Abstract
This study was performed to investigate the association between four BP measurements (systolic blood pressure [SBP], diastolic blood pressure [DBP], mean arterial pressure [MAP], and pulse pressure [PP]) and four TOD parameters (left ventricular mass index [LVMI], E/e′, estimated glomerular filtration rate [eGFR], and obstructive coronary artery disease [CAD]). Data were obtained from a nation‐wide registry, composed of 652 patients (471 women, 58.4 ± 10.5 years) with suspected CAD who underwent elective invasive coronary angiography (CAG). A total of 217 patients (33.2%) had obstructive CAD (≥50%). In multivariable analyses, E/e′ was associated with SBP, MAP and PP, and CAD was associated with SBP and PP (P < 0.05 for each). All four BP measurements were not associated with LVMI and eGFR (P > 0.05 for each). In patients undergoing elective invasive CAG, SBP, and PP had stronger relationships with E/e′ and CAD than DBP and MAP.
Keywords: blood pressure measurements, coronary angiography, target organ damage
1. INTRODUCTION
High blood pressure (BP) is the leading cause of cardiovascular mortality and morbidity.1, 2 Sustained high blood pressure causes structural and functional changes in major organs, such as the heart, kidneys, brain, and eyes, which is generally called “target organ damage (TOD).”3 As subclinical TOD is an important predictor as an intermediate endpoint for cardiovascular events,4, 5, 6, 7, 8, 9 early detection and proper management of TOD, as well as BP control, are key determinants of cardiovascular prognosis.10 Fortunately, high BP‐associated subtle damage to several organs can be detected by modern medical technologies before an overt clinical event occurs.10
There are four main BP measurements: systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and pulse pressure (PP). As the association between elevated SBP and worse cardiovascular outcome has been well‐documented, SBP is commonly used as the major criteria for diagnosis, staging, and treatment of hypertension.9, 11, 12 DBP is related to coronary perfusion, and its predictive value on coronary heart disease has been reported especially in younger subjects.13, 14 MAP is an average blood pressure in an individual, and it is considered a classic indicator of perfusion to vital organs.15, 16 PP is the difference between SBP and DBP, and it increases with age. It is well‐known that PP reflects arterial stiffness, and PP is considered a strong independent predictor of cardiovascular complications and mortality.17, 18
Information on the association between each BP measurement and TOD is clinically important. If we know which BP measurement is more correlated with certain types of TOD, we can use it to treat hypertension in practice. However, data from systemic evaluation of the influence of each BP measurement on various TOD has been limited. Therefore, this study was performed to investigate the association between BP components and several types of TOD. Our hypothesis is that each BP measurement has a different influence on TOD. More specifically, SBP and PP rather than DBP and MAP are more strongly associated with TOD.
2. METHODS
2.1. Patient population
Our study data were obtained from a prospective nation‐wide registry, the KoRean wOmen'S chest pain rEgistry (KoROSE). The registry was constructed for multicenter study aims to investigate characteristics and outcomes of Korean women with or without coronary artery disease (CAD).19 Women with stable chest pain undergoing invasive coronary angiography (CAG) for the evaluation of CAD were enrolled in the registry. Men were also enrolled in the ongoing registry for gender comparison with the same enroll criteria of women.19, 20 Due to the small number of enrolled men, gender comparison was not performed in the present study. Patients with the end‐stage renal disease, chronic obstructive pulmonary disease, primary pulmonary hypertension, malignancy, and autoimmune disease were excluded. Between February 2011 and Jun 2015, a total of 933 patients were registered into the registry database from well‐qualified 29 cardiac centers in Korea. Among the patients, 652 (men = 181, women = 471) with available data on brachial BP and results of TOD measurement, including renal function, transthoracic echocardiography (TTE), and invasive CAG, were included in this study. All the patients provided their written informed consent to this study. This study protocol was approved by the Institutional Review Board (IRB) of each center. The IRB number of Seoul National University Boramae Medical Center (Seoul, Korea) was 06‐2011‐222.
