Abstract
New‐onset diabetes mellitus (NOD) refers to forms of diabetes mellitus that develop during the therapeutic processes of other diseases such as hypertension. This study has been conducted in a network meta‐analysis to compare antihypertensive drugs by identifying both the advantages and disadvantages on NOD by focusing on their respective effect rates. Odd ratios and corresponding 95% confidence intervals or credible intervals were calculated within pairwise and network meta‐analysis. A total of 38 articles with 224 140 patients were included to evaluate the preventive effect of hypertension drugs on NOD. From the network meta‐analysis it was evident that both angiotensin‐converting enzyme inhibitor as well as angiotensin receptor blocker treatments are associated with a lower risk of developing NOD compared with placebo, with ranking probabilities of 79.81% and 72.77%, respectively, while β‐blockers and calcium channel blockers may significantly increase the probability of developing NOD (β‐blockers: odds ratio, 2.18 [95% credible intervals: 1.36–3.50]; calcium channel blockers: odds ratio, 1.16 [95% credible intervals, 1.05–1.29]). In conclusion, angiotensin receptor blockers have an advantage over the other treatments regarding the NOD.
Keywords: hypertension, network meta‐analysis, new‐onset diabetes mellitus
1. INTRODUCTION
In recent decades, diabetes mellitus (DM) has become one of the most prevalent chronic diseases and significant public health problem around the world.1 New‐onset DM (NOD, usually type 2 DM) is the development of DM during a therapeutic process of other diseases (eg, hypertension). NOD is attributable to the increasing proportion of the total cases of DM in recent years.2 Hypertension is closely related to DM, with approximately 50% of patients who have hypertension developing hyperinsulinemia and 75% of patients who have type 2 DM developing hypertension.3 With hypertension, the age‐related rise in blood pressure, and the coexistence of obesity and hypertension, it is not surprising that DM—both at onset and during its treatment—is so common among persons with treated hypertension.4 Patients with both hypertension and DM have a higher risk of developing cardio‐cerebral‐vascular system diseases compared with patients who have either one of alone.5, 6, 7 Therefore, hypertension has to be controlled by drugs in patients’ daily life.
Common antihypertensive drugs include: (1) angiotensin‐converting enzyme inhibitors (ACEIs), (2) angiotensin II receptor blockers (ARBs), (3) β‐blockers, (4) calcium channel blockers (CCBs), and (5) diuretics, with all their different targeting sites. Antihypertensive drugs influence the patient's insulin sensitivity, which is responsible for the development of NOD.8 Although the full mechanism that causes NOD is uncertain, reported studies have revealed some evidence. The traditional mechanism for diuretics, for example, is a reduction in serum potassium. Low plasma potassium could impair insulin secretion and thereby increase plasma glucose.9 What is increasingly recognized is that differing antihypertensive agents have been shown to have varying effects on glucose tolerance.10 For patients treated with CCBs, 0.9% to 2.0% have NOD; for patients treated with ACEIs and β‐blockers, approximately 1.0% have NOD; and for patients treated with other types of antihypertensive drugs, the value lies between 1.5% and 3%.11 In addition, previous research has found that both ACEIs and ARBs could reduce the incidence of NOD by up to 25%, indicating that these drugs could serve as a potential treatment for DM.3
Few studies, however, have made a comprehensive conclusion on the NOD effect for most antihypertensive drugs and are unable to rank these drugs. Even today, physicians face the challenge of prescribing the appropriate antihypertensive drug for their patients. Complications might arise with different drugs and the safety of the medications in particular cases remains unclear. Therefore, it is necessary to make a comprehensive comparison of the previous findings.
This study compares different types of antihypertensive drugs, including ACEIs, ARBs, β‐blockers, CCBs, diuretics, and combinations of the drugs. In order to assess the characteristics of the drugs, the NOD rates were analyzed by network meta‐analysis.
2. MATERIAL AND METHODS
2.1. Search strategy
PubMed, Embase, and the Cochrane Library were consulted regarding the preventive effect of antihypertensive agents on NOD. Only English articles were searched. Based on the information derived from these databases, five common hypertension drugs with sufficient evidence supporting their effect on hypertension were included in this study: (1) ACEIs, (2) ARBs, (3) β‐blockers, (4) CCBs, and (5) diuretics. Randomized controlled trials on the preventive effect of antihypertensive drugs on NOD published between January 1, 1980, and May 1, 2016, were included in the primary search of relevant articles. The following key words were applied using conjunctions in all databases: “diuretics, adrenergic β‐antagonists, angiotensin‐converting enzyme inhibitors, angiotensin receptor antagonists, calcium channel blockers, diabetes mellitus, new‐onset diabetes, hypertension, randomized controlled trial.” Two researchers independently evaluated the articles derived from the databases and the reference lists of the retrieved articles were manually reviewed for related articles in order to further improve the integrity of the analysis. When discrepancies arose, the result would be made by discussion. This systematic review was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.12
2.2. Study selection
The selection of articles was further narrowed down by defining the following inclusion criteria: (1) randomized controlled trail design; (2) diagnosis of NOD confirmed by American Diabetes Association criteria,13 diabetic symptoms, glucose levels on fasting or oral glucose tolerance testing; and (3) clearly described treatment information including medication and doses. Studies that were in accordance with these inclusion criteria were included in the analysis.
2.3. Data extraction
Two authors independently extracted relevant data from the included articles. Name of first author, year of publication, study design, duration of treatment, primary diseases, number of patients and average age, blood pressure, and medications were documented. The number of NOD cases was considered as the clinical outcome in the current meta‐analysis.
