1. INTRODUCTION
Cardiovascular disease (CVD) is the world's leading cause of death, killing 18 million people per year—about one‐third of all deaths.1 Hypertension is the leading cause of CVD and contributes to 10 million deaths per year—the same number of people killed by all infectious diseases combined.2 Whereas the burden of hypertension and CVD afflict predominantly older aged persons in high‐income countries, younger populations are disproportionately affected in low‐ and middle‐income countries.1 It is estimated that CVD costs 2%‐4% of gross national income in these countries.3
Hypertension is amenable to treatment with generic, once‐daily, low‐cost medications that are safe and effective, yet currently, fewer than 15% of adults with hypertension worldwide have their blood pressure controlled to 140/90 or lower.4 Some countries and health systems have achieved control rates approaching 70%;5, 6 accomplishing this on a global basis could save millions of lives.
The Resolve to Save Lives Cardiovascular Health Initiative has been established to help improve hypertension management. Resolve to Save Lives — a 5‐year, $225 million initiative based at Vital Strategies, a global health nonprofit organization, is supporting key organizations and engaging new partners to help low‐ and middle‐income countries accelerate and scale‐up implementation of proven tools and strategies to prevent CVD.7 These tools, together with the elimination of artificial trans‐fats and a reduction of sodium consumption, could improve hypertension management and save 100 million lives over the next 30 years.
2. USING TREATMENT PROTOCOLS
Practical treatment protocols can increase rates of hypertension control by reducing treatment cost; facilitating team‐based care, training, and supportive supervision; and reducing both unwarranted clinical variability and inappropriate therapeutic inertia.8 Widespread adoption of treatment protocols that specify a limited set of carefully selected, effective medications facilitates large‐volume purchases, which will reduce medication costs as well as improve supply chain reliability.9 We estimate, based on preliminary information from large procurements by various organizations, that medication costs of hypertension treatment could be as little as $2 per patient per year. Use of treatment protocols can also enhance incorporation of non‐physician health workers into clinical care, a practice that can improve consistency of care, further reduce health system costs, and make treatment more accessible to patients.10 Protocols can also ensure the use of evidence‐based, long‐acting, and inexpensive medications that are maximally compatible with each other—ideally in a single‐pill formulation, which increases patient adherence11—and when combined, may have a lower prevalence of adverse effects and require less laboratory monitoring.12
The treatment protocols recommended here are intended for use in a variety of primary care community settings, where the vast majority of people seek medical care and most individuals with hypertension, including people with comorbidities such as diabetes and chronic obstructive pulmonary disease, can be effectively managed. Before treatment initiation, it is required that each patient have an assessment to determine whether there are contraindications to drugs in the protocol and if there are specific indications for alternative drugs not included in the treatment protocol. The assessment will also identify patients who may require further evaluation and individualized treatment by specialists.
3. RATIONALE FOR USING A SIMPLIFIED TREATMENT PROTOCOL
During the 1970s, hypertension was treated in the community using a stepped care approach developed in clinical trials. This approach was demonstrated to be more effective than usual care, with improved blood pressure control and reduced mortality.13 Despite the availability of many clinical guidelines that recommended this stepped care approach, control rates have remained low over the past several decades. Recently, there has been interest in expanding the use of hypertension treatment protocols, often including the use of single‐pill combination (SPC) medications. SPC medications have been demonstrated to result in improved blood pressure control due to a reduction in clinical inertia associated with complex treatment regimens and increased prescriber adherence to the treatment protocols.14, 15, 16
This approach is analogous to that of specific treatment protocols successfully used to scale up treatment of chronic communicable diseases (eg, HIV and tuberculosis).17, 18 The WHO HEARTS technical package,19 which is endorsed by the World Hypertension League, Resolve to Save Lives, and many other partners, strongly emphasize and recommend the use of a practical and precise hypertension treatment protocol.20, 21, 22
4. RATIONALE FOR SELECTED TREATMENT PROTOCOLS
The treatment protocols recommended here are based on published clinical trial evidence, guidance from evidence‐based treatment guidelines, implementation experience in large populations, and experiences with in‐country authorities and hypertension experts when selecting country or subnational treatment protocols. According to the recent hypertension treatment guidelines issued by the American College of Cardiology and American Heart Association, 4 medication classes (angiotensin‐converting enzyme inhibitors [ACEI], angiotensin receptor blockers [ARB], calcium channel blockers [CCB], and diuretics) are effective, most patients will require more than 1 medication for control, and single‐pill (fixed dose) combination medications have important advantages.23, 24 The best scientific evidence suggests that these and other regimens outlined in the WHO HEARTS technical package, all of which use various combinations of the 4 drug classes previously mentioned (with the exception that ACEI and ARB should not be used together in the same individual), can reduce blood pressure and prevent cardiovascular disease in a high proportion of the treated patients.