2.2. Clinical data collection
Body mass index (BMI) was calculated by weight (kg)/height (m2). Hypertension was defined based on the following criteria: (a) a history of hypertension diagnosis, or (b) the use of anti‐hypertensive medications, or (c) SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg in repeated measurements. Diabetes mellitus was defined based on following criteria: (a) a history of diagnosis of diabetes mellitus, or (b) the use of anti‐glycemic medications, or (c) serum fasting glucose level ≥126 mg/dL at least two times. Dyslipidemia was defined based on following criteria: (a) a history of diagnosis of dyslipidemia, or (b) the use of anti‐dyslipidemic medications, or (c) serum low‐density lipoprotein (LDL)‐cholesterol level ≥130 mg/dL. Current smoker was defined as a person who smoked regularly for the past 12 months. Blood samples were collected after overnight fasting to obtain baseline data for laboratory tests, including hemoglobin, total cholesterol, LDL‐cholesterol, and creatinine for estimated glomerular filtration rate (eGFR). Then, eGFR was calculated by the Modification of Diet in Renal Disease equation: 186 × SCr−1.154 × age−0.203 (×0.742, if women).21 Data on current medications, including renin‐angiotensin system (RAS) blockers, beta‐blocker, calcium channel blocker, and diuretics at registry enrollment, were also recorded in detail.
2.3. BP measurement
BP was measured on the right upper arms, after at least 5 minutes of rest in the sitting position on the day of invasive CAG. SBP and DBP were read by using phase I and V (disappearance) Korotkoff sounds, respectively. MAP was calculated as 1/3 (SBP) + 2/3 (DBP). PP was calculated as the difference between SBP and DBP.
2.4. TTE
Data of left ventricular ejection fraction (LVEF), left ventricular mass index (LVMI), and the ratio of mitral peak velocity of early filling (E) to early diastolic mitral annular velocity (e′) at septal annulus (E/e′) were obtained using TTE based on the recommendations of current guidelines.22, 23
2.5. Invasive CAG
Obstructive CAD was defined as a ≥50% stenosis of one or more major epicardial coronary arteries in CAG by visual estimation. The extent of CAD was defined as 1‐, 2‐ or 3‐vessel disease. After diagnostic CAG, percutaneous coronary intervention (PCI), or optimal medical treatment was done appropriately for each patient in accordance with diagnosis.
2.6. TOD
TOD parameters investigated in our study were selected based on data availability and current guidelines.10 Several TOD parameters of the heart and the kidney were focused in this study: LVMI for LV hypertrophy, E/e′ for LV diastolic dysfunction, eGFR for renal dysfunction and the presence of obstructive CAD in CAG.
2.7. Statistical analysis
Statistical analyses were performed using the SPSS software (version 20.0; IBM, Armonk, NY). Data are presented as the mean ± standard deviation for continuous variables and percentage for categorical variables. The linear relationship between two continuous variables was analyzed by Pearson's correlation. Multiple linear regression analyses were performed to evaluate independent relationships of each BP measurement with LVMI, E/e′, and eGFR. Mean values of BP measurements between groups with or without obstructive CAD were compared with Student t test. The mean values of BP measurements according to CAD extent were compared using analysis of variance (ANOVA). Multiple binary logistic regression analysis was performed to determine independent relationships between each BP measurements and CAD. Age, gender, BMI, heart rate, diabetes mellitus, hypercholesterolemia, smoking, eGFR, and concomitant cardiovascular medications were adjusted in each multivariable model. Each BP measurement was input to multiple binary logistic regression analysis using tertile stratification. A P value of less than 0.05 was defined as statistically significant.
3. RESULTS
3.1. Clinical characteristics of study patients
The clinical characteristics of 652 patients are shown in Table 1. Their mean age was 58.4 ± 10.5 years, and 471 patients (72.2%) were female. Proportions of patients with hypertension, diabetes mellitus, dyslipidemia, and current smoking were 46.5%, 15.5%, 19.8%, and 11.8%, respectively. Mean values of SBP, DBP, MAP, and PP were 127 ± 17 mm Hg, 79.1 ± 11.0 mm Hg, 95.0 ± 11.8 mm Hg, and 47.9 ± 14.0 mm Hg, respectively. The distributions of values of each BP measurement are demonstrated in Figure S1. There was no significant abnormality in the blood test results, including hemoglobin, total cholesterol, LDL‐cholesterol, and eGFR. The major parameters of TTE also showed no significant abnormality. A total of 217 patients (32.2%) had obstructive CAD in CAG. The proportions of patients with 1‐, 2‐, and 3‐vessel disease were 19.6%, 7.5%, and 6.1%, respectively. The proportions of patients with current medications, including RAS blockers, beta‐blockers, calcium channel blockers, and diuretics were 25.0%, 16.7%, 32.1%, and 6.1%, respectively.
