Abstract
Hypertension is the leading cause of cardiovascular disease in the United States and worldwide. It also provides a useful model for team‐based chronic disease management. This article describes the M.A.P. checklists: a framework to help practice teams summarize best practices for providing coordinated, evidence‐based care to patients with hypertension. Consisting of three domains—Measure Accurately; Act Rapidly; and Partner With Patients, Families, and Communities—the checklists were developed by a team of clinicians, hypertension experts, and quality improvement experts through a multistep process that combined literature review, iterative feedback from a panel of internationally recognized experts, and pilot testing among a convenience sample of primary care practices in two states. In contrast to many guidelines, the M.A.P. checklists specifically target practice teams, instead of individual clinicians, and are designed to be brief, cognitively easy to consume and recall, and accessible to healthcare workers from a range of professional backgrounds.
1. Background
Recent policy changes emphasizing value‐based payment—primarily through passage of the 2009 Patient Protection and Affordable Care Act and the 2015 Medicare Access and CHIP Reauthorization Act—have incentivized health systems to transition towards population health–based care models that seek to provide higher quality care while avoiding unnecessary cost.1, 2 Within this environment, healthcare providers can no longer rely on simply examining and treating patients with a particular condition, instead they are now expected to ensure that their patients achieve desirable outcomes for that condition. One targeted condition is hypertension.3
Hypertension is the leading cause of cardiovascular disease and premature deaths worldwide. In the United States, it affects nearly one in three adults and costs an estimated $42.9 billion in direct medical spending annually.4 It is also among the most treatable conditions since a wide variety of lifestyle modification strategies and medications have been shown to improve blood pressure (BP) control. Despite the intense attention directed toward this condition and a wealth of effective treatments, hypertension control remains suboptimal. The 2011–2012 National Health and Nutrition Examination Survey (NHANES), the most recent data available, reported that only 51.8% of hypertensive adults had their BP under control, leaving approximately 15% of the population still at unnecessary risk for strokes, heart attacks, kidney disease, and premature death.5 Almost 90% of patients with hypertension who remain uncontrolled say they have a usual source of health care, yet 16% of persons nationwide are unaware that they have the condition. Of those with a usual source of care who are aware of their hypertension, 7% remain untreated and another 31% are treated but still do not have their BP under control, suggesting that multipronged, systems‐based strategies are needed.6
In recent decades, exemplary organizations have implemented innovative approaches, such as the Chronic Care Model and the Patient‐Centered Medical Home, which redistribute role responsibilities among teams of clinicians, professional staff, and nonprofessional staff, so that they can efficiently deliver evidence‐based treatment and promote patients’ ability to self‐manage their health.7, 8, 9 While these care models often set goals for how members of a primary care team should interact with each other, they typically do not describe what clinical tasks the teams must perform to effectively care for a condition. Instead, it is assumed that for each clinical condition, teams will review the literature, identify best practices, and integrate those practices into their daily workflow—even though many lack the staffing, resources, and access to specialized expertise needed to do this. Our Working Group, comprising leadership from the American Medical Association's (AMA's) Improving Health Outcomes Program, the Johns Hopkins Center to Eliminate Cardiovascular Health Disparities, and the Armstrong Institute for Patient Safety and Quality, developed the M.A.P. checklists to address this gap.
2. Development Process
Effective treatment of chronic diseases such as hypertension must overcome three challenges: diagnostic uncertainty, defined as lack of clarity as to whether a condition exits; clinical inertia, defined as failure to initiate or escalate treatment when patients have not met treatment goals; and nonadherence to prescribed treatments. The M.A.P. checklists (Figure 1) address these challenges by organizing best practices into three intuitive domains for managing patients with hypertension: Measure Accurately: Act Rapidly: and Partner With Patients, Families, and Communities. Although many clinicians will already recognize some or all of the best practices described, the M.A.P. checklists summarize hypertension care in a way that describes what the entire practice team must do to help patients achieve hypertension control. This provides a framework for building out role‐specific tasks, so that each member of the care team understands not only what tasks he or she must perform but also what other team members should be doing, thereby applying core components of team‐based chronic care, such as “situational monitoring” and “mutual support.”10 For example, a medical assistant (MA) who becomes familiar with a limited set of standardized antihypertensive medications used by his or her practice may feel more able to reinforce patient education, provide anticipatory guidance related to treatment, and recognize when patients have deviated from the treatment plan. By explicating what care the practice team must provide, the M.A.P. checklist helps all members of a practice team to actively participate in managing patients with high BP.
Figure 1.
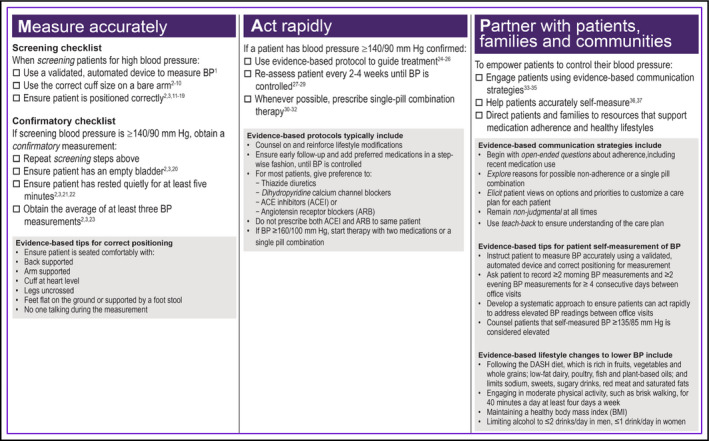
The 2015 M.A.P. checklist for improving blood pressure (BP) control
2.1. Model development
The M.A.P. checklists were developed through a multistep process (Figure 2) that incorporated a review of peer‐reviewed and “gray” literature, feedback, and validation from a panel of internationally recognized scientific experts and stakeholders (the Hypertension Control Advisory Group [HCAG]), and pilot testing with a sample of 10 primary care practices of varying sizes and patient populations in Illinois and Maryland. The overarching aims guiding the checklists’ development were that they would: (1) describe evidence‐based strategies, (2) promote strategies that would be feasible in most primary care settings, and (3) present recommendations in an accessible format. One of the coauthors (MW) conducted a literature review of best practices in hypertension care between January 2013 and May 2013. Findings from this review were synthesized into five domains that were then presented to members of the HCAG for feedback on their validity and feasibility in June 2013.