2.4. Statistical analysis
In this study, both pairwise traditional meta‐analysis and network meta‐analysis were performed. First, a pairwise analysis was performed to evaluate the preventive effect of antihypertensive drugs on NOD. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated. The heterogeneity was assessed with I 2 test, with an I 2 >50% indicating the existence of heterogeneity. The random‐effects model (DerSimonian‐Laird method) was used for data sets. STATA version 12.0 (StataCorp) software was used to perform the statistical analysis.
In addition, a Bayesian model network meta‐analysis was conducted to combine both direct and indirect evidences into one single comparison. The network meta‐analysis was performed with a random‐effects model within a Bayesian framework using Markov chain Monte Carlo methods in WinBUGS (MRC Biostatistics Unit, Cambridge University). Cumulative ORs and corresponding 95% credible intervals (CrIs) were calculated. The rank probabilities of different treatments regarding the occurrence of NOD were illustrated by the surface under the cumulative ranking curve.14 Moreover, the consistency was checked by node‐splitting plot. P values were calculated to identify the difference between direct and indirect evidences. In addition, publication bias of articles involved in the analysis was evaluated by funnel plot and Egger's test. The existence of publication bias was indicated by a P value <.1.
3. RESULTS
3.1. Study characteristics
A total of 38 articles with 224 140 patients were included in this network meta‐analysis to evaluate the preventive effect of hypertension drugs on NOD. The PRIMSA flow chart is shown in Figure S1. Among all studies, 18 231 cases of NOD were identified during the trials. Treatments were categorized into: (1) β‐blockers, (2) β‐blockers and diuretics, (3) diuretics, (4) ARBs, (5) ACEIs, and (6) CCBs. An overview of the studies included in the network meta‐analysis is presented in Table 1. Among the studies, the patients’ baseline blood glucose level was normal, and the duration of the treatments were 1 to 6 years. A PEDro scale was provided to evaluate the quality of trials included (Table S1). Conflicts of interest of the included trials are shown in Table S2.
Table 1.
Summary of the studies included in the network meta‐analysis
| Trials/author | Year | Duration, y | Blinding | Treatment 1 | Treatment 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drugs | Size | Age | BP, mm Hg | NOD | Drugs | Size | Age | BP, mm Hg | NOD | |||||
| Park et al | 2015 | 3.0 | – | ACEI | 409 | 60 | – | 7 | Placebo | 409 | 61 | – | 49 | |
| ADaPT | 2012 | 4.0 | Nonblind | ACEI | 896 | 69 | 147/87 | 151 | Diuretic | 423 | 69 | 144/87 | 76 | |
| EMPHASIS‐HF | 2012 | 1.8 | – | ARB | 894 | 69 | – | 33 | Placebo | 952 | 69 | – | 36 | |
| COPE <60‐1 | 2012 | 3.0 | Nonblind | ARB | 612 | 55 | 153/92 | 14 | BB | 555 | 54 | 153/92 | 19 | |
| COPE <60‐2 | 2012 | 3.0 | Nonblind | ARB | 612 | 55 | 153/92 | 14 | Diuretics | 593 | 55 | 153/92 | 17 | |
| COPE <60‐3 | 2012 | 3.0 | Nonblind | BB | 555 | 54 | 153/92 | 19 | Diuretics | 593 | 55 | 153/92 | 17 | |
| COPE >60‐1 | 2012 | 3.0 | Nonblind | ARB | 498 | 73 | 155/85 | 7 | BB | 534 | 72 | 155/85 | 18 | |
| COPE >60‐2 | 2012 | 3.0 | Nonblind | ARB | 498 | 73 | 155/85 | 7 | Diuretics | 501 | 73 | 155/85 | 15 | |
| COPE > 60‐3 | 2012 | 3.0 | Nonblind | BB | 534 | 72 | 155/85 | 18 | Diuretics | 501 | 73 | 155/85 | 15 | |
| SPARCL | 2011 | 1.0 | ‐ | ACEI | 1905 | 61 | 136/80 | 166 | Placebo | 1898 | 61 | 136/80 | 115 | |
| NAVIGAROR | 2010 | 6.0 | Double‐blind | ARB | 4631 | 64 | 139.4/82.5 | 1532 | Placebo | 4675 | 64 | 139.9/82.6 | 1722 | |
| HIJ‐CREATE | 2009 | 4.2 | – | ARB | 645 | 65 | 135.5/75.8 | 7 | Placebo | 624 | 65 | 135/75.6 | 18 | |
| Kyoto Heart | 2009 | 3.3 | – | ARB | 1126 | 66 | 157/88 | 58 | Placebo | 1108 | 66 | 157/88 | 86 | |
| CASE‐J | 2008 | 3.2 | – | ARB | 1343 | 64 | 162.5/91.6 | 38 | CCB | 1342 | 64 | 163.2/91.8 | 59 | |
| IMAGINE | 2008 | 3.0 | Double‐blind | ACEI | 1159 | 61 | 122/70 | 28 | Placebo | 1141 | 61 | 121/70 | 35 | |