Because the goal of treatment protocols is to substantially increase the number of patients on treatment, there is a strong preference for regimens that minimize the need for laboratory monitoring and minimize the rates of adverse effects, particularly events that could be serious or life‐threatening. Because patients who experience symptomatic adverse effects may discontinue treatment, it is important to minimize adverse events, even if they do not pose a serious health risk.
Therefore, Resolve to Save Lives and partners recommend 2 protocols for treatment of hypertension. One protocol uses a fixed‐dose single combination pill, and the other uses single medications in combination as needed. Both recommend the same 3 core medications—amlodipine 5 mg, telmisartan 40 mg, and chlorthalidone 12.5 mg—in slightly different sequences. Both are modifiable by substituting another renin‐angiotensin system (RAS) inhibitor for telmisartan, other diuretics for chlorthalidone, and modifying the sequence of treatment. SPC therapy has the advantage of greater overall effectiveness, reduced rates of adverse effects, greater patient adherence, improved rates of blood pressure control, and lower risks of hypertension‐related cardiovascular complications.25 However, these protocols are essentially equivalent and would be expected to have similar impact reducing blood pressure. A country or health system may prefer 1 protocol to the other because of local prescribing practices, availability of drugs, and other factors. This article provides an overview of the rationale for these recommended protocols.
5. RECOMMENDED MEDICATIONS FOR PROTOCOLS
The CCB amlodipine is recommended as a 5 mg tablet because it can be taken once daily, is available in a low‐cost generic form, has been studied in clinical outcome trials, and can be split in half and taken as half tablet (2.5 mg), 1 tablet (5 mg), or 2 tablet (10 mg) dosages. Because it does not cause hypo‐ or hyperkalemia, there is a reduced need for laboratory testing. Other once daily dihydropyridine CCB drugs (eg, long‐acting nifedipine) can be substituted. CCB is the most frequently used antihypertensive medication in many low‐ and middle‐income countries because of its low rate of serious adverse events and lack of impact on potassium levels, and local health care providers are familiar with this class of medications. One disadvantage of amlodipine is that at 10 mg/d, 25% of patients may experience pedal edema, which many patients find bothersome, and although not a serious adverse effect it increases the rate of treatment discontinuation. This can usually be mitigated by a dose reduction or by adding a RAS inhibitor (ACEI or ARB).26
The ARB telmisartan is recommended because of its low rate of adverse effects27 and long duration of action that enables once‐daily dosing, its availability in a low‐cost generic form, favorable experience in clinical outcome trials,28, 29 and convenient dosing as a half tablet (20 mg), 1 tablet (40 mg), or 2 tablets (80 mg). When used in combination with a CCB or a diuretic, it is appropriate for all races, but it should not be used for women who are or may become pregnant. As noted above, a RAS inhibitor (ACEI or ARB) can reduce pedal edema from a CCB when used simultaneously.30 Although ACEIs are better documented to reduce cardiovascular events than are ARBs, ARBs are recommended due to the much lower incidence of cough and angioedema, particularly in darker‐skinned patients. Any once‐daily ARB (eg, losartan, irbesartan, candesartan, olmesartan, or valsartan, all of which are available in generic formulations) or once‐daily ACEI (eg, lisinopril) can be substituted for telmisartan. Although rare, therapy with RAS inhibitors (ACEI or ARB) can cause hyperkalemia, especially among patients with reduced kidney function, and therefore periodic laboratory testing is advised.