Figure 2.
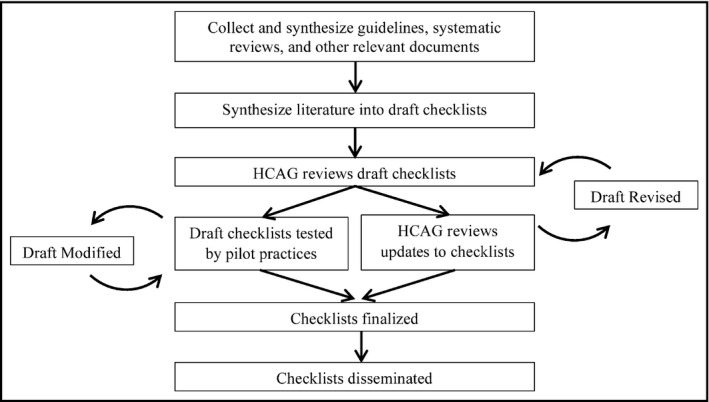
Process for developing the M.A.P. checklists. HCAG indicates Hypertension Control Advisory Group
Building on feedback from the HCAG, our team of clinicians and quality improvement experts refined the concepts iteratively between June 2013 and October 2013, ultimately producing a three‐domain framework that closely resembles the current checklists. The refinement process was guided by the psychological principles of “working memory” and “chunking.”11 These principles state that human beings can cognitively manipulate only a limited number of items (eg, guideline recommendations) at one time, but that they can work with more items if individual items are grouped together in a logical or intuitive manner. Additionally, recommendations were written so that they would be understandable to workers with at least a high school education.
In October 2013, we recruited a convenience sample of 10 primary care practices of varying sizes and characteristics to participate in Improving Health Outcomes: Blood Pressure (IHO:BP), a quality improvement collaborative in which the M.A.P. checklists provided the objectives for process improvement. Based on feedback from clinicians and office staff, we continued to iteratively refine the checklists until December 2014, when a penultimate version was presented to members of HCAG for final comment and validation.
The rationale underlying each M.A.P. checklist domain and examples for how primary care teams can apply them in practice are described in the following sections.
3. The M.A.P. checklists
3.1. Measure accurately
3.1.1. Why BP measurement is important
All efforts to improve hypertension care must begin with ensuring that patients receive accurate BP measurements (Figure 1). Despite their importance in deciding whether or not to escalate treatment and in monitoring response to therapy, overwhelming evidence suggests that the typical office‐based BP is imprecise.12, 13 For example, a study by Powers and colleagues,14 comparing office‐based BP measurements with readings obtained by research nurses using gold standard techniques, concluded that primary care providers (PCPs) relying on a single office‐based BP reading would have <80% certainty in correctly classifying a patient as hypertensive or nonhypertensive if the systolic BP reading was between 120 and 157 mm Hg.
The disparity between the quality of research BP measurements and those obtained in typical primary care practice may explain a common dilemma faced by many PCPs. On one hand, some experts have cautioned against aggressively treating high BP among older patients given anecdotal reports and observational studies linking aggressive treatment with falls and other medication‐related adverse effects.15, 16 However, others have called on practice teams to ensure that they treat patients with any BP value above goal (BP ≥140/90 mm Hg for most patients), citing evidence that every 2‐mm Hg decrease in systolic BP leads to a 7% reduction in cardiovascular events and a 10% reduction in stroke.17 Furthermore, rigorous clinical trials have shown no increased risk for falls among patients randomized to receive intensive antihypertensive treatment instead of placebo.18, 19 One explanation for these seemingly contradictory findings is that in real‐world settings, where BP measurements are obtained haphazardly, some providers may overtreat patients by intensifying treatment for patients with BP values that only appear to be high; whereas other providers may be undertreating patients when they defer treating so‐called borderline high BP values. For these reasons, it is critical that practice teams obtain precise and reliable BP measurements in order to make appropriate treatment decisions.
3.1.2. The “screen and confirm” process
A variety of environmental factors can influence BP measurements, thus guidelines specify several specific steps, including positioning patients in a chair with their back, arms, and feet supported and legs uncrossed; using an appropriate cuff size; providing patients with a rest period before the procedure; using the correct measurement technique; and obtaining multiple BP readings (Table 1).20 While researchers routinely adhere to these standards in tightly controlled clinical trials, healthcare workers in real‐world practices are often hindered by suboptimal conditions, such as patients registering late or arriving in pain, as well as by competing demands, including measuring patients’ temperatures, tracking down laboratory results, and performing dozens of other tasks unrelated to hypertension.
Table 1.