Table 1.
Clinical characteristics of study patients
| Characteristic | Value (n = 652) |
|---|---|
| Age, y | 58.4 ± 10.5 |
| Women, n (%) | 471 (72.2) |
| Body mass index, kg/m2 | 25.2 ± 3.3 |
| Traditional risk factors, n (%) | |
| Hypertension | 303 (46.5) |
| Diabetes mellitus | 101 (15.5) |
| Dyslipidemia D | 129 (19.8) |
| Current smoker | 77 (11.8) |
| Blood pressure measurements, mm Hg | |
| Systolic blood pressure | 127 ± 17 |
| Diastolic blood pressure | 79.1 ± 11.0 |
| Mean arterial pressure | 95.0 ± 11.8 |
| Pulse pressure | 47.9 ± 14.0 |
| Major laboratory findings | |
| Hemoglobin, g/dL | 13.3 ± 1.5 |
| Total cholesterol, mg/dL | 179 ± 41 |
| LDL‐cholesterol, mg/dL | 107 ± 35 |
| Estimated GFR, mL/min/1.73 m2 | 82.8 ± 22.1 |
| Echocardiographic findings | |
| Left ventricular ejection fraction, % | 61.7 ± 6.4 |
| Left ventricular mass index, g/m2 | 92.3 ± 25.0 |
| Septal E/e′ ratio | 10.2 ± 3.4 |
| Coronary angiographic findings, n (%) | |
| Insignificant | 435 (66.7) |
| One‐vessel disease | 128 (19.6) |
| Two‐vessels disease | 49 (7.5) |
| Three‐vessels disease | 40 (6.1) |
| Current medications history, n (%) | |
| RAS blocker | 163 (25.0) |
| Beta‐blocker | 109 (16.7) |
| Calcium channel blocker | 209 (32.1) |
| Diuretics | 40 (6.1) |
GFR, glomerular filtration rate; LDL, low‐density lipoprotein; RAS, renin‐angiotensin system.
3.2. Associations of BP measurements with LVMI, E/e′, and eGFR
The results of simple correlation analyses showing the associations between each BP component and TOD are summarized in Table 2. LVMI was positively correlated with DBP (r = 0.102, P = 0.026) and MAP (r = 0.098, P = 0.031), but not with SBP and PP (P > 0.05). Septal E/e′ was positively correlated with SBP (r = 0.227, P < 0.001), MAP (r = 0.135, P = 0.001), and PP (r = 0.257, P < 0.001), but not with DBP (P > 0.05). eGFR was negatively correlated with SBP (r = −0.088, P = 0.040), but not with DBP, MAP, and PP (P > 0.05). The results of multiple linear regression analyses showing independent associations between each BP measurement and TOD are summarized in Table 3. Significant correlations of E/e′ with SBP (β = 0.183, P < 0.001), MAP (β = 0.110, P = 0.023), and PP (β = 0.202, P < 0.001) remained even after controlling for potential confounders including age, gender, BMI, heart rate, diabetes mellitus, dyslipidemia, smoking, and medications. LVMI and eGFR were not associated with any of the BP measurements in these multivariable analyses (P > 0.05 for each).
Table 2.
Simple correlation analyses showing the associations between each blood pressure measurement and TOD (n = 652)
| TOD parameter | SBP | DBP | MAP | PP | |
|---|---|---|---|---|---|
| LVMI | r | 0.072 | 0.102 | 0.098 | 0.010 |
| P | 0.118 | 0.026 | 0.031 | 0.827 | |
| E/e′ | r | 0.227 | 0.035 | 0.135 | 0.257 |
| P | <0.001 | 0.401 | 0.001 | <0.001 | |
| eGFR | r | −0.088 | −0.041 | −0.069 | −0.076 |
| P | 0.040 | 0.333 | 0.103 | 0.076 | |
DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; LVMI, left ventricular mass index; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure; TOD, target organ damage.