The diuretic chlorthalidone is recommended because it has a long duration of action, can be taken once daily, is available in a low‐cost generic form, and has been documented to reduce cardiovascular events and mortality. Based on a reluctance to prescribe diuretics in many countries as well as the risk of hypokalemia in settings with limited access to routine monitoring of serum potassium and inconsistent availability of potassium supplementation, chlorthalidone is not recommended as the initial treatment step. Both protocols recommend referral to a specialist if blood pressure is not controlled by a combination of amlodipine 10 mg, telmisartan 80 mg, and chlorthalidone 12.5 mg.
Some countries may choose to recommend an increase in the dosage of chlorthalidone to 25 mg before referring to a specialist, particularly if monitoring for serum potassium levels and management of hypokalemia can be done in primary care. A suitable alternative to chlorthalidone is indapamide 1.25 mg, but if it is unavailable or costly, hydrochlorothiazide 25 mg can be considered as it is widely available and familiar to most practitioners. Some jurisdictions may choose to substitute one of these diuretics for amlodipine in the initial regimen if potassium levels can be monitored and managed and there is no clinician resistance to the use of diuretics.
6. SINGLE‐PILL COMBINATION HYPERTENSION TREATMENT PROTOCOL WITH AMLODIPINE 5 mg AND TELMISARTAN 40 mg
The recommended single‐pill combination (SPC) hypertension treatment protocol starts with a half pill of amlodipine 5 mg and telmisartan 40 mg in a combination scored tablet, which permits up‐titration to control using single pill and 2 pill doses (Figure 1). If the 2 pills do not control blood pressure, the protocol recommends adding chlorthalidone 12.5 mg. The combination pill has advantages of simplicity of procurement, lower prices through large‐quantity purchases, simplified logistics and titration, the potential for increased adherence, and a lower risk of some adverse effects (such as pedal edema) when a CCB is used in combination with a RAS inhibitor. Moreover, a recent meta‐analysis suggested superiority of a RAS inhibitor plus CCB to other combination therapies (eg, lower risk of cardiovascular events as well as serious adverse effects).31 The advisability of laboratory monitoring is a disadvantage, and some patients whose blood pressure would have been controlled on amlodipine alone would be treated with a second medication unnecessarily.
Figure 1.
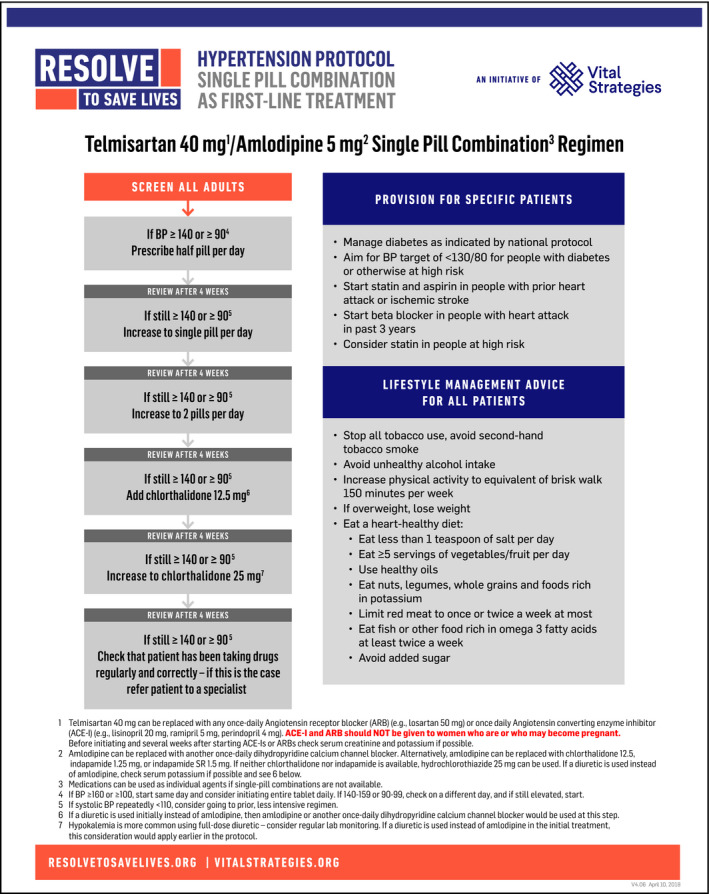
Recommended single‐pill combination hypertension treatment regimen
The use of SPC therapy containing 3 drugs is a potential alternative. Due to limited experience with 3 anti‐hypertensive drug SPC medications, a 2‐drug SPC therapy has been recommended at this time. However, as additional experience accrues, 3‐drug SPC therapy can be reconsidered. A low dosage of chlorthalidone or another diuretic in addition to telmisartan and amlodipine might reduce the incidence of hyperkalemia relative to the current protocol, but to what extent it increases the rate of idiosyncratic reactions and hypokalemia is unknown.