Recommendations for Performing Office‐Based Blood Pressure Measurements20
| Recommendation | Explanation |
|---|---|
| 1. Use a valid sphygmomanometer or automated BPM device | Manual and automated BPM devices should be calibrated at least annually |
| 2. Seat patient in chair with back support | Lack of back support can falsely raise BP readings |
| 3. Ensure patient's feet are supported | Dangling feet can falsely raise BP readings; patients with short legs may require a foot stool |
| 4. Ensure patient's legs are not crossed | Crossed legs can falsely raise BP readings |
| 5. Place appropriate cuff size on patient's bare arm | The rubber bladder inside the cuff's covering cloth should wrap around 80%–100% of the patient's upper arm; cuffs falsely raise BP if too small and falsely lower BP if too large |
| 6. Ensure arm is supported on a flat surface with middle of cuff at heart level | BP can be falsely raised or lowered if the middle of the cuff is lower or higher than heart level; BP can be falsely raised when patients hold up their arms during BP measurement |
| 7. Allow patient to rest for 5 minutes | Resting periods allow BP to “settle” and provides a more stable BP reading |
| 8. Identify the MIL by inflating cuff to a pressure where the radial artery pulsation disappears, then deflate cuff | Identifying the MIL ensures that the cuff will be inflated high enough to identify the first Korotkoff sound, which corresponds to SBP |
| 9. Reinflate cuff to 30 mm Hg higher than the MIL pressure | This step ensures that the observer will be able to identify the first Korotkoff sound |
| 10. Slowly deflate cuff, no faster than 2 mm Hg per second | Rapid cuff deflation can lead observers to falsely underestimate SBP and overestimate DBP because the needle falls faster than the observer's ability to hear Korotkoff sounds |
| 11. Record SBP when the first Korotkoff sound appears | The pain where blood begins to flow past the cuff corresponds to the SBP |
| 12. Record DBP when fifth Korotkoff sound disappears | The point where blood flows freely past the cuff corresponds to the DBP |
| 13. Continue to deflate cuff another 20 mm Hg, then completely deflate cuff | This step helps observers avoid mistaking the “auscultatory gap” for the fifth Korotkoff sound. The auscultatory gap can be eliminated by raising the patients arm overhead for 30 seconds before inflating the cuff. It is not an issue with automated BPM devices |
| 14. Wait 1 minute, then repeat steps 1 through 13 twice | Multiple BPMs allow observers to obtain a more stable and precise estimate of “true” BP |
| 15. Record the mean value of the second and third BP measurements | The first BP reading can be falsely higher than the true estimate; calculating the average value of multiple BP readings accounts for normal variability among individual BP readings and provides a more precise estimate of true BP |
Abbreviations: BP, blood pressure; BPM, blood pressure measurement; DBP, diastolic blood pressure; MIL, minimum inflation level; SBP, systolic blood pressure.
The M.A.P. checklists seek to balance the clinical value of applying careful, deliberate measurement technique with the real‐world need to rapidly room and assess patients by recommending a “screen and confirm” process analogous to how public health officials test for HIV. Patients who undergo HIV testing initially receive a rapid, sensitive test that can reliably exclude HIV infection. But if the screening test result is positive, they then undergo a specific but more complex test to confirm the results of the screening test. (If the confirmatory test result is negative, the patient is considered to be HIV negative.)
In an analysis of NHANES data, Handler and colleagues21 found that few patients with an initial reading below the treatment cutoff of 140/90 mm Hg would have been classified as having high BP if the measurements were repeated, but approximately one third of patients with a high initial reading (≥140/90 mm Hg) would have been classified as not requiring treatment if BP measurements were repeated. Thus, the M.A.P. checklists recommend a two‐tiered BP measurement process that “screens” all patients for high BP and applies more time‐intensive “confirmatory” steps only for patients whose first BP is ≥140/90 mm Hg. In this way, practice teams can minimize workflow disruptions while at the same time improve the likelihood that patients with “true” uncontrolled BP will receive treatment.
Primary care teams can put these principles into practice by training MAs and other staff to position patients appropriately and use the correct cuff size (steps that increase reliability of BP measurements but require little or no added time) when they screen for high BP, and also to automatically confirm high BP readings with a process that also ensures the patient's bladder is empty, provides an appropriate resting period, and calculates the average of multiple BP readings (Table 2 and Figure 3). Teams can support this process by using an automated BP measurement device that can be programmed to provide patients with a resting period and automatically perform multiple measurements, so that healthcare workers are free to multitask while obtaining confirmatory BP measurements.
Table 2.
Recommendations for Using Self‐Measured Blood Pressure Monitoring44
| Recommendation | Explanation |
|---|---|
| 1. Patients should use an automated or a semiautomated device | Automated devices require less patient training, avoid observer bias, and are often equipped with features to electronically store and transmit BP measurement data |
| 2. Patients should use a device that measures BP from the upper arm whenever possible | Wrist devices are vulnerable to inaccurate measurement if the cuff is held below or above heart level. They should only be used for patients with arms that are too large for upper arm devices |
| 3. Patients should use SMBP in the morning (before taking medications) and evening for at least 3, and preferably 7, days before a clinic visit | Obtaining multiple consecutive BP readings smooths out the expected variability in patients’ BP, thus providing a more accurate estimate of their “true” BP |
| 4. Patients should use SMBP at least 30 minutes without smoking, caffeine, eating, or exercise | Smoking, caffeine, recent meals, or recent exercise can all falsely raise BP readings |
| 5. Patients should use SMBP while seated in a quiet room in a chair with back support | Loud or uncomfortable rooms can falsely raise BP; lack of back support can falsely raise BP |
| 6. While seated, patients’ arms and feet should be supported and their legs uncrossed | Unsupported arms, dangling feet, or crossed legs can all falsely raise BP readings |
| 7. Patients should apply the cuff 1–2 inches above the bend of the elbow; the inflatable bladder inside cover cloth should wrap around 80%–100% of their upper arm | Cuff bladders smaller than 80% of the upper arm circumference can lead to falsely high BP readings; cuff bladders that are too large can lead to falsely low BP readings |
| 8. Patients should rest at least 5 minutes before activating the SMBP device | Premeasurement resting periods allow BP to “settle” and provides a more stable BP reading |
| 9. Patients should record the date, time, and SMBP reading in a log | Recording these data will help ensure that the BP readings are interpreted correctly |
| 10. Patients and providers should calculate the mean average of all BP readings to use for making treatment decisions. SMBP values ≥135/85 mm Hg indicate a high BP | Calculating a mean average BP value accounts for normal variability among individual SMBP readings; the threshold SMBP 135/85 mm Hg corresponds with the threshold BP 140/90 mm Hg in clinic readings |
Abbreviations: BP, blood pressure; SMBP, self‐measured blood pressure monitoring.
Figure 3.