Table 3.
Multiple linear regression analyses showing the associations between each blood pressure measurement and TOD (n = 652)
| TOD parameter | SBP | DBP | MAP | PP | |
|---|---|---|---|---|---|
| LVMI | β | 0.057 | 0.114 | 0.100 | −0.026 |
| P | 0.343 | 0.052 | 0.092 | 0.665 | |
| E/e′ | β | 0.183 | 0.037 | 0.110 | 0.202 |
| P | <0.001 | 0.440 | 0.023 | <0.001 | |
| eGFR | β | 0.010 | 0.029 | 0.023 | 0.023 |
| P | 0.845 | 0.568 | 0.651 | 0.651 | |
DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; LVMI, left ventricular mass index; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure; TOD, target organ damage.
Each blood pressure measure as independent variable was entered into separate model. Age, gender, body mass index, heart rate, diabetes mellitus, dyslipidemia, smoking, and medications were adjusted in each model.
3.3. Association between BP measurements and CAD
SBP (133 ± 18 vs 123 ± 16 mm Hg, P < 0.001), MAP (94.5 ± 12.2 vs 93.8 ± 11.2 mm Hg, P < 0.001), and PP (54.5 ± 15.5 vs 44.6 ± 11.9 mm Hg, P < 0.001) were significantly higher in patients with obstructive CAD than those without (Figure 1). SBP, MAP, and PP showed a gradual increase according to the increase of CAD extent (P < 0.05 for each) (Figure 2). Multiple binary logistic regression analyses showed that SBP (third vs first tertile; odds ratio [OR], 2.43; 95% confidence interval [CI], 1.28‐4.61; P = 0.020) and PP (third vs first tertile; OR, 2.52; 95% CI, 1.29‐4.93; P = 0.018) were independent risk factors for obstructive CAD even after adjusting for potential confounding factors, including age, BMI, diabetes mellitus, dyslipidemia and smoking, renal function and current medications (Table 4). MAP and DBP were not associated with CAD in the same multivariable analyses (P > 0.05 for each). Scatter plots showing the linear associations between E/e′ and each BP measurement are demonstrated in Figure 3.
Figure 1.
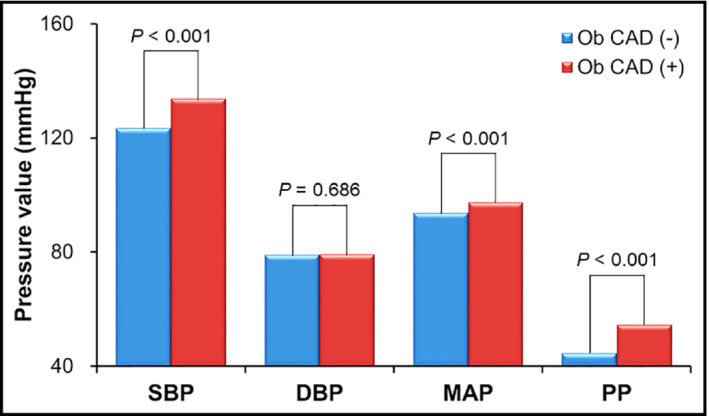
Blood pressure values according to the presence of obstructive coronary artery disease (n = 652). DBP, diastolic blood pressure; MAP, mean arterial pressure; Ob CAD, obstructive coronary artery disease; PP, pulse pressure; SBP, systolic blood pressure [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 2.
