 2005). Following stimulation via TLR7 or TLR9, pDCs rapidly mature from a plasmacytoid morphology into a classic dendritic cell morphology with associated antigen presentation capability, which stop producing IFN-I and can activate T cells (Colonna et al., 2004; Duramad et al., 2003; Ito et al., 2005). Due to their roles in both innate and adaptive responses, pDCs have been extensively studied and implicated in various diseases (Reizis, 2019; Reizis et al., 2011a). However, persistent activation of pDCs through TLR7/TLR9 contributes to a number of human inflammatory and autoimmune diseases, including cancers and chronic viral infections (Barrat and Su, 2019).
One major challenge to understanding the role of human pDCs in vivo results from the significant differences between human pDCs and mouse pDCs (Boonstra et al., 2003; Duramad et al., 2003). Thus, activation of TLR9 in human leads to a strong IFN-I response whereas in the mouse the TLR9 response is predominantly IL-12, IL-6, and other cytokines (Friedberg et al., 2005). Humanized mice transplanted with human immune tissues or cells have been developed (McCune et al., 1988; Namikawa et al., 1988). We and others have reported that human pDCs with normal percentage and function are developed in lymphoid tissues in humanized mice (Traggiai et al., 2004; Zhang et al., 2011). Humanized mice are thus relevant to study human pDC biology in vivo (Li et al., 2014; Li et al., 2017b; Zhang et al., 2015).
Interestingly, human pDCs express high levels of CD4. Productive HIV-1 infection of pDCs is detected in humanized mice in vivo (Zhang et al., 2011), although pDCs are relatively resistant to HIV-1 infection in vitro (Bloch et al., 2014). Even without productive HIV-1 infection, HIV-1 virions can efficiently bind CD4+ pDCs and be endocytose to the endosome, leading to activation of TLR7, IRF7 and the IFN-I genes (Beignon et al., 2005). Therefore, CD4-mediated endocytosis of HIV-1 is sufficient to activate human pDCs via the TLR7 receptor in the endosome (O’Brien et al., 2016; Reszka-Blanco et al., 2015).
Tumor associated pDCs are detected in multiple human cancers including breast cancers (Treilleux et al., 2004) and ovarian cancers (Conrad et al., 2012), associated with worse survival and metastasis. In human multiple myeloma, pDCs are shown to suppress anti-tumor CD8 T cells and promote tumor cell growth (Chauhan et al., 2009; Ray et al., 2017). Therapeutic strategies that target tumor associated pDC will likely provide a novel immunotherapeutic approach to treating human cancers with high levels of pDCs.
CRITICAL PARAMETERS:
First of all, to conduct these protocols for human blood or humanized mice, bio-safety cabinets in BSL2 lab are required. For pDC isolation, avoid using anti-BDCA2 antibody or beads, because binding of BDCA2 will exert a functional inhibition on pDCs (Dzionek et al., 2001). During activation of purified pDCs, human IL-3 (10ng/ml) is important to maintain pDC viability in the culture.
For activating pDCs in PBMC or cells from humanized mouse tissues, it is important to measure the relative frequency of pDCs in the samples, in order to have reproducible or comparable results between different donors or experiments. For a successful stimulation of pDCs, titration of each new batch of the TLR7/9 agonists is necessary, as products vary from batch to batch. Titration of the agonist by detecting interferon will ensure reproducibility and comparison between experiments using different batches of agonists. For the reconstituted TLR7/9 agonists with long-term storage (more than 6 months) at −20°C or −80°C, titration is also necessary to determine the most effective dose.
STATISTICAL ANALYSIS: (optional)
When applicable, discuss the most appropriate statistical approaches used to analyze the data. Discuss alternative statistical approaches, if any are available.