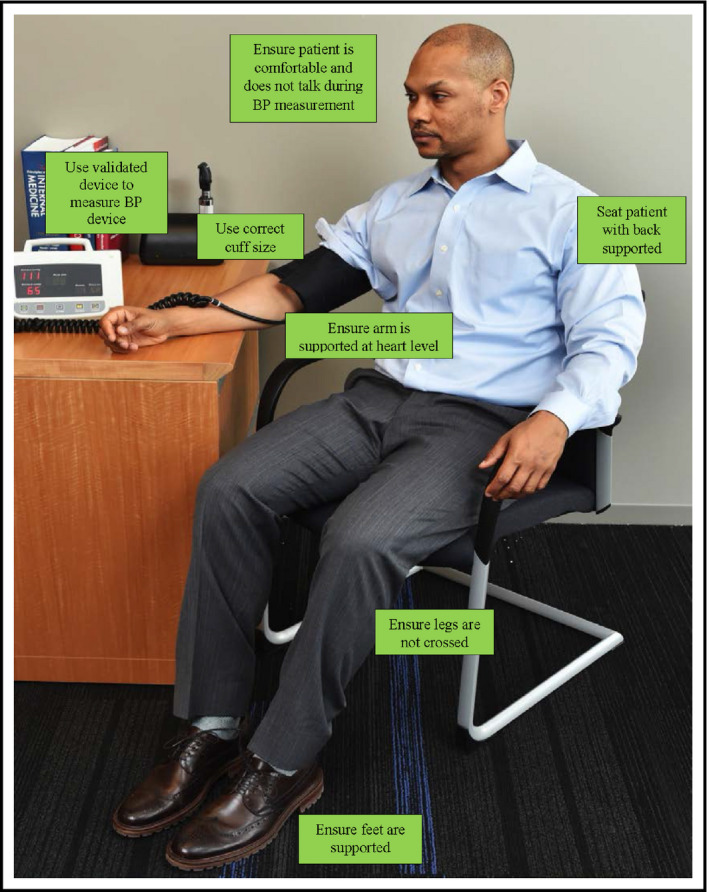
Key elements for patient positioning during office‐based blood pressure (BP) measurements
3.2. Act rapidly
3.2.1. Why proactively treating high BP is important
Despite the importance of BP measurement, accurately measuring BP does not, by itself, control hypertension (Figure 1). Healthcare workers must also prescribe effective therapies and titrate treatment until patients achieve BP control. Although most hypertensive patients require treatment with two or more medication classes,22 clinical inertia (ie, failing to start treatment or failing to intensify it when BP is found to be high) is common and is the leading factor behind suboptimal BP control rates.23
Why does clinical inertia occur? One study of 1169 patients seen in Veteran Administration (VA) primary care clinics found that many factors contribute to clinical inertia, including lack of confidence in the accuracy of the office BP measurement, competing priorities during the office visit, patient reluctance to intensify treatment, and difficulty in arranging follow‐up after treatment has been adjusted. Among these factors, clinical inertia was most strongly linked to uncertainty about the patient's true BP, followed by PCPs giving priority to other issues during the office visit.24
It follows, then, that the most direct way for practices to improve BP control is to implement systems to ensure that PCPs have accurate BP readings before the patient's visit ends. While the Measure Accurately checklist provides valuable guidance for reducing uncertainty about the accuracy of BP measurements, the Act Rapidly checklist offers three additional approaches to overcoming clinical inertia.
3.2.2. Evidence‐based treatment protocols
First, practice teams can facilitate proactive treatment by adopting a standardized hypertension management protocol that describes what treatment patients should receive, when they should receive it, and how they should follow up.25 Although protocols have sometimes been disparaged as “cookbook medicine,” experience from exemplary practices suggests that having a systematic approach to selecting antihypertensive medications is superior to prescribing antihypertensive drugs on an ad hoc basis because protocols allow practice teams to plan treatment strategies in a thoughtful, deliberative way instead of haphazardly selecting medications during the hustle and bustle of a clinic session.26 Also, by encouraging use of a few preferred medications and recommended series of actions, protocols make it easier for nonclinician staff to learn details about each option and assume responsibility for tasks such as counseling patients about medications, screening for potential side effects, and providing anticipatory guidance to promote adherence.27
The Million Hearts Campaign has made several treatment algorithms available on its website (Box). While minor differences exist among them, each describes a stepwise strategy that systematically addresses the major mechanisms underlying hypertension, including renin‐angiotensin system dysfunction, sodium/volume excess, and excess sympathetic tone.28 For example, an algorithm used by Kaiser Permanente and available on the Million Hearts Campaign website begins treatment by combining a low dose of an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker (which addresses renin‐angiotensin system dysfunction) with a thiazide diuretic (which addresses excess sodium/volume), and then gradually increases the dose as needed. If BP remains uncontrolled after the initial prescription has been maximized, the algorithm adds a dihydropyridine calcium channel blocker (strengthening treatment for excess sodium/volume), before adding either spironolactone (optimizing diuretic therapy) or a β‐blocker (treating excess sympathetic tone).27
Box 1. Online resources for “Act Rapidly” and “Partner with Patients” Domains.