| PRoFESS | 2008 | 2.5 | – | ARB | 7306 | 66 | 144.1/83.8 | 125 | Placebo | 7283 | 66 | 144.2/83.8 | 151 | |
| TRANSCEND | 2008 | 4.7 | Double‐blind | ARB | 1895 | 67 | 140.7/81.8 | 209 | Placebo | 1913 | 67 | 141.3/82 | 245 | |
| AASK‐1 | 2006 | 3.8 | Double‐blind | ACEI | 410 | 54 | 150/96 | 45 | BB | 405 | 54 | 150/96 | 70 | |
| AASK‐2 | 2006 | 3.8 | Double‐blind | ACEI | 410 | 54 | 150/96 | 45 | CCB | 202 | 54 | 150/96 | 32 | |
| AASK‐3 | 2006 | 3.8 | Double‐blind | BB | 405 | 54 | 150/96 | 70 | CCB | 202 | 54 | 150/96 | 32 | |
| DREAM | 2006 | 3.0 | Double‐blind | ACEI | 2623 | 55 | 136.1/83.4 | 449 | Placebo | 2646 | 55 | 136/83.4 | 489 | |
| ASCOT | 2005 | 5.5 | – | BB | 7071 | 63 | 163.0/94.5 | 799 | CCB | 7087 | 63 | 164.1/94.8 | 567 | |
| CHARM | 2005 | 3.1 | Single‐blind | ARB | 2715 | 66 | 130/‐ | 163 | Placebo | 2721 | 66 | 131/‐ | 202 | |
| FEVER | 2005 | 3.3 | Double‐blind | CCB | 4841 | 62 | 159/92 | 177 | Placebo | 4870 | 62 | 159/93 | 154 | |
| PEACE | 2004 | 4.8 | Double‐blind | ACEI | 3432 | 64 | 134/78 | 335 | Placebo | 3472 | 64 | 133/78 | 399 | |
| VALUE | 2004 | 4.2 | Double‐blind | ARB | 5032 | 67 | 154.5/87.4 | 580 | CCB | 4963 | 67 | 154.8/87.6 | 718 | |
| ALPINE | 2003 | 1.0 | Double‐blind | ARB | 196 | 55 | – | 1 | Diuretic | 196 | 55 | – | 8 | |
| ANBP2 | 2003 | 4.1 | – | ACEI | 2800 | 72 | 167/91 | 138 | Diuretic | 2826 | 72 | 168/91 | 200 | |
| CHARM | 2003 | 3.1 | Double‐blind | ARB | 1630 | 66 | 130.6/76.6 | 163 | Placebo | 1646 | 66 | 131.1/76.7 | 202 | |
| EUROPA | 2003 | 4.3 | Double‐blind | ACEI | 5389 | 60 | 137/82 | 389 | Placebo | 5327 | 60 | 137/82 | 397 | |
| INVEST | 2003 | 2.7 | – | CCB | 8098 | 66 | 149.5/86.3 | 665 | Placebo | 8078 | 66 | 149.5/86.3 | 569 | |
| SCOPE | 2003 | 3.7 | Double‐blind | ARB | 2168 | 76 | 166/90.3 | 93 | Placebo | 2175 | 76 | 166.5/90.4 | 115 | |
| SOLVD | 2003 | 2.9 | Double‐blind | ACEI | 153 | 56 | 127.4/77.8 | 9 | Placebo | 138 | 57 | 128.2/79.7 | 31 | |
| ALLHAT‐1 | 2002 | 4.0 | Double‐blind | ACEI | 4096 | 67 | 146/84 | 119 | CCB | 3954 | 67 | 146/84 | 154 | |
| ALLHAT‐2 | 2002 | 4.0 | Double‐blind | CCB | 3954 | 67 | 146/84 | 154 | Diuretic | 6766 | 67 | 146/84 | 302 | |
| ALLHAT‐3 | 2002 | 4.0 | Double‐blind | ACEI | 4096 | 67 | 146/84 | 119 | Diuretic | 6766 | 67 | 146/84 | 302 | |
| LIFE | 2002 | 4.8 | Double‐blind | ARB | 4019 | 67 | 174.3/97.9 | 241 | BB | 3979 | 67 | 174.5/97.7 | 319 | |
| HOPE | 2001 | 4.5 | Double‐blind | ACEI | 2837 | 66 | 136.4/78.2 | 102 | Placebo | 2883 | 66 | 136.7/78.7 | 155 | |
| INSIGHT | 2000 | 3.0 | Double‐blind | CCB | 2508 | 60‐70 | – | 96 | Diuretic | 2511 | 60‐70 | – | 137 | |
| NOEDIL | 2000 | 4.5 | – | BB+diuretic | 5122 | 60 | 173.4/105.7 | 251 | CCB | 5023 | 61 | 173.5/105.8 | 216 | |
| CAPPP | 1999 | 6.1 | – | ACEI | 5183 | 52 | 161.8/99.8 | 337 | BB+diuretic | 5230 | 53 | 159.6/98.1 | 380 | |
| STOP‐1 | 1999 | 4.0 | Double‐blind | ACEI | 1970 | 76 | 187/101 | 93 | BB | 1960 | 76 | 187/101 | 97 | |
| STOP‐2 | 1999 | 4.0 | Double‐blind | ACEI | 1970 | 76 | 187/101 | 93 | CCB | 1965 | 76 | 187/101 | 95 | |
| STOP‐3 | 1999 | 4.0 | Double‐blind | BB | 1960 | 76 | 187/101 | 97 | CCB | 1965 | 76 | 187/101 | 95 | |
| SHEP | 1998 | 3.0 | Double‐blind | Diuretic | 1631 | >60 | 190/90 | 140 | Placebo | 1578 | >60 | 190/90 | 118 | |
| MRC‐E‐1 | 1992 | 5.8 | Single‐blind | BB | 1102 | 70 | 183/91 | 37 | Diuretic | 1081 | 70 | 183/91 | 43 | |
| MRC‐E‐2 | 1992 | 5.8 | Single‐blind | BB | 1102 | 70 | 183/91 | 37 | Placebo | 2213 | 70 | 183/91 | 34 | |
| MRC‐E‐3 | 1992 | 5.8 | Single‐blind | Diuretic | 1081 | 70 | 183/91 | 43 | Placebo | 2213 | 70 | 183/91 | 34 | |
| EWPHE | 1991 | 4.7 | – | Diuretic | 416 | >60 | – | 29 | Placebo | 424 | >60 | – | 20 | |
| HAPPHY | 1987 | 3.8 | – | BB | 3297 | 52 | 166/107 | 86 | Diuretic | 3272 | 52 | 166/107 | 75 | |
| MRC | 1985 | 4.9 | Single‐blind | BB | 4300 | 51 | 158/98 | 43 | Diuretic | 4240 | 51 | 158/98 | 106 | |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BB, β‐blocker; BP, blood pressure; CCB, calcium channel blocker; NOD, new‐onset diabetes mellitus.