7. CALCIUM CHANNEL BLOCKER‐FIRST TREATMENT PROTOCOL
The second recommended protocol starts with the calcium channel blocker amlodipine (Figure 2). This protocol has advantages of decreased medication cost and reduced need for laboratory monitoring in the initial steps. This protocol has disadvantages due to the increased number of titrations, which may increase the time until blood pressure is controlled, decreased adherence due to more pills, less logistical simplicity, and increased pedal edema when a calcium channel blocker (CCB) is used without an ACEI or an ARB. As with the other protocol, any once‐daily RAS inhibitor and once‐daily dihydropyridine CCB can be substituted for amlodipine and telmisartan; also, indapamide or hydrochlorothiazide can be substituted for chlorthalidone.
Figure 2.

Recommended single‐agent hypertension treatment regimen
8. ADHERENCE
Lack of adherence to antihypertensive drug therapy is a major reason for lack of control of hypertension. Some patients will not collect medications and others will either not take the drugs or will not take them as prescribed. Non‐adherence is more common when starting therapy, and is especially common in people who are taking 3 or more drugs. It is important to ask patients about any difficulties adhering to prescribed medication at each visit and assess their adherence in greater depth if blood pressure remains elevated; a high level of adherence to treatment substantially decreases the risk of cardiovascular events.32, 33
9. LABORATORY MONITORING
Many guidelines suggest regular laboratory monitoring for metabolic effects of anti‐hypertensive treatment. However, in many resource‐constrained settings, particularly rural locations, the options are either treatment without reliable laboratory monitoring or no treatment for hypertension. In actual experience, even in high‐income countries, the proportion of patients who undergo laboratory monitoring may be far lower than recommended, and among patients who did not undergo testing, no increase in serious adverse events was identified.34, 35
The risk of hypokalemia and other complications of diuretic treatment has led to the recommendation of laboratory monitoring, which makes diuretics less attractive, especially in resource‐constrained settings, and has led to their placement as the third medication to be added in both recommended protocols. The risk of hyperkalemia with an ACEI or ARB is lower than that of hypokalemia with a diuretic, but is higher among people with chronic kidney disease;36, 37 laboratory monitoring is recommended for patients receiving either of these classes of medication. The use of a thiazide diuretic with a RAS inhibitor (ACEI or ARB) is associated with lower incidence of hypokalemia than a thiazide diuretic when used alone and lower incidence of hyperkalemia than when a RAS inhibitor is used alone,12 but this reduction does not eliminate the need for laboratory monitoring.
The goal of a scalable protocol is to maximize the control of blood pressure while minimizing both adverse events and the need for monitoring. Countries, regions, and health care systems might modify these protocols or select other protocols based on factors such as access to laboratory testing, cost and availability of specific medications, and prevailing practice patterns established in the community. As large numbers of patients are treated with different protocols in different regions of the world, more information will be obtained about the effectiveness, adherence, and adverse events with different regimens in different contexts.