Resources with hypertension care tools and protocols
The Million Hearts Campaign – http://millionhearts.hhs.gov/tools-protocols/index.html
American Medical Group Foundation: Measure Up Pressure Down toolkit – http://www.measureuppressuredown.com/hcprof/toolkit.pdf
Resources to support patient‐provider communication
Agency for Healthcare Research and Quality: “Training to advance physicians’ communication skills” – http://www.ahrq.gov/cahps/quality-improvement/improvement-guide/6-strategies-for-improving/communication/strategy6gtraining.html
Johns Hopkins University Center to Eliminate Cardiovascular Health Disparities: Project ReDCHiP Communication Skills Program – http://www.projectredchip.com/
Motivational Interviewing Network of Trainers (MINT) – http://motivationalinterviewing.org/
Institute for Healthcare Communication: Training program – http://healthcarecomm.org/training/
American Academy on Communication in Healthcare: Workshops – http://healthcarecomm.org/training/
Resources to support patient lifestyle modification
National Institutes of Health: Your guide to lowering your blood pressure with DASH – https://www.nhlbi.nih.gov/files/docs/public/heart/dash_brief.pdf
DASH for good health southern style, 2nd edition” cookbook – http://academicdepartments.musc.edu/cme/resources/obesity_resources/dashcookbook2008.pdf
American Heart Association. Baltimore Kitchen – http://www.heart.org/HEARTORG/Affiliate/BaltimoreKitchen_UCM_453841_SubHomePage.jsp
3.2.3. Arranging for early and frequent follow‐up until BP is controlled
Second, teams can overcome clinical inertia by following up with patients early and frequently until they achieve BP control. Close follow‐up allows practice teams to efficiently gather feedback on whether treatments are working, adjust treatment, and strengthen the patient‐provider relationship, thereby increasing patient engagement and treatment adherence.29 One observational study found that following up with hypertensive patients within 6 weeks is linked to shorter time to BP control, higher rates of BP control,30 and lower incidence of cardiovascular events, while another reported benefit from even earlier follow‐up.31
One way that teams can put this principle into practice is by offering patients follow‐up with nonclinician members of the team. While tightly booked schedules often prevent PCPs from reexamining patients early, nonclinicians are often available and can address many follow‐up issues just as effectively. At Kaiser Permanente, for example, patients identified as having BP above goal are typically scheduled to return for a BP check by an MA every 2 to 4 weeks until their BP is controlled.32 During these return visits, an MA will measure the patient's BP using a standardized process and, if the BP remains high, inform the PCP, who can immediately adjust treatment and arrange for follow‐up care if needed. A key feature of this approach is that patients are able drop in at their convenience, since any available MA could perform the BP check, and PCPs can direct medication changes and follow‐up by telephone instead of intervening in person.
When combined with a well‐designed protocol, practices can also use nonclinician BP visits to make routine medication adjustments without direct clinician input. For example, PCPs in a practice within the Johns Hopkins Community Physicians network refer hypertensive patients to a protocol‐based nurse visit, guided by a treatment plan that was developed in advance by the practice's PCPs. The protocol explicitly describes when the patient should return for a BP visit, what potential side effects the nurse should screen for, and how medications should be adjusted if the patient adhered to medications but their BP is still elevated. If the patient's BP is still elevated after the maximum dose of a medication has been prescribed (typically by the second or third BP visit), the nurse informs the PCP, who then prescribes another medication and activates a protocol for it. Along with adjusting treatment according to protocol, the nurse also counsels patients about their medications and discusses potential barriers to adherence.
3.2.4. Single‐pill combination therapy
Third, teams can address clinical inertia by encouraging the use of single‐pill combination therapy,33 which accelerates time to BP control and promotes medication adherence. For example, the Kaiser Permanente treatment algorithm described earlier begins drug therapy for most patients with a relatively low‐dose combination pill and adds a third class of medications only if needed. Designed to minimize risk for side effects, promote adherence, and let PCPs write as few prescriptions as possible, this protocol also allows patients to achieve maximal doses of up to three medication classes with only two prescriptions and in six or fewer titration steps, instead of the three prescriptions and eight steps that would otherwise be required if PCPs prescribed one antihypertensive medication at a time (ie, a 25% gain in treatment efficiency, and a 33% reduction in the number of times a patient would need to obtain a prescription from their PCP).27
3.3. Partner with patients, families, and communities
3.3.1. Why engaging patients in their health is important
Of course, prescribing effective medications and actively following up with patients to titrate doses will not improve hypertension control rates if patients do not adhere to treatment or keep their appointments (Figure 1). Fortunately, evidence suggests that practice teams can apply several effective strategies to promote patients’ adherence and ability to self‐manage their health. The Partner With Patients Checklist focuses on three approaches in this area.
3.3.2. Collaborative communication
The extent to which patients adhere to treatment and effectively manage their health is often thought of as being influenced by factors outside of clinicians’ control, including social and economic factors; patients’ health beliefs, attitudes, knowledge; and their ability to participate in care and manage their illness. However, evidence shows that when patients feel their provider involves them in decision‐making or that their provider knows them as a person, they are significantly more likely to adhere to treatment and experience better outcomes. For example, researchers have long known that engaged, “activated” patients experience better health outcomes than disengaged patients.34 Yet, Naik and colleagues35 found that when high‐risk, disengaged patients felt their provider collaborated with them to make treatment decisions, their BP control improved to levels equivalent to those of highly engaged patients. Thus, the M.A.P. checklists encourage clinicians, clinical staff, and other members of the team to obtain training in evidence‐based communication skills so they can effectively engage patients in their own care.
Practice teams may access online resources, as well as more intensive training programs to keep abreast of best practices in the evolving science of patient‐provider communication (Box). However, a common thread that connects all effective communication strategies is that patients should feel respected as a whole person.36 For example, patients are more likely to report not taking medications when asked with a nonjudgmental, normalizing manner, such as “How many doses would you say you missed since your last visit?”37 Similarly, motivational interviewing strategies, such as using open‐ended questions and reflective listening, are also effective for identifying nonadherence and why it occurred.38 Thus, instead of encouraging clinicians and staff to use rote phrases or specific communication techniques, practice teams may find it more effective to flexibly employ a combination of strategies, as long as they build a therapeutic relationship in which patients feel respected and understood.39
3.3.3. Self‐measured BP monitoring
Practice teams can also support patients by helping them use self‐measured BP monitoring (SMBP), also known as home BP monitoring. SMBP is increasingly affordable for many patients, predicts risk for cardiovascular events better than clinic‐based BP measurements, and can clarify patients’ concerns about the accuracy of clinic‐based BP readings, such as when they report, “My blood pressure is lower at home.”40 It also engages patients in self‐managing their health by providing invaluable feedback as to what factors in their day‐to‐day lives lead to good BP control, thus reinforcing positive lifestyle changes.41 For these reasons, the M.A.P. checklists recommend that practice teams encourage patients to use SMBP.