To show the comparisons in the meta‐analysis, a network plot of the included studies is presented in Figure 1. The row numbers indicate the number of studies comparing treatment pairs and the width of the lines is proportional to the number.
Figure 1.
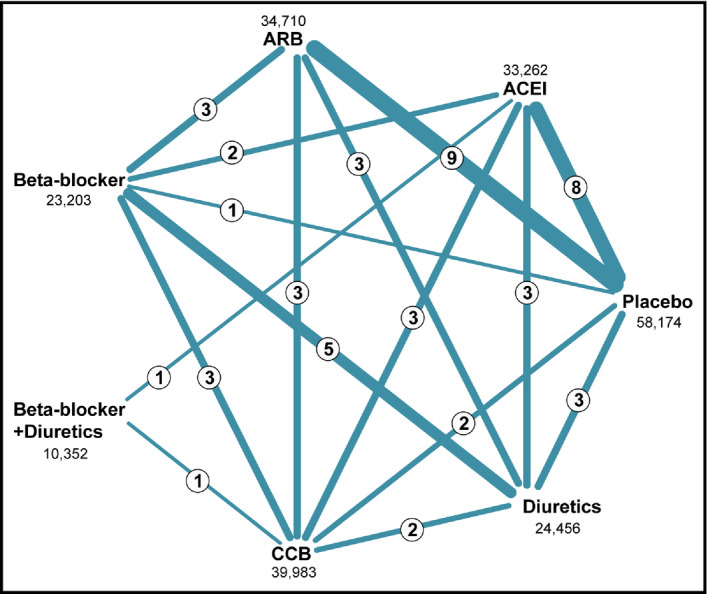
Comparisons of the included studies in the network meta‐analysis. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker
3.2. Results of pairwise meta‐analysis
Traditional pairwise meta‐analysis was performed to measure the preventive effect of different treatments (Table 2. It was observed that compared with placebo and CCBs, patients using ACEIs and ARBs had a lower probability of developing NOD (ACEI vs placebo: OR, 0.77; 95% CI, 0.61–0.97; ARB vs placebo: OR, 0.86; 95% CI, 0.81–0.91; CCB vs ACEI: OR, 1.23; 95% CI, 1.01–1.50; CCB vs ARB: OR, 1.27; 95% CI, 1.14–1.43), whereas β‐blockers and CCBs may increase the risk of developing NOD (β‐blocker vs placebo: OR, 2.18; 95% CI, 1.36–3.50; CCB vs placebo: OR, 1.16; 95% CI, 1.05–1.29). Patients taking diuretics were also observed to have higher incidence rates of NOD than those taking ACEIs, β‐blockers, and β‐blockers plus diuretics (ACEI vs diuretic: OR, 1.36; 95% CI, 1.12–1.66; β‐blocker vs diuretic: OR, 2.50; 95% CI, 1.75–3.57; β‐blocker plus diuretic vs diuretic: OR, 1.25; 95% CI, 1.01–1.55). In addition, ARBs were associated with a lower risk than β‐blockers in the development of NOD (β‐blocker vs ARB: OR, 1.37; 95% CI, 1.16–1.62). Moreover, random‐effects model across all analyses were performed in this study, generating the most conservative estimate of statistical significance. P value, I 2, and τ2 of all comparisons are provided in Table S3.
Table 2.
Results for new‐onset diabetes mellitus from the network meta‐analysis (lower diagonal part) and pairwise meta‐analysis (upper diagonal part)
| Treatment | Placebo | ACEI | ARB | β‐Blocker | β‐Blocker+diuretic | CCB | Diuretic |
|---|---|---|---|---|---|---|---|
| Placebo | 1 | 0.77 (0.61–0.97) | 0.86 (0.81–0.91) | 2.18 (1.36–3.50) | – | 1.16 (1.05–1.29) | 1.61 (0.95–2.72) |
| ACEI | 1.22 (1.01–1.52) | 1 | – | 1.25 (0.84–1.86) | 1.12 (0.96–1.30) | 1.23 (1.01–1.50) | 1.36 (1.12–1.66) |
| ARB | 1.30 (1.07–1.60) | 1.07 (0.82–1.39) | 1 | 1.37 (1.16–1.62) | – | 1.27 (1.14–1.43) | 1.90 (0.90–4.01) |
| β‐Blocker | 0.83 (0.63–1.08) | 0.68 (0.51–0.90) | 0.64 (0.47–0.84) | 1 | – | 0.82 (0.65–1.04) | 2.50 (1.75–3.57) |
| β‐Blocker+diuretic | 0.95 (0.59–1.55) | 0.79 (0.49–1.25) | 0.73 (0.44–1.21) | 1.15 (0.70–1.93) | 1 | 0.88 (0.73–1.06) | 1.25 (1.01–1.55) |
| CCB | 0.96 (0.76–1.22) | 0.79 (0.61–1.01) | 0.73 (0.56–0.95) | 1.16 (0.89–1.54) | 1.01 (0.63–1.60) | 1 | |
| Diuretic | 0.73 (0.57–0.92) | 0.59 (0.45–0.76) | 0.55 (0.41–0.73) | 0.88 (0.67–1.14) | 0.76 (0.45–1.25) | 0.76 (0.58–0.98) | 1 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker.