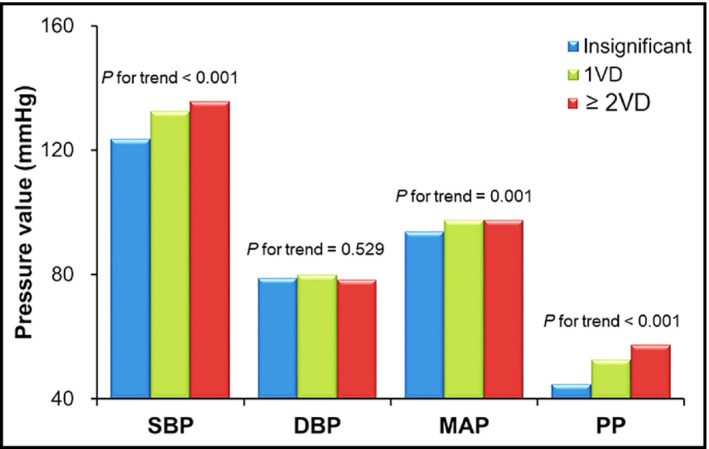
Blood pressure values according to the severity of coronary artery disease (n = 652). DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure; VD, vessel disease [Colour figure can be viewed at wileyonlinelibrary.com]
Table 4.
Multiple binary logistic regression analyses showing the associations between each blood pressure measurement and coronary artery disease (n = 652)
| Blood pressure measurement | OR (95% CI) | P value |
|---|---|---|
| SBP (3rd vs 1st tertile) | 2.43 (1.28‐4.61) | 0.020 |
| DBP (3rd vs 1st tertile) | 0.91 (0.48‐1.73) | 0.779 |
| MAP (3rd vs 1st tertile) | 1.36 (0.71‐2.61) | 0.357 |
| PP (3rd vs 1st tertile) | 2.52 (1.29‐4.93) | 0.018 |
CI, confidence interval; DBP, diastolic blood pressure; MAP, mean arterial pressure; OR, odds ratio; PP, pulse pressure; SBP, systolic blood pressure.
Dependent variable is obstructive coronary artery disease (≥50% stenosis). Each independent variable was entered into separate model. Age, sex, body mass index, heart rate, diabetes mellitus, dyslipidemia, smoking, renal function, and concomitant medications were adjusted in each model.
Figure 3.
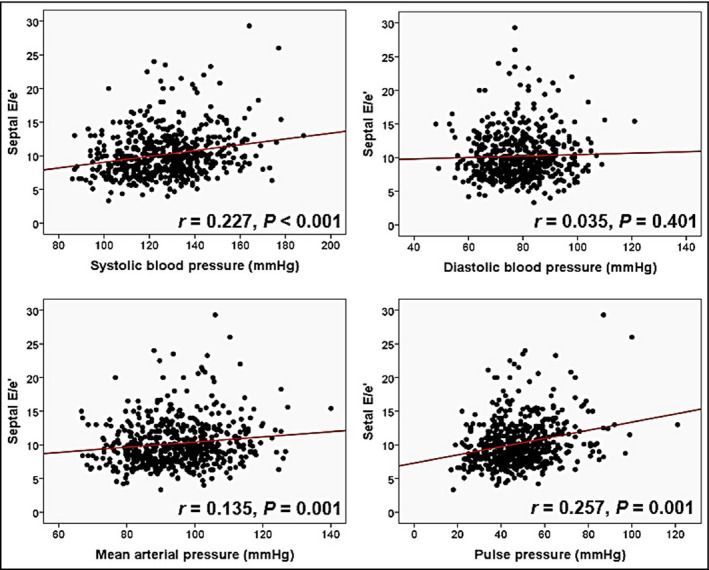
Scatter plots showing linear correlations between septal E/e′ and blood pressure measurements (n = 652). DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure; VD, vessel disease [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
Among patients undergoing elective CAG, we investigated the association between four BP measurements and several TOD parameters. Among three TOD parameters including LVMI, E/e′, and eGFR, E/e′ was correlated with SBP, MAP, and PP even after controlling for potential confounders including age, gender, BMI, heart rate, diabetes mellitus, dyslipidemia, smoking, and cardiovascular medications. On the other hand, there were lack of significant associations of BP measurements with LVMI and eGFR. We also found that SBP and PP had a stronger relationship with CAD than DBP and MAP.