As with clinic‐based BP measurements, however, practice teams should ensure that patients use SMBP correctly. Studies have found that 60% to 70% of patients use SMBP incorrectly when their technique is evaluated in practice settings, and that lack of patient education on how to use it is the primary reason for this.42 Practice teams and patients should both be aware that BP cutoffs for treatment are typically 5 mm Hg lower with SMBP than with office‐based BP measurements (ie, SMBP 135/85 mm Hg is equivalent to office‐based BP 140/90 mm Hg for treatment purposes).43 Additionally, guidelines recommend that patients use validated automated SMBP devices, with an upper arm cuff, while seated in the recommended position, and obtain multiple averaged measurements twice a day for 3 to 7 consecutive days before results are used to adjust treatment (Table 2).44 Finally, practice teams should provide patients with between‐visit support (eg, providing telephone‐based guidance on what to do with different SMBP readings), as patients are significantly more likely to achieve BP control when practices support them. The AMA offers a variety of tools to help practice teams implement comprehensive SMBP programs on its website.45
The experience of the VA provides an example of an efficacious clinic‐based SMBP initiative. In this program, VA nurses identified patients with uncontrolled BP and educated them on how to use SMBP. Patients then self‐measured their BP every other day and submitted the results by telephone. VA nurses then used the submitted data to calculate an average BP reading every 2 weeks. If the average BP was above a specified treatment threshold, the nurse informed the patient's PCP, who then reviewed the treatment plan and made adjustments as needed. The nurse then sent new prescriptions to the patient's pharmacy and informed them of treatment changes by telephone. During an 18‐month evaluation of this program, investigators reported significantly improved BP control rates among enrolled patients, who also received health education from nurses.46
3.3.4. Evidence‐based lifestyle modifications
Guidelines consistently recommend that all hypertension treatment begin with “lifestyle modification,” and patients often ask for alternatives to taking medicine. Yet, when asked what lifestyle modifications lower BP, clinicians and patients often are aware of only a few strategies, such as reducing sodium intake and avoidance of “hidden” salt. Few are aware of other evidence‐based strategies, such as aerobic exercise,47, 48 and fewer recognize the relative magnitude that various lifestyle changes have on BP. For example, the Dietary Approaches to Stop Hypertension (DASH) diet, which is rich in fruits, vegetables, nuts, low‐fat dairy products, and lean protein, lowers BP nearly five times more than reducing sodium intake alone (Table 3).49
Table 3.
| Intervention | AHA Recommendation Expected SBP Change | Comment |
|---|---|---|
| Antihypertensive medications |
Should be recommended 7–20 mm Hg per medication |
First‐line therapies for most patients include thiazide diuretics, ACEIs, ARBs, and dihydropyridine CCBs |
| Reduce weight |
Should be recommended 5–20 mm Hg per 10‐kg weight reduction |
Aim to maintain normal body weight (BMI 18.5–24.9 kg/m2) |
| Aerobic exercise |
Should be recommended 4–9 mm Hg |
At least 30 min/d of aerobic activity (such as brisk walking) most days of the week |
| Resistance exercise |
Reasonable to offer 2–3 mm Hg |
Two to three sessions per week. Examples include weight lifting and circuit training |
| Low sodium diet |
Should be recommended 2–8 mm Hg |
a. Consume ≤2400 mg sodium per day b. Consuming ≤1500 mg sodium per day associated with even greater BP reduction c. Reducing intake to at least 1000 mg/d lowers BP even if desired daily intake is not achieved |
| Moderate alcohol intake |
Should be recommended 2–4 mm Hg |
Limit consumption to ≤2 drinks (24 oz of beer, 10 oz of wine, 3 oz of 80‐proof spirits) per day for men and ≤1 drink per day for women |
| DASH diet |
Should be recommended 8–10 mm Hg |
A diet rich in fruits, vegetables, and low‐fat dairy products with reduced saturated/total fat |
| DASH+very low sodium diet |
Should be recommended 11–14 mm Hg |
DASH diet combined with consuming ≤1100 mg sodium per day |
| Device‐guided breathing |
Reasonable to offer 3–5 mm Hg |
FDA‐approved device monitors breathing rate and offers real‐time feedback to promote relaxation. Device costs $150–$200 |
| Acupuncture | No benefit | Limited evidence finds that acupuncture offers no benefit for hypertension; more evidence would be needed to demonstrate positive effect |
| Yoga | No benefit | Little evidence exists to recommend for or against use of yoga for treating hypertension |
| Transcendental meditation | May be considered | Limited evidence suggests transcendental meditation may have positive effects on hypertension but more evidence is needed to fully recommend it |
| Other meditation techniques | No benefit | Little evidence exists to recommend for or against other meditation techniques |
| Biofeedback | May be considered | Limited evidence suggests that biofeedback may have positive effects on hypertension but more evidence is needed to fully recommend it |
| Other relaxation techniques | No benefit | Limited evidence finds relaxation techniques offer no benefit for hypertension; more evidence would be needed to demonstrate positive effect |
Abbreviations: AHA, American Heart Association; ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; BMI, body mass index; CCB, calcium channel blocker; DASH, Dietary Approaches to Stop Hypertension; SBP, systolic blood pressure.
In order to effectively counsel patients about lifestyle modifications, the M.A.P. checklists encourage teams to explicitly identify which specific dietary and lifestyle changes are evidence based and help patients integrate this information into their daily lives. Many tools and resources are publicly available to practice teams and their patients, including DASH diet–specific brief handouts, culturally appropriate DASH cookbooks, and in some communities, DASH cooking classes (Box).
4. Conclusions
Effective management of chronic diseases increasingly demands team‐based primary care. The M.A.P. checklists provide a memorable, easily disseminated framework for communicating and coordinating evidence‐based hypertension management strategies among all members of a care team. It also provides the central principles for implementing the IHO:BP program, a national hypertension improvement collaborative program sponsored by the AMA and Johns Hopkins Medicine. Although hypertension is only one of many chronic conditions managed in primary care settings, the principles applied in this framework—summarizing effective, evidence‐based strategies to address diagnostic uncertainty, clinical inertia, and patient engagement—may also be applied to other diseases, such as diabetes mellitus and chronic kidney disease. Using this checklist may help primary care practices to increase hypertension control rates and improve patient care overall.