Values are expressed as odds ratios (ORs) and 95% credible intervals (CrIs) or confidence intervals (CIs). In the upper diagonal part, the OR (95% CI) compares the column condition with the row condition, and in the lower diagonal part, this OR (95% CrI) compares the column condition with the row condition.
Bold values indicate significance.
3.3. Results of the network meta‐analysis
Network meta‐analysis facilitates the combination of both direct and indirect evidence into a single comparison. Placebo showed a higher risk compared with both ACEI and ARB treatments (placebo vs ACEI: OR, 1.22; 95% CrI, 1.01–1.52; placebo vs ARB: OR, 1.30; 95% CrI, 1.07–1.60). β‐Blockers were also associated with a lower probability than ACEIs and ARBs (ACEI vs β‐blocker: OR, 0.68; 95% CrI, 0.51–0.90; ARB vs β‐blocker: OR, 0.64; 95% CrI, 0.47–0.84) as well as ARBs alone in preventing NOD more effectively than CCBs (ARB vs CCB: OR, 0.73; 95% CrI, 0.56–0.95). Results were consistent for diuretics, with four treatments showing a significant advantage over diuretics including placebo (placebo vs diuretic: OR, 0.73; 95% CrI, 0.57–0.92; placebo vs ACEI: OR, 0.59; 95% CrI, 0.45–0.76; placebo vs ARB: OR, 0.55; 95% CrI, 0.41–0.73; placebo vs CCB: OR, 0.76; 95% CrI, 0.58–0.98). Results of the network meta‐analysis are shown in Figure 2 and Table 2. In addition, the surface under the cumulative ranking curve was generated to calculate the cumulative rank probability of all medications for the risk of NOD (Figure 3). It was observed that ACEIs and ARBs have high ranking probabilities (72.77% and 79.81%, respectively) in preventing NOD. Meanwhile, diuretics showed the lowest ranking probabilities of 4.44%, even less than placebo, which had a ranking probability of 45.19%. The results indicate that ACEIs and ARBs play a relatively stronger role in preventing NOD, while diuretics, β‐blockers, and CCBs may increase the risk of NOD during the treatment of hypertension compared with placebo.
Figure 2.
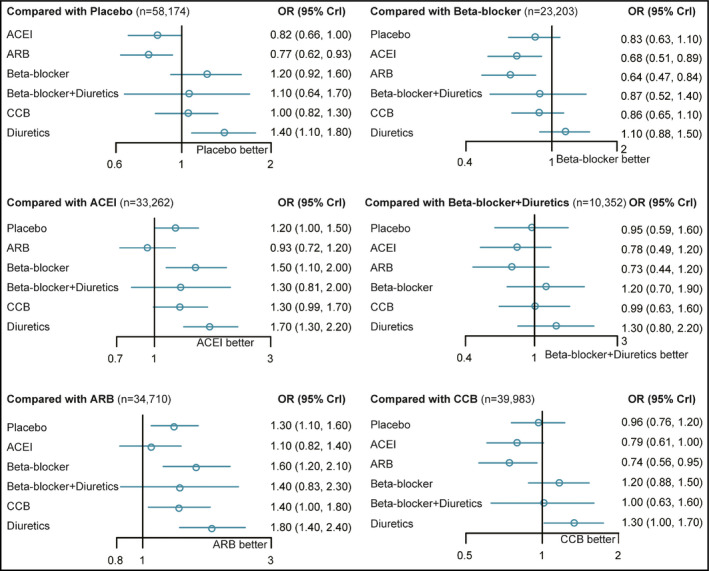
Results of the network meta‐analysis. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; CrI, credible interval; OR, odds ratio
Figure 3.
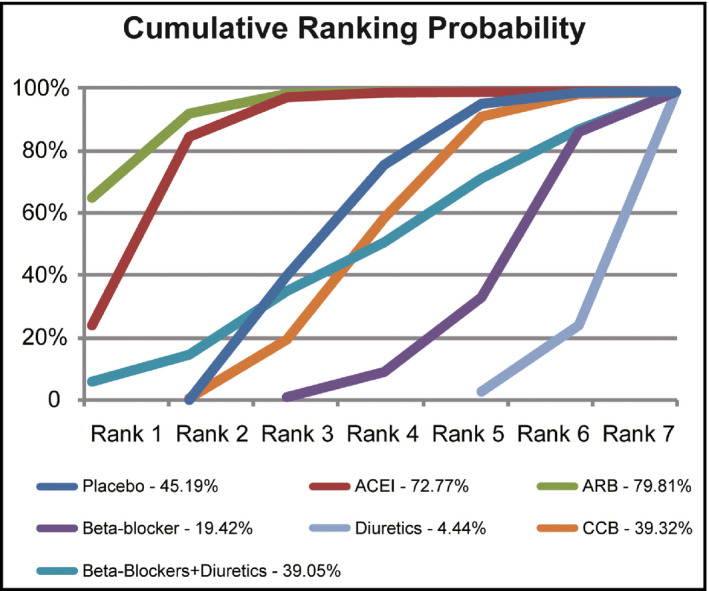
The cumulative rank probability of all medications on the prevention of new‐onset diabetes mellitus. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker
3.4. Consistency and publication bias
The consistency of direct and indirect evidences has been assessed by the node‐splitting method. As presented in Table 3, no significant difference was observed between direct and indirect evidence. A funnel plot from publication bias analysis is presented in Figure 4. No significant publication bias was identified. The validity and credibility of this meta‐analysis were thus confirmed.