UNDERSTANDING RESULTS:
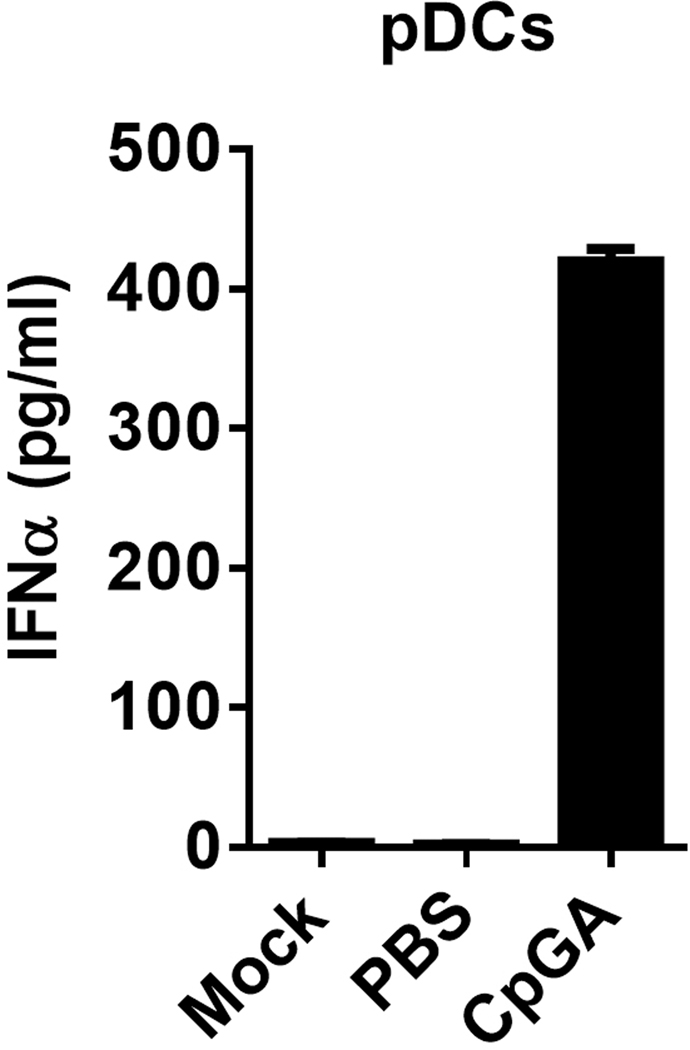
pDCs typically comprise 0.2–0.5% of all human PBMC and should be clearly identified by flow cytometry using either the BDCA2 (CD303) mAb or CD123 mAb, combined with CD4 mAb as well as other lineage antibodies like CD3, CD19, CD14 and CD11c. Figure 1 shows an example of multiple color staining of human PBMC and identification of pDCs using CD4 and CD123 markers in lineage negative cells. Check if pDC frequency is in the normal range by back-calculating pDC percentage in the human CD45+ cells (~0.2–0.4%). For pDC purification, one common method is to isolate cells using the MACS magnet beads. The commercial MACS pDC kit for isolating human pDCs uses positive, negative selection or negative/positive combined isolation strategy. Figure 2 shows an example for the purification of pDC from human PBMC using the commercial kit from miltenyi biotec with one round of positive selection of BDCA4+ cells after excluding lineage cells by negative selection. The representative results show pDC (identified by CD123 and BDCA2 double staining) percentage before enrichment, after first round negative selection (pDC purity reached 90%), and the second-round positive selection further increased the purity to 97.5%. pDCs respond to TLR7 or TLR9 agonist stimulation and rapidly produce large amount of type I interferon. Figure 3 shows the representative results of IFN-α production after stimulating pDCs (1×103 cell) with CpG A in 200 μL cell culture media for 12 hours. Alternatively, PBMC can be used for pDCs function assay since type I interferon induction to TRL-7 and TLR-9 agonist stimulation is restricted to pDCs within 16–24 hours post stimulation in vitro, with a peak type I interferon production by pDCs around 8–12 hours. Figure 4 shows an example of the specific activation of pDCs in human PBMC culture by GpG A at 8 to 10 hours post stimulation. Thus, depending on the aim and need of the specific experiment, either PBMC or purified pDCs can be used.
Figure 1.
Flow cytometry gating strategy for identifying human pDCs in PBMCs. Gating strategy is described in Basic protocol 1, Flow cytometry data analysis.
Figure 3.
Representative results of human pDC activation. pDCs isolated using MACS beads were stimulated with CpG A for 12 hours. IFN-α in culture supernatants was detected by ELISA using commercial kit.
Figure 4.
Representative plots show IFN-α expression in different cells types. Human PBMCs were cultured with CpG A for 8–10 hours. (1) CD45+CD3-CD11c+CD14+ monocytes; (2) CD45+CD3-CD14-CD11c+ mDC; (3) CD45+CD3-CD14-CD11c-CD4+CD123+ pDCs.