Statement of Financial Disclosure
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
Acknowledgments
IHO:BP is sponsored by the AMA and is conducted as a partnership between the AMA and Johns Hopkins Medicine. At the time of this work, Drs Daniel, Hasan, and Wynia were with the AMA. The authors wish to thank Stephanie Busam, Una Charley, Shahid Choudhry, and Erin Kirley for their invaluable support during the development and pilot testing of the concepts and tools described in this paper.
Members of the AMA's HCAG: Robert Davis, MS (Care Coordination Institute); Brent Egan, MD (Care Coordination Institute); Joel Handler, MD (Kaiser Permanente of Southern California); Jacquelyn Hunt, PharmD, MS (Bellin Health); Judith Monroe, MD, FAAFP (US Centers for Disease Control and Prevention); William Oetgen, MD, MBA (American College of Cardiology); Olugbenga Ogedegbe, MD, MPH (New York University School of Medicine); Jerry Penso, MD (American Medical Group Foundation); Stephen Persell, MD, MPH (Northwestern University Feinberg School of Medicine); Jesse Singer, DO, MPH (New York City Department of Health and Mental Hygiene); and Sarah Woolsey, MD, FAAFP (HealthInsight).
Practices participating in the IHO:BP pilot phase: Advocate Medical Group–Metrodocs (Mount Prospect, IL); Advocate Medical Group–Midwest Heart Specialists (Batavia, IL); Erie Family Health Center (Chicago, IL); Northwestern Medical Group (Evanston, IL); Quality Primary Care (Chicago, IL); Drs Edwards and Stephens, LTD (Baltimore, MD); Johns Hopkins Community Physicians (Frederick, MD); Medstar Franklin Square Family Medical Center (Baltimore, MD); Mitchell S. Gittelman, DO, PA (Salisbury, MD); Power Street Medical Center (Salisbury, MD).
Boonyasai RT, Rakotz MK, Lubomski LH, et al. Measure accurately, Act rapidly, and Partner with patients: An intuitive and practical three‐part framework to guide efforts to improve hypertension control. J Clin Hypertens. 2017;19:684‐694. 10.1111/jch.12995
References
- 1. Clough JD, McClellan M. Implementing MACRA: implications for physicians and for physician leadership. JAMA. 2016;315:2397‐2398. [DOI] [PubMed] [Google Scholar]
- 2. Blumenthal D, Abrams M, Nuzum R. The Affordable Care Act at 5 years. N Engl J Med. 2015;372:2451‐2458. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Medicare and Medcaid Services . CMS and major commerical health plans, in concert with physician groups and other stakeholders, announce alignment and simplification of quality measures [Press Release]; 2016. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2016-Press-releases-items/2016-02-16.html. Accessed October 4, 2016.
- 4. Davis KE. Expenditures for hypertension among adults age 18 and older, 2010: estimates for the U.S. civilian noninstitutionalized population. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [PubMed] [Google Scholar]
- 5. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011‐2012. NCHS Data Brief. 2013;133:1‐8. [PubMed] [Google Scholar]
- 6. Wozniak G, Khan T, Gillespie C, et al. Hypertension control cascade: a framework to improve hypertension awareness, treatment, and control. J Clin Hypertens (Greenwich). 2016;18:232‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ladden MD, Bodenheimer T, Fishman NW, et al. The emerging primary care workforce: preliminary observations from the primary care team: learning from effective ambulatory practices project. Acad Med. 2013;88:1830‐1834. [DOI] [PubMed] [Google Scholar]
- 8. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775‐1779. [DOI] [PubMed] [Google Scholar]
- 9. Grumbach K, Bodenheimer T. A primary care home for Americans: putting the house in order. JAMA. 2002;288:889‐893. [DOI] [PubMed] [Google Scholar]
- 10. Lomax SW, White D. Interprofessional collaborative care skills for the frontline nurse. Nurs Clin North Am. 2015;50:59‐73. [DOI] [PubMed] [Google Scholar]
- 11. Miller GA. The magical number seven plus or minus two: some limits on our capacity for processing information. Psychol Rev. 1956;63:81‐97. [PubMed] [Google Scholar]
- 12. Hodgkinson J, Mant J, Martin U, et al. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ. 2011;342:d3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim JW, Bosworth HB, Voils CI, et al. How well do clinic‐based blood pressure measurements agree with the mercury standard? J Gen Intern Med. 2005;20:647‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Powers BJ, Olsen MK, Smith VA, Woolson RF, Bosworth HB, Oddone EZ. Measuring blood pressure for decision making and quality reporting: where and how many measures? Ann Intern Med. 2011;154:781‐788, W‐289‐790. [DOI] [PubMed] [Google Scholar]
- 15. Butt DA, Mamdani M, Austin PC, Tu K, Gomes T, Glazier RH. The risk of hip fracture after initiating antihypertensive drugs in the elderly. Arch Intern Med. 2012;172:1739‐1744. [DOI] [PubMed] [Google Scholar]
- 16. Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174:588‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903‐1913. [DOI] [PubMed] [Google Scholar]
- 18. Margolis KL, Palermo L, Vittinghoff E, et al. Intensive blood pressure control, falls, and fractures in patients with type 2 diabetes: the ACCORD trial. J Gen Intern Med. 2014;29:1599‐1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williamson JD, Supiano MA, Applegate WB, et al; SPRINT Research Group. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673‐2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697‐716. [DOI] [PubMed] [Google Scholar]
- 21. Handler J, Zhao Y, Egan BM. Impact of the number of blood pressure measurements on blood pressure classification in US adults: NHANES 1999–2008. J Clin Hypertens (Greenwich). 2012;14:751‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2002;288:2981‐2997. [DOI] [PubMed] [Google Scholar]