The monitoring system being recommended through the WHO HEARTS initiative tracks blood pressure control 6‐9 months after the start of treatment and can promptly identify problems with control or treatment discontinuation. Also, hypertension management program implementers at sentinel treatment facilities plan to conduct an in‐depth analysis of medication safety and efficacy under program conditions. It is important to highlight, however, that all medications recommended by these protocols have been in widespread clinical use in high‐income countries for decades, they are all safe and effective, and all classes are included in the WHO Essential Medication List.38
The value of SPC therapy using currently listed essential medicines, including dual agents for hypertension, was recently highlighted by WHO in 2017.38 Low‐ and middle‐income countries have already included several of these treatments on their lists of national essential medicines to inform priorities for procurement, reimbursement, and central distribution.39 The scale‐up of WHO essential medicines for hypertension protocols follows the success of adapting national level scale‐up priorities for other medication types.40
10. CONCLUSIONS
Of all adult primary care interventions, improvement in the management of hypertension treatment can save the most lives41 and can also reduce spending on the management of patients with heart attacks, strokes, kidney failure, and other preventable complications of uncontrolled hypertension. It is time to implement simple, practical, and specific hypertension treatment protocols to improve blood pressure control and reserve individualized approaches to the small proportion of patients who have complicated medical conditions or who experience adverse events and require a more tailored approach. As treatment programs expand in various clinical and epidemiological contexts, Resolve to Save Lives and its partners will evaluate hypertension control, treatment adherence, adverse events, medication cost, supply chain consistency, and implementation effectiveness and suggest improvements in recommended protocols accordingly.
Improving the global hypertension control rate from < 15% as it is at present to 50% would save more than 1 million lives per year. However, this would require effective treatment of an additional 500 million patients worldwide. The World Health Organization has proposed an increase in treatment of more than 200 million patients between 2018 and 2023, which would be important progress.42 Massive as this challenge is, it can be done—but only if practical and specific treatment protocols are adopted, used, and optimized based on actual experience. Improvement of hypertension treatment in primary care will not only save millions of lives but also provide a foundation for effective care of a range of health conditions that require long‐term care.43 We hope that jurisdictions will consider adopting 1 of the 2 protocols recommended here and accelerate progress achieving this goal.
ACKNOWLEDGMENTS
The authors thank Dr Sandeep Kishore of the Arnhold Institute for Global Health at Icahn School of Medicine at Mount Sinai and Young Professionals Chronic Disease Network and Dr Roopa Shivashankar of Resolve to Save Lives for their careful review, insightful comments, and suggested edits and additions to the manuscript. Resolve to Save Lives, including support for development of this paper, is funded in part by grants from Bloomberg Philanthropies; the Bill and Melinda Gates Foundation; and the Chan Zuckerberg Initiative DAF, an advised fund of Silicon Valley Community Foundation.