In humanized mice reconstitute with human hematopoietic stem cells, human pDCs are functionally developed in all lymphoid tissues. As described in BASIC PROTOCOL 4, the protocols for disassociating distinct human lymphoid tissues are different. However, the same gating strategy can be used to identify human pDCs in different lymphoid tissues using flow cytometry. Staining of both human and mouse CD45 and cell viability dye is necessary, since mouse and/or dead cells may consist of a significant fraction of the total suspension cells, especially when processing tissues from multiple animals. Figure 5 shows an example of identifying pDCs in mouse CD45 negative and human CD45+ and lineage marker negative live cell population by flow cytometry. As shown in Figure 5, pDCs in bone marrow consist of the highest percentage of total human CD45 leukocytes, while lymph nodes contain the lowest frequency of pDCs in humanized mice. We have reported that pDCs play an important role in virus infection and chronic viral immune pathogenesis (Li et al., 2014; Li et al., 2017b; Zhang et al., 2011; Zhang et al., 2015). Figure 6 is one representative result showing that pDCs function is impaired in HIV infected humanized mice, displaying a reduced type I interferon production when stimulated by CpG A as compared to pDCs from mock-infected animals. In order to study the role of pDCs in HIV infection, we developed an mAb (15B) specific to human BDCA2 and can specifically and efficiently induce human pDCs depletion in humanized mice. In addition, the BDCA2 antibody can also bind BDCA2 to suppress pDCs function. Figure 7 shows that the 15B treatment will abolish human type I interferon production during acute HIV infection. It is important to check pDCs frequency as recommended in Figure 5, to confirm pDCs is physically depleted.
Figure 5.
Representative results of human pDCs in developed in humanized mice. Leukocytes from lymph node, spleen and bone marrow of humanized mice were analyzed for human pDCs using the gating strategy described in Flow cytometry data analysis in Basic Protocol 4. Singlet gate setup is as described in Basic Protocol 2 and Alternative Protocol 3.
Figure 6.
Representative results of human pDCs defined as described in Basic protocol 4. Expression of IFN-α or TNF-α in pDCs in response to CpG A stimulation is analyzed. The representative plots show a lower level of cytokine expression in pDCs from HIV-infected humanized mice, as compare to pDC from mock-infected mice.
Figure 7.
Humanized mice were pre-treated with pDC-depleting antibody or control IgG, and then infected with HIV. IFN-α levels in plasma were detected at 7 days after infection. IFN-α in animal plasma was detected by ELISA.
TIME CONSIDERATIONS:
Processing human buffy coat for PBMC usually takes about 1.5h for 1 to 4 donor samples, more donors will take longer time accordingly. The processing of mouse lymph nodes is the quickest among the tissues (spleen, bone marrow, lymph node and liver). It typically takes 10 min for one mouse. Spleen and bone marrow processing will take about 25 to 30 min individually for one mouse (one spleen and two femurs/tibias). Liver disassociation takes the longest time, compared to other tissues processing, about 45 to 60 min for one liver. For the flow cytometric staining, staining with surface antibodies takes 30 min, and washing step will be about 10 min. For intracellular staining of cytokines such as interferon and TNFa in pDCs, it typically takes 3.5 hr to complete (including 30 min surface staining time), with multiple 30-min to 1-hr incubation steps interspersed with short practical times required to add antibody as well as approximately total 20 min wash steps. Once the cells are stained and fixed, they can be left for 1 day in the dark at 4◦C before flow cytometric analysis. The interferon ELISA assay will take about 6 hours to complete. The pDC activation in vitro will take 8 to 12 hours before harvesting of cell culture for interferon ELISA in the supernatant or flow cytometry detection of interferon in pDCs.
Acknowledgements
This work is supported in part by grants from the National Institutes of Health (AI076142, AA018009 to L.S.).