- 23. Okonofua EC, Simpson KN, Jesri A, Rehman SU, Durkalski VL, Egan BM. Therapeutic inertia is an impediment to achieving the Healthy People 2010 blood pressure control goals. Hypertension. 2006;47:345‐351. [DOI] [PubMed] [Google Scholar]
- 24. Kerr EA, Zikmund‐Fisher BJ, Klamerus ML, Subramanian U, Hogan MM, Hofer TP. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. 2008;148:717‐727. [DOI] [PubMed] [Google Scholar]
- 25. Frieden TR, King SM, Wright JS. Protocol‐based treatment of hypertension: a critical step on the pathway to progress. JAMA. 2014;311:21‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Godwin M, Birtwhistle R, Seguin R, et al. Effectiveness of a protocol‐based strategy for achieving better blood pressure control in general practice. Fam Pract. 2010;27:55‐61. [DOI] [PubMed] [Google Scholar]
- 27. Handler J. Commentary in support of a highly effective hypertension treatment algorithm. J Clin Hypertens (Greenwich). 2013;15:874‐877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mann SJ, Parikh NS. A simplified mechanistic algorithm for treating resistant hypertension: efficacy in a retrospective study. J Clin Hypertens (Greenwich). 2012;14:191‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turchin A, Conlin PR. The doctor needs to see you now: accelerating the care of patients with uncontrolled hypertension. Expert Rev Cardiovasc Ther. 2010;8:1501‐1503. [DOI] [PubMed] [Google Scholar]
- 30. Xu W, Goldberg SI, Shubina M, Turchin A. Optimal systolic blood pressure target, time to intensification, and time to follow‐up in treatment of hypertension: population based retrospective cohort study. BMJ. 2015;350:h158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Turchin A, Goldberg SI, Shubina M, Einbinder JS, Conlin PR. Encounter frequency and blood pressure in hypertensive patients with diabetes mellitus. Hypertension. 2010;56:68‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Handler J, Lackland DT. Translation of hypertension treatment guidelines into practice: a review of implementation. J Am Soc Hyperten. 2011;5:197‐207. [DOI] [PubMed] [Google Scholar]
- 33. Gradman AH, Basile JN, Carter BL, Bakris GL; American Society of Hypertension Writing Group. Combination therapy in hypertension. J Clin Hypertens (Greenwich). 2011;13:146‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hibbard JH, Greene J, Shi Y, Mittler J, Scanlon D. Taking the long view: how well do patient activation scores predict outcomes four years later? Med Care Res Rev. 2015;72:324‐337. [DOI] [PubMed] [Google Scholar]
- 35. Naik AD, Kallen MA, Walder A, Street RL Jr. Improving hypertension control in diabetes mellitus: the effects of collaborative and proactive health communication. Circulation. 2008;117:1361‐1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bodenheimer T. A 63‐year‐old man with multiple cardiovascular risk factors and poor adherence to treatment plans. JAMA. 2007;298:2048‐2055. [DOI] [PubMed] [Google Scholar]
- 37. Callon W, Saha S, Korthuis PT, et al. Which clinician questions elicit accurate disclosure of antiretroviral non‐adherence when talking to patients? AIDS Behav. 2016;20:1108‐1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Levensky ER, Forcehimes A, O'Donohue WT, Beitz K. Motivational interviewing: an evidence‐based approach to counseling helps patients follow treatment recommendations. Am J Nurs. 2007;107:50‐58; quiz 58–59. [DOI] [PubMed] [Google Scholar]
- 39. Rosenbaum L. Beyond belief–how people feel about taking medications for heart disease. N Engl J Med. 2015;372:183‐187. [DOI] [PubMed] [Google Scholar]
- 40. Uhlig K, Patel K, Ip S, Kitsios GD, Balk EM. Self‐measured blood pressure monitoring in the management of hypertension: a systematic review and meta‐analysis. Ann Intern Med. 2013;159:185‐194. [DOI] [PubMed] [Google Scholar]
- 41. Pickering TG, Miller NH, Ogedegbe G, et al. Call to action on use and reimbursement for home blood pressure monitoring: executive summary: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1‐9. [DOI] [PubMed] [Google Scholar]
- 42. Campbell NR, Milkovich L, Burgess E, McKay DW. Self‐measurement of blood pressure: accuracy, patient preparation for readings, technique and equipment. Blood Press Monit. 2001;6:133‐138. [DOI] [PubMed] [Google Scholar]
- 43. Thijs L, Staessen JA, Celis H, et al. Reference values for self‐recorded blood pressure: a meta‐analysis of summary data. Arch Intern Med. 1998;158:481‐488. [DOI] [PubMed] [Google Scholar]
- 44. Parati G, Stergiou GS, Asmar R, et al. European Society of Hypertension practice guidelines for home blood pressure monitoring. J Hum Hypertens. 2010;24:779‐785. [DOI] [PubMed] [Google Scholar]
- 45. American Medical Association . STEPS forward: improving blood pressure control; 2015. https://www.stepsforward.org/modules/hypertension-blood-pressure-control. Accessed August 23, 2016.
- 46. Bosworth HB, Powers BJ, Olsen MK, et al. Home blood pressure management and improved blood pressure control: results from a randomized controlled trial. Arch Intern Med. 2011;171:1173‐1180. [DOI] [PubMed] [Google Scholar]
- 47. Brook RD, Appel LJ, Rubenfire M, et al. Beyond medications and diet: alternative approaches to lowering blood pressure: a scientific statement from the American Heart Association. Hypertension. 2013;61:1360‐1383. [DOI] [PubMed] [Google Scholar]
- 48. Appel LJ, Brands MW, Daniels SR, et al. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296‐308. [DOI] [PubMed] [Google Scholar]
- 49. Gay HC, Rao SG, Vaccarino V, Ali MK. Effects of different dietary interventions on blood pressure: systematic review and meta‐analysis of randomized controlled trials. Hypertension. 2016;67:733‐739. [DOI] [PubMed] [Google Scholar]
- 50. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta‐analyses, and meta‐regression analyses of randomized trials. J Hypertens. 2014;32:2285‐2295. [DOI] [PubMed] [Google Scholar]