Jaffe MG, Frieden TR, Campbell NRC, et al. Recommended treatment protocols to improve management of hypertension globally: A statement by Resolve to Save Lives and the World Hypertension League (WHL). J Clin Hypertens. 2018;20:829–836. 10.1111/jch.13280
REFERENCES
- 1. Roth GA, Johnson C, Abajobir A, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;71:1‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2013 Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2287‐2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gaziano TA. Reducing the growing burden of cardiovascular disease in the developing world. Health Aff (Millwood). 2007;26:13‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population‐based studies from 90 countries. Circulation. 2016;134:441‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Padwal RS, Bienek A, McAlister FA, Campbell NR. Outcomes research task force of the Canadian Hypertension Education Program. Epidemiology of hypertension in Canada: an update. Can J Cardiol. 2016;32:687‐694. [DOI] [PubMed] [Google Scholar]
- 6. Luepker RV, Steffen LM, Jacobs DR Jr, Zhou X, Blackburn H. Trends in blood pressure and hypertension detection, treatment, and control 1980 to 2009: the Minnesota heart survey. Circulation. 2012;126:1852‐1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frieden TR, Bloomberg MR. Saving an additional 100 million lives. Lancet. 2018;391:709‐712. [DOI] [PubMed] [Google Scholar]
- 8. Frieden TR, King SM, Wright JS. Protocol‐based treatment of hypertension: a critical step on the pathway to progress. JAMA. 2014;311:21‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tran DN, Njuguna B, Mercer T, et al. Ensuring patient‐centered access to cardiovascular disease medicines in low‐income and middle‐income countries through health‐system strengthening. Cardiol Clin. 2017;35:125‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ogedegbe G, Gyamfi J, Plange‐Rhule J, et al. Task shifting interventions for cardiovascular risk reduction in low‐income and middle‐income countries: a systematic review of randomised controlled trials. BMJ Open. 2014;4:e005983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamdidouche I, Jullien V, Boutouyrie P, Billaud E, Azizi M, Laurent S. Drug adherence in hypertension: from methodological issues to cardiovascular outcomes. J Hypertens. 2017;35:1133‐1144. [DOI] [PubMed] [Google Scholar]
- 12. Reboldi G, Gentile G, Angeli F, Verdecchia P. Choice of ACE inhibitor combinations in hypertensive patients with type 2 diabetes: update after recent clinical trials. Vasc Health Risk Manag. 2009;5:411‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Five‐year findings of the hypertension detection and follow‐up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. Hypertension Detection and Follow‐up Program Cooperative Group. JAMA. 1979;242:2562‐2571. [PubMed] [Google Scholar]
- 14. Feldman RD, Zou GY, Vandervoort MK, Wong CJ, Nelson SA, Feagan BG. A simplified approach to the treatment of uncomplicated hypertension: a cluster randomized, controlled trial. Hypertension. 2009;53:646‐653. [DOI] [PubMed] [Google Scholar]
- 15. Stewart S, Stocks NP, Burrell LM, et al. More rigorous protocol adherence to intensive structured management improves blood pressure control in primary care: results from the Valsartan Intensified Primary carE Reduction of Blood Pressure study. J Hypertens. 2014;32:1342‐1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. Improved blood pressure control associated with a large‐scale hypertension program. JAMA. 2013;310:699‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ford N, Ball A, Baggaley R, et al. The WHO public health approach to HIV treatment and care: looking back and looking ahead. Lancet Infect Dis. 2018;18:e76‐e86. [DOI] [PubMed] [Google Scholar]
- 18. Frieden TR, Munsiff SS. The DOTS strategy for controlling the global tuberculosis epidemic. Clin Chest Med. 2005;26:197‐205. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization . HEARTS: Technical Package for Cardiovascular Disease Management in Primary Health Care. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 20. Angell SY, De Cock KM, Frieden TR. A public health approach to global management of hypertension. Lancet. 2015;385:825‐827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Campbell NR, Berbari AE, Cloutier L, et al. Policy statement of the world hypertension league on noninvasive blood pressure measurement devices and blood pressure measurement in the clinical or community setting. J Clin Hypertens (Greenwich). 2014;16:320‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Frieden TR, Jaffe MG. Saving 100 million lives by improving global treatment of hypertension and reducing cardiovascular disease risk factors. J Clin Hypertens (Greenwich). 2018;20:208‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017. 10.1161/hyp.0000000000000065. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24. Reboussin DM, Allen NB, Griswold ME, et al. Systematic review for the 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017; pii: HYP.0000000000000067. 10.1161/hyp.0000000000000067. [Epub ahead of print] [DOI] [Google Scholar]