Literature Cited
- Abel K, Wang Y, Fritts L, Sanchez E, Chung E, Fitzgerald-Bocarsly P, Krieg AM, and Miller CJ 2005. Deoxycytidyl-deoxyguanosine oligonucleotide classes A, B, and C induce distinct cytokine gene expression patterns in rhesus monkey peripheral blood mononuclear cells and distinct alpha interferon responses in TLR9-expressing rhesus monkey plasmacytoid dendritic cells. Clinical and diagnostic laboratory immunology 12:606–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara-Hernandez M, Leylek R, Wagar LE, Engleman EG, Keler T, Marinkovich MP, Davis MM, Nolan GP, and Idoyaga J. 2017. High-Dimensional Phenotypic Mapping of Human Dendritic Cells Reveals Interindividual Variation and Tissue Specialization. Immunity 47:1037–1050 e1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrat FJ and Su L. 2019. A pathogenic role of plasmacytoid dendritic cells in autoimmunity and chronic viral infection. The Journal of experimental medicine 216:1974–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, Larsson M, Gorelick RJ, Lifson JD, and Bhardwaj N. 2005. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. The Journal of clinical investigation 115:3265–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch N, O’Brien M, Norton TD, Polsky SB, Bhardwaj N, and Landau NR 2014. HIV type 1 infection of plasmacytoid and myeloid dendritic cells is restricted by high levels of SAMHD1 and cannot be counteracted by Vpx. AIDS research and human retroviruses 30:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, Liu YJ, and O’Garra A. 2003. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential toll-like receptor ligation. The Journal of experimental medicine 197:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, and Colonna M. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nature medicine 5:919–923. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Singh AV, Brahmandam M, Carrasco R, Bandi M, Hideshima T, Bianchi G, Podar K, Tai YT, Mitsiades C, Raje N, Jaye DL, Kumar SK, Richardson P, Munshi N, and Anderson KC 2009. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer cell 16:309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Ma J, Li J, Li D, Li G, Li F, Zhang Q, Yu H, Yasui F, Ye C, Tsao LC, Hu Z, Su L, and Zhang L. 2017a. Blocking type I interferon signaling enhances T cell recovery and reduces HIV-1 reservoirs. The Journal of clinical investigation 127:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Yu H, Li G, Li F, Ma J, Li J, Chi L, Zhang L, and Su L. 2017b. Type I interferons suppress viral replication but contribute to T cell depletion and dysfunction during chronic HIV-1 infection. JCI insight 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, Trinchieri G, and Liu YJ 2004. Plasmacytoid dendritic cells in immunity. Nature immunology 5:1219–1226. [DOI] [PubMed] [Google Scholar]
- Conrad C, Gregorio J, Wang YH, Ito T, Meller S, Hanabuchi S, Anderson S, Atkinson N, Ramirez PT, Liu YJ, Freedman R, and Gilliet M. 2012. Plasmacytoid dendritic cells promote immunosuppression in ovarian cancer via ICOS costimulation of Foxp3(+) T-regulatory cells. Cancer research 72:5240–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duramad O, Fearon KL, Chan JH, Kanzler H, Marshall JD, Coffman RL, and Barrat FJ 2003. IL-10 regulates plasmacytoid dendritic cell response to CpG-containing immunostimulatory sequences. Blood 102:4487–4492. [DOI] [PubMed] [Google Scholar]
- Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Gunther G, Johnston I, Lanzavecchia A, Nagasaka T, Okada T, Vermi W, Winkels G, Yamamoto T, Zysk M, Yamaguchi Y, and Schmitz J. 2001. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. The Journal of experimental medicine 194:1823–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg JW, Kim H, McCauley M, Hessel EM, Sims P, Fisher DC, Nadler LM, Coffman RL, and Freedman AS 2005. Combination immunotherapy with a CpG oligonucleotide (1018 ISS) and rituximab in patients with non-Hodgkin lymphoma: increased interferon-alpha/beta-inducible gene expression, without significant toxicity. Blood 105:489–495. [DOI] [PubMed] [Google Scholar]
- Grage-Griebenow E, Loseke S, Kauth M, Gehlhar K, Zawatzky R, and Bufe A. 2007. Anti-BDCA-4 (neuropilin-1) antibody can suppress virus-induced IFN-alpha production of plasmacytoid dendritic cells. Immunology and cell biology 85:383–390. [DOI] [PubMed] [Google Scholar]
- Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, and Liu YJ 1997. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. The Journal of experimental medicine 185:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiducci C, Coffman RL, and Barrat FJ 2009. Signalling pathways leading to IFN-alpha production in human plasmacytoid dendritic cell and the possible use of agonists or antagonists of TLR7 and TLR9 in clinical indications. Journal of internal medicine 265:43–57. [DOI] [PubMed] [Google Scholar]
- Hornung V, Rothenfusser S, Britsch S, Krug A, Jahrsdorfer B, Giese T, Endres S, and Hartmann G. 2002. Quantitative expression of toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. Journal of immunology 168:4531–4537. [DOI] [PubMed] [Google Scholar]
- Ito T, Wang YH, and Liu YJ 2005. Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by Toll-like receptor (TLR) 7 and TLR9. Springer seminars in immunopathology 26:221–229. [DOI] [PubMed] [Google Scholar]
- Kerkmann M, Rothenfusser S, Hornung V, Towarowski A, Wagner M, Sarris A, Giese T, Endres S, and Hartmann G. 2003. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. Journal of immunology 170:4465–4474. [DOI] [PubMed] [Google Scholar]
- Kim T, Pazhoor S, Bao M, Zhang Z, Hanabuchi S, Facchinetti V, Bover L, Plumas J, Chaperot L, Qin J, and Liu YJ 2010. Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proceedings of the National Academy of Sciences of the United States of America 107:15181–15186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug A, Rothenfusser S, Hornung V, Jahrsdorfer B, Blackwell S, Ballas ZK, Endres S, Krieg AM, and Hartmann G. 2001a. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. European journal of immunology 31:2154–2163. [DOI] [PubMed] [Google Scholar]
- Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, Giese T, Engelmann H, Endres S, Krieg AM, and Hartmann G. 2001b. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. European journal of immunology 31:3026–3037. [DOI] [PubMed] [Google Scholar]
- Lee J, Chuang TH, Redecke V, She L, Pitha PM, Carson DA, Raz E, and Cottam HB 2003. Molecular basis for the immunostimulatory activity of guanine nucleoside analogs: activation of Toll-like receptor 7. Proceedings of the National Academy of Sciences of the United States of America 100:6646–6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennert K. and Remmele W. 1958. [Karyometric research on lymph node cells in man. I. Germinoblasts, lymphoblasts & lymphocytes]. Acta haematologica 19:99–113. [DOI] [PubMed] [Google Scholar]
- Li G, Cheng M, Nunoya J, Cheng L, Guo H, Yu H, Liu YJ, Su L, and Zhang L. 2014. Plasmacytoid dendritic cells suppress HIV-1 replication but contribute to HIV-1 induced immunopathogenesis in humanized mice. PLoS pathogens 10:e1004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Nunoya JI, Cheng L, Reszka-Blanco N, Tsao LC, Jeffrey J, and Su L. 2017a. Regulatory T Cells Contribute to HIV-1 Reservoir Persistence in CD4+ T Cells Through Cyclic Adenosine Monophosphate-Dependent Mechanisms in Humanized Mice In Vivo. The Journal of infectious diseases 216:1579–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Zhao J, Cheng L, Jiang Q, Kan S, Qin E, Tu B, Zhang X, Zhang L, Su L, and Zhang Z. 2017b. HIV-1 infection depletes human CD34+CD38− hematopoietic progenitor cells via pDC-dependent mechanisms. PLoS pathogens 13:e1006505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ 2005. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annual review of immunology 23:275–306. [DOI] [PubMed] [Google Scholar]
- Lo CC, Schwartz JA, Johnson DJ, Yu M, Aidarus N, Mujib S, Benko E, Hyrcza M, Kovacs C, and Ostrowski MA 2012. HIV delays IFN-alpha production from human plasmacytoid dendritic cells and is associated with SYK phosphorylation. PloS one 7:e37052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JD, Fearon KL, Higgins D, Hessel EM, Kanzler H, Abbate C, Yee P, Gregorio J, Cruz TD, Lizcano JO, Zolotorev A, McClure HM, Brasky KM, Murthy KK, Coffman RL, and Nest GV 2005. Superior activity of the type C class of ISS in vitro and in vivo across multiple species. DNA and cell biology 24:63–72. [DOI] [PubMed] [Google Scholar]
- McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, and Weissman IL 1988. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science 241:1632–1639. [DOI] [PubMed] [Google Scholar]
- Namikawa R, Kaneshima H, Lieberman M, Weissman IL, and McCune JM 1988. Infection of the SCID-hu mouse by HIV-1. Science 242:1684–1686. [DOI] [PubMed] [Google Scholar]