Table 3.
Results of consistency analysis by node‐splitting plot
| Study | P value | Odds ratio (95% CI/CrI) |
|---|---|---|
| ACEIs vs placebo | ||
| Direct | .072 | 0.73 (0.55–0.91) |
| Indirect | 1.10 (0.76–1.50) | |
| Network | 0.82 (0.66–0.99) | |
| ARBs vs placebo | ||
| Direct | .809 | 0.78 (0.60–0.97) |
| Indirect | 0.73 (0.46–1.10) | |
| Network | 0.77 (0.63–0.92) | |
| β‐Blockers vs placebo | ||
| Direct | .108 | 2.20 (1.10–4.40) |
| Indirect | 1.10 (0.83–1.50) | |
| Network | 1.20 (0.92–1.60) | |
| CCBs vs placebo | ||
| Direct | .568 | 1.20 (0.72–1.90) |
| Indirect | 1.00 (0.74–1.30) | |
| Network | 1.00 (0.82–1.30) | |
| Diuretics vs placebo | ||
| Direct | .361 | 1.60 (1.00–2.50) |
| Indirect | 1.30 (0.94–1.80) | |
| Network | 1.40 (1.10–1.80) | |
| β‐Blockers vs ACEIs | ||
| Direct | .575 | 1.30 (0.78–2.20) |
| Indirect | 1.60 (1.10–2.30) | |
| Network | 1.50 (1.10–2.00) | |
| β‐Blockers+diuretics vs ACEIs | ||
| Direct | .535 | 1.10 (0.58–2.10) |
| Indirect | 1.50 (0.73–3.10) | |
| Network | 1.30 (0.82–2.10) | |
| CCBs vs ACEIs | ||
| Direct | .970 | 1.30 (0.81–1.90) |
| Indirect | 1.30 (0.89–1.90) | |
| Network | 1.30 (0.99–1.70) | |
| Diuretics vs ACEIs | ||
| Direct | .127 | 1.40 (0.92–2.00) |
| Indirect | 2.00 (1.50–2.90) | |
| Network | 1.70 (1.30–2.20) | |
| β‐Blockers vs ARBs | ||
| Direct | .948 | 1.60 (0.96–2.60) |
| Indirect | 1.60 (1.10–2.30) | |
| Network | 1.60 (1.20–2.10) | |
| CCBs vs ARBs | ||
| Direct | .846 | 1.40 (0.84–2.30) |
| Indirect | 1.30 (0.99–1.90) | |
| Network | 1.40 (1.00–1.80) | |
| Diuretics vs ARBs | ||
| Direct | .722 | 2.00 (1.00–4.10) |
| Indirect | 1.80 (1.30–2.50) | |
| Network | 1.8 (1.40–2.40) | |
| CCBs vs β‐blockers | ||
| Direct | .796 | 0.83 (0.54–1.20) |
| Indirect | 0.90 (0.59–1.30) | |
| Network | 0.86 (0.65–1.10) | |
| Diuretics vs β‐blockers | ||
| Direct | .624 | 1.20 (0.87–1.70) |
| Indirect | 1.10 (0.72–1.60) | |
| Network | 1.10 (0.88–1.50) | |
| CCBs vs β‐blockers+diuretics | ||
| Direct | .584 | 0.89 (0.48–1.70) |
| Indirect | 1.10 (0.58–2.20) | |
| Network | 0.99 (0.63–1.60) | |
| Diuretics vs CCBs | ||
| Direct | .911 | 1.30 (0.80–2.10) |
| Indirect | 1.30 (0.93–1.90) | |
| Network | 1.30 (1.00–1.70) | |
Abbreviations: ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; CCBs, calcium channel blockers; CI, confidence interval; CrI, credible interval.
Figure 4.