- 25. Feldman RD. Single pill combinations as initial therapy in the management of hypertension: What is taking you so long? Am J Hypertens. 2018;31:263‐264. [DOI] [PubMed] [Google Scholar]
- 26. Makani H, Bangalore S, Romero J, et al. Peripheral edema associated with calcium channel blockers: incidence and withdrawal rate – a meta‐analysis of randomized trials. J Hypertens. 2011;29:1270‐1280. [DOI] [PubMed] [Google Scholar]
- 27. Mancia G, Schumacher H. Incidence of adverse events with telmisartan compared with ACE inhibitors: evidence from a pooled analysis of clinical trials. Patient Prefer Adherence. 2012;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. ONTARGET Investigators , Yusuf S, Teo KK, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547‐1559. [DOI] [PubMed] [Google Scholar]
- 29. The Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators . Effects of the angiotensin‐receptor blocker telmisartan on cardiovascular events in high‐risk patients intolerant to angiotensin‐converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372:1174‐1183. [DOI] [PubMed] [Google Scholar]
- 30. Sica D. Calcium channel blocker‐related peripheral edema: can it be resolved? J Clin Hypertens (Greenwich). 2003;5:291‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chi C, Tai C, Bai B, et al. Angiotensin system blockade combined with calcium channel blockers is superior to other combinations in cardiovascular protection with similar blood pressure reduction: a meta‐analysis in 20,451 hypertensive patients. J Clin Hypertens (Greenwich). 2016;18:801‐808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mazzaglia G, Ambrosioni E, Alacqua M, et al. Adherence to antihypertensive medications and cardiovascular morbidity among newly diagnosed hypertensive patients. Circulation. 2009;120:1598‐1605. [DOI] [PubMed] [Google Scholar]
- 33. Rao CR, Kamath VG, Shetty A, Kamath A. Treatment compliance among patients with hypertension and type 2 diabetes mellitus in a coastal population of southern India. Int J Prev Med. 2014;5:992‐998. [PMC free article] [PubMed] [Google Scholar]
- 34. Bandak G, Sang Y, Gasparini A, et al. Hyperkalemia after initiating renin‐angiotensin system blockade: the Stockholm Creatinine Measurements (SCREAM) project. J Am Heart Assoc. 2017;6:pii: e005428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chang AR, Sang Y, Leddy J, et al. Antihypertensive medications and the prevalence of hyperkalemia in a large health system. Hypertension. 2016;67:1181‐1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Franse LV, Pahor M, Di Bari M, Somes GW, Cushman WC, Applegate WB. Hypokalemia associated with diuretic use and cardiovascular events in the Systolic Hypertension in the Elderly Program. Hypertension. 2000;35:1025‐1030. [DOI] [PubMed] [Google Scholar]
- 37. ALLHAT Officers and Coordinators for the ALLHAT Collaborative . Research Group. The antihypertensive and lipid‐lowering treatment to prevent heart attack trial. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2002;288:2981‐2997. [DOI] [PubMed] [Google Scholar]
- 38. World Health Organization . WHO Model List of Essential Medicines, 20th List. March 2017 (amended August 2017). Geneva, Switzerland: World Health Organization; 2017. [Google Scholar]
- 39. Wirtz VJ, Kaplan WA, Kwan GF, Laing RO. Access to medications for cardiovascular diseases in low‐ and middle‐income countries. Circulation. 2016;133:2076‐2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kishore SP, Blank E, Heller DJ, et al. Modernizing the World Health Organization list of essential medicines for preventing and controlling cardiovascular diseases. J Am Coll Cardiol. 2018;71:564‐574. [DOI] [PubMed] [Google Scholar]
- 41. Farley TA, Dalal MA, Mostashari F, Frieden TR. Deaths preventable in the US by improvements in use of clinical preventive services. Am J Prev Med. 2010;38:600‐609. [DOI] [PubMed] [Google Scholar]
- 42. World Health Organization, Executive Board . Draft Thirteenth General Programme of Work, 2019‐2023. Executive Board, 142nd session. EB142/3, 5 January 2018. Geneva, Switzerland: World Health Organization; 2018. http://apps.who.int/gb/ebwha/pdf_files/EB142/B142_3-en.pdf. Accessed April 5, 2018. [Google Scholar]
- 43. Ordunez P, Martinez R, Niebylski ML, Campbell NR. Hypertension prevention and control in Latin America and the Caribbean. J Clin Hypertens (Greenwich). 2015;17:499‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]