- O’Brien M, Manches O, Sabado RL, Baranda SJ, Wang Y, Marie I, Rolnitzky L, Markowitz M, Margolis DM, Levy D, and Bhardwaj N. 2011. Spatiotemporal trafficking of HIV in human plasmacytoid dendritic cells defines a persistently IFN-alpha-producing and partially matured phenotype. The Journal of clinical investigation 121:1088–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien M, Manches O, Wilen C, Gopal R, Huq R, Wu V, Sunseri N, and Bhardwaj N. 2016. CD4 Receptor is a Key Determinant of Divergent HIV-1 Sensing by Plasmacytoid Dendritic Cells. PLoS pathogens 12:e1005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Das DS, Song Y, Macri V, Richardson P, Brooks CL, Chauhan D, and Anderson KC 2017. A novel agent SL-401 induces anti-myeloma activity by targeting plasmacytoid dendritic cells, osteoclastogenesis and cancer stem-like cells. Leukemia 31:2652–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizis B. 2019. Plasmacytoid Dendritic Cells: Development, Regulation, and Function. Immunity 50:37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizis B, Bunin A, Ghosh HS, Lewis KL, and Sisirak V. 2011a. Plasmacytoid dendritic cells: recent progress and open questions. Annual review of immunology 29:163–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizis B, Colonna M, Trinchieri G, Barrat F, and Gilliet M. 2011b. Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system? Nature reviews. Immunology 11:558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reszka-Blanco NJ, Sivaraman V, Zhang L, and Su L. 2015. HIV-1 Env and Nef Cooperatively Contribute to Plasmacytoid Dendritic Cell Activation via CD4-Dependent Mechanisms. Journal of virology 89:7604–7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnblom L. and Alm GV 2001. A pivotal role for the natural interferon alpha-producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. The Journal of experimental medicine 194:F59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, and Liu YJ 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 284:1835–1837. [DOI] [PubMed] [Google Scholar]
- Sivaraman V, Zhang L, and Su L. 2011. Type I interferon contributes to CD4+ T cell depletion induced by infection with HIV-1 in the human thymus. Journal of virology 85:9243–9246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers KL, Hock BD, McKenzie JL, and Hart DN 2001. Phenotypic characterization of five dendritic cell subsets in human tonsils. The American journal of pathology 159:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiecki M. and Colonna M. 2015. The multifaceted biology of plasmacytoid dendritic cells. Nature reviews. Immunology 15:471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, and Manz MG 2004. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science 304:104–107. [DOI] [PubMed] [Google Scholar]
- Treilleux I, Blay JY, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla JP, Bremond A, Goddard S, Pin JJ, Barthelemy-Dubois C, and Lebecque S. 2004. Dendritic cell infiltration and prognosis of early stage breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 10:7466–7474. [DOI] [PubMed] [Google Scholar]
- Zhang L, Jiang Q, Li G, Jeffrey J, Kovalev GI, and Su L. 2011. Efficient infection, activation, and impairment of pDCs in the BM and peripheral lymphoid organs during early HIV-1 infection in humanized rag2(−)/(−)gamma C(−)/(−) mice in vivo. Blood 117:6184–6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Cheng L, Zhao J, Li G, Zhang L, Chen W, Nie W, Reszka-Blanco NJ, Wang FS, and Su L. 2015. Plasmacytoid dendritic cells promote HIV-1-induced group 3 innate lymphoid cell depletion. The Journal of clinical investigation 125:3692–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Cheng L, Wang H, Yu H, Tu B, Fu Q, Li G, Wang Q, Sun Y, Zhang X, Liu Z, Chen W, Zhang L, Su L, and Zhang Z. 2018. Infection and depletion of CD4+ group-1 innate lymphoid cells by HIV-1 via type-I interferon pathway. PLoS pathogens 14:e1006819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Key References
- Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, and Liu YJ 1997. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. The Journal of experimental medicine 185:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Jiang Q, Li G, Jeffrey J, Kovalev GI, and Su L. 2011. Efficient infection, activation, and impairment of pDCs in the BM and peripheral lymphoid organs during early HIV-1 infection in humanized rag2(−)/(−)gamma C(−)/(−) mice in vivo. Blood 117:6184–6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Cheng M, Nunoya J, Cheng L, Guo H, Yu H, Liu YJ, Su L, and Zhang L. 2014. Plasmacytoid dendritic cells suppress HIV-1 replication but contribute to HIV-1 induced immunopathogenesis in humanized mice. PLoS pathogens 10:e1004291. [DOI] [PMC free article] [PubMed] [Google Scholar]