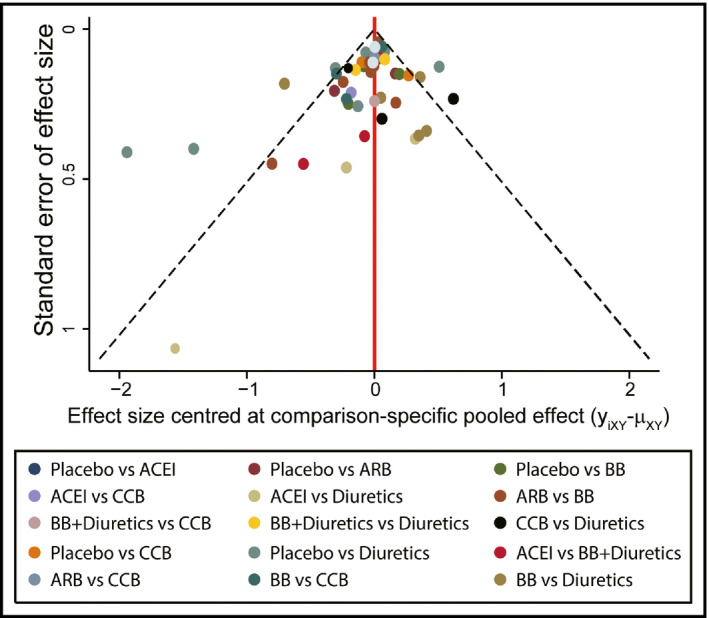
Funnel plots from publication bias analysis. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, β‐blocker; CCB, calcium channel blocker
4. DISCUSSION
In this study, the pairwise meta‐analysis and the network meta‐analysis showed that compared with placebo, ACEIs and ARBs showed a significant advantage, CCBs showed a mild or no impact, and β‐blockers showed an opposite effect on the risk of NOD. The surface under the cumulative ranking curve rank showed that ARBs had an obvious advantage over the other six treatments and ACEIs showed good performance. It is worth mentioning that there were two articles from the 1980s published in the included studies. When performing analysis without these two studies, the outcome did not change.
Previous studies have demonstrated that ARBs exert a beneficial effect on decreasing cardiovascular events and lead to a lowered incidence of NOD.6, 15, 16, 17, 18 Despite the higher price, ACEIs may be more cost‐effective compared with diuretics in elderly patients with hypertension.19 One study showed that ACEIs and ARBs reduced the risk of NOD in patients with hypertension or congestive heart failure and ithat ts mechanism may be complex, which might involve the improvements of both insulin secretion and insulin sensitivity.20 It has been demonstrated that the renin‐angiotensin system is activated in all insulin‐resistant states, including type II DM and hypertension, which are associated with insulin‐resistant states. Angiotensin (Ang) II has been shown to increase the production of hepatic glucose, decrease insulin sensitivity, and contribute to insulin resistance. Renin‐angiotensin system blockade may not only improve blood circulation and cellular ionic balance of skeletal muscle and pancreatic cells but also improve the effect of peripheral insulin and insulin secretion and prevent DM by promoting the recruitment and differentiation of adipocytes via Ang II type 1 receptors.21 However, the mechanisms of preventing insulin resistance between ACEIs and ARBs are not the same. ACEIs not only inhibit the conversion of Ang I to Ang II but also block the degradation of bradykinin. ARBs could completely inhibit the effects of Ang II by selectively binding the receptor site, leading to an accumulation of Ang II and contributing to insulin resistance.22 Therefore, compared with ACEIs, ARBs may exert more stimuli for the prevention of NOD. CCBs as a first‐line antihypertensive drug choice are effective in preventing cardiovascular events23 and are generally prescribed in the treatment of hypertension.24 It has been shown that CCBs have mild or no impact on the risk of NOD.25 In addition, findings from the surface under the cumulative ranking curve show that the prevention effects of β‐blockers and diuretics on NOD are inferior to placebo. Meanwhile, it has been reported that diuretics and β‐blockers are more likely to evoke hyperglycemia compared with ACEIs, ARBs, and CCBs despite their antihypertensive effects.4
5. STUDY STRENGTHS AND LIMITATIONS
A previous network meta‐analysis has published similar findings.26 Compared with previous research, we included more articles and one more combination treatment in our Bayesian network meta‐analysis. Meanwhile, our results show a consistent conclusion with the previous study, making the results more reliable. In the first Bayesian network meta‐analysis, seven treatments for hypertension were pooled to assess the incidence of NOD and synthesize the available data including direct and indirect evidence of traditional meta‐analysis.
There are some limitations, however, that affect the results of our study. First, the studies of the prevention effects on NOD of β‐blockers, β‐blockers+diuretics, CCBs, and diuretics were less than those of ACEIs and ARBs, leading to larger deviations in the sample size among seven treatments of hypertension. Second, there existed heterogeneity of the patients in the included studies, eg, patients treated with combination therapy might have had a longer duration of hypertension, which could have resulted in a higher risk of NOD. Third, NOD was not a predefined outcome, and therefore may not have been accurately evaluated. It was difficult to assess whether the reported NOD developed after antihypertensive treatment or whether it was present before taking the antihypertensive treatment. Fourth, we searched only English articles because of our limitation of language. This may have resulted in the omission of useful data. Finally, we did not evaluate the effect of the drugs on their ability to prevent complications such as diabetic angiopathies because few of the included studies reported complications. This may have limited the assessment of these agents.
6. CONCLUSIONS
Based on the present results from this network meta‐analysis, ARBs show an obvious advantage over the other six treatments associated with of NOD and seems to be the optimal choice in clinical practice. In addition, ACEIs also show better performance regarding the prevention of NOD in patients with hypertension. Future studies should focus on the mechanisms of prevention of NOD among these treatments.
AUTHOR CONTRIBUTIONS
Zimeng Li: research conception and article writing; Yi Li and Yulong Liu: literature search and data extraction; Wenbo Xu and Qing Wang: article revision. All authors have read and approved the final article.
CONFLICTS OF INTEREST
None of the authors have any conflicts of interest to declare.
Supporting information
Li Z, Li Y, Liu Y, Xu W, Wang Q. Comparative risk of new‐onset diabetes mellitus for antihypertensive drugs: A network meta‐analysis. J Clin Hypertens. 2017;19:1348–1356. 10.1111/jch.13108
REFERENCES
- 1. Kannel WB, McGee DL. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the Framingham study. Diabetes Care. 1979;2:120‐126. [DOI] [PubMed] [Google Scholar]
- 2. Messerli FH, Grossman E, Leonetti G. Antihypertensive therapy and new onset diabetes. J Hypertens. 2004;22:1845‐1847. [DOI] [PubMed] [Google Scholar]
- 3. Abuissa H, Jones PG, Marso SP, O'Keefe JH Jr. Angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta‐analysis of randomized clinical trials. J Am Coll Cardiol. 2005;46:821‐826. [DOI] [PubMed] [Google Scholar]
- 4. Alderman MH. New onset diabetes during antihypertensive therapy. Am J Hypertens. 2008;21:493‐499. [DOI] [PubMed] [Google Scholar]
- 5. Barzilay JI, Gao P, Ryden L, et al. Effects of telmisartan on glucose levels in people at high risk for cardiovascular disease but free from diabetes: the TRANSCEND study. Diabetes Care. 2011;34:1902‐1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liou YS, Chen HY, Tien L, Gu YS, Jong GP. Antihypertensive drug use and new‐onset diabetes in female patients with coronary artery disease: a population‐based longitudinal cohort study. Medicine (Baltimore). 2015;94:e1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barzilay JI, Davis BR, Pressel SL, et al. Long‐term effects of incident diabetes mellitus on cardiovascular outcomes in people treated for hypertension: the ALLHAT Diabetes Extension Study. Circ Cardiovasc Qual Outcomes. 2012;5:153‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilkins RW. New drugs for the treatment of hypertension. Ann Intern Med. 1959;50:1‐10. [DOI] [PubMed] [Google Scholar]
- 9. Dronavalli S, Bakris GL. Mechanistic insights into diuretic‐induced insulin resistance. Hypertension. 2008;52:1009‐1011. [DOI] [PubMed] [Google Scholar]
- 10. Vasisht KP, Bakris G. New onset diabetes: can it be delayed? J Hum Hypertens. 2008;22:517‐519. [DOI] [PubMed] [Google Scholar]
- 11. Grimm C, Koberlein J, Wiosna W, Kresimon J, Kiencke P, Rychlik R. New‐onset diabetes and antihypertensive treatment. GMS Health Technol Assess. 2010;6:Doc03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta‐analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. 2011;39:91‐92. [DOI] [PubMed] [Google Scholar]
- 13. Fox CS, Golden SH, Anderson C, et al. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2015;132:691‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163‐171. [DOI] [PubMed] [Google Scholar]
- 15. Mancia G, Grassi G, Zanchetti A. New‐onset diabetes and antihypertensive drugs. J Hypertens. 2006;24:3‐10. [DOI] [PubMed] [Google Scholar]
- 16. Gupta AK, Dahlof B, Dobson J, Sever PS, Wedel H, Poulter NR. Determinants of new‐onset diabetes among 19,257 hypertensive patients randomized in the Anglo‐Scandinavian Cardiac Outcomes Trial–Blood Pressure Lowering Arm and the relative influence of antihypertensive medication. Diabetes Care. 2008;31:982‐988. [DOI] [PubMed] [Google Scholar]
- 17. Chowdhury EK, Owen A, Ademi Z, et al. Short‐ and long‐term survival in treated elderly hypertensive patients with or without diabetes: findings from the Second Australian National Blood Pressure study. Am J Hypertens. 2014;27:199‐206. [DOI] [PubMed] [Google Scholar]
- 18. Hasvold LP, Bodegard J, Thuresson M, et al. Diabetes and CVD risk during angiotensin‐converting enzyme inhibitor or angiotensin II receptor blocker treatment in hypertension: a study of 15,990 patients. J Hum Hypertens. 2014;28:663‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chowdhury EK, Ademi Z, Moss JR, Wing LM, Reid CM. Cost‐utility of angiotensin‐converting enzyme inhibitor‐based treatment compared with thiazide diuretic‐based treatment for hypertension in elderly Australians considering diabetes as comorbidity. Medicine (Baltimore). 2015;94:e590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scheen AJ. Renin‐angiotensin system inhibition prevents type 2 diabetes mellitus. Part 1. A meta‐analysis of randomised clinical trials. Diabetes Metab. 2004;30:487‐496. [DOI] [PubMed] [Google Scholar]
- 21. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840‐846. [DOI] [PubMed] [Google Scholar]
- 22. Basile JN. Antihypertensive therapy, new‐onset diabetes, and cardiovascular disease. Int J Clin Pract. 2009;63:656‐666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Costanzo P, Perrone‐Filardi P, Petretta M, et al. Calcium channel blockers and cardiovascular outcomes: a meta‐analysis of 175,634 patients. J Hypertens. 2009;27:1136‐1151. [DOI] [PubMed] [Google Scholar]
- 24. Yamamoto Y, Sonoyama K, Matsubara K, et al. The status of hypertension management in Japan in 2000. Hypertens Res. 2002;25:717‐725. [DOI] [PubMed] [Google Scholar]
- 25. Taylor EN, Hu FB, Curhan GC. Antihypertensive medications and the risk of incident type 2 diabetes. Diabetes Care. 2006;29:1065‐1070. [DOI] [PubMed] [Google Scholar]
- 26. Elliott WJ, Meyer PM. Incident diabetes in clinical trials of antihypertensive drugs: a network meta‐analysis. Lancet. 2007;369:201‐207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