Our study has several strengths compared to prior other studies. First, prior studies have usually determined the association between some BP measurements and only one of the markers for TOD.12, 24, 25 However, our study evaluated the association between all of the BP measurements and several markers for TOD. Secondly, since CAD was confirmed by invasive CAG, we could more accurately define CAD than prior studies. Thirdly, given that most of the previous studies have been performed in Western countries, our data from a nationwide Korean registry may deserve attention.
The DBP has classically been thought of as an important target of anti‐hypertensive treatment and prognostic value of cardiovascular disease until 1980s. In 1977, the first guideline of Joint National Committee recommended DBP as the basis of diagnosis and treatment for hypertension.26 In this context, the treatment of hypertension was based on DBP in some studies.27, 28 However, in the 1990s, the Framingham cohort showed that the cardiovascular event risk was more strongly associated with SBP than with DBP.29 Moreover, numerous studies have proved that SBP is associated with cardiovascular outcomes and TOD.9, 12, 19, 30, 31 In a cross‐sectional analysis of 1315 Chinese subjects who were either normotensive or had untreated hypertension, SBP was the most powerful determinant of LV mass among other BP measurements including DBP.30 Likewise, Lekakis et al31 investigated 48 untreated hypertension and showed that LV hypertrophy was associated with SBP but not with DBP in univariable analysis. In line with these findings, our study showed that SBP had a stronger association with TOD including CAD and LV diastolic dysfunction than with DBP. Otherwise, studies investigating the relationship between MAP and TOD have been limited. A few studies have attempted to demonstrate the association between MAP and LV mass; however, their results were negative.30, 31 Supporting this finding, MAP showed weaker association with only E/e′ among several TOD in our study.
Recently, the importance of PP has been recognized as an independent risk factor for cardiovascular morbidity and mortality.17, 18 The association between PP and TOD has also been demonstrated. In a 10‐year follow‐up study, Jokiniitty et al32 evaluated 97 men and showed that PP was the most significant BP parameter related to future LVMI and its change. Abhayaratna et al33 showed that PP had a significant correlation with LV diastolic dysfunction in their 233 subjects, although the correlation power was weaker than pulse wave velocity. Cirillo et al34 performed a large cross‐sectional analysis (n = 1567) and demonstrated that PP was significantly related to the prevalence of microalbuminuria in nondiabetic middle‐aged adults. Similarly, our study showed that PP had more strong associations with E/e′ as LV diastolic dysfunction and CAD than the other BP measurements, including SBP, DBP, and MAP.
Our results showed that SBP was not associated with LVMI. These unexpected results may be due to the fact that mean SBP in our subjects was lower (127 ± 17 mm Hg) than that of previous studies (≥140 mm Hg).9, 31 In addition, about half of the patients were untreated with vasoactive drugs in our study, which might have an impact on the association between SBP and LVMI. Elevated SBP is well‐known risk factors for renal dysfunction.35 Our study also showed significant and negative correlation between SBP and eGFR in univariable analysis. However, the significance disappeared when controlling for several clinical covariates. This suggests that several cardiovascular risk factors such as age and diabetes mellitus play a significant role as confounding factors, and emphasizes the importance of controlling the effects of these potential confounders.
There are some theoretical backgrounds that SBP and PP are better indicators of TOD. Generally, an increase in SBP increases end‐systolic stress, leading to an increase in LV mass.36 An increase in PP causes vascular endothelial damage and mechanical fatigue, leading to atherosclerosis.37 Increased PP is also associated with increased afterload and reduced coronary perfusion.37 Arterial stiffness is a key mechanism for ventricular remodeling, diastolic dysfunction, and CAD.33 Especially, it has well‐known that SBP and PP have better implication power than DBP and MAP for diastolic dysfunction and CAD, both consequences of increased arterial stiffness.38, 39 Increased arterial stiffness promotes an increase in SBP and a decrease in DBP, leading to an increase in PP, but not MAP, as an offsetting effect. These processes can at least partially explain that SBP and PP have a more pronounced impact on TODs and cardiovascular events than MAP and DBP.
4.1. Study limitations
There are several limitations in our study. First, our cross‐sectional study could not clarify the causal relationship between BP measurements and TOD. Second, BP was measured from brachial pressure, not central hemodynamics in our study. Several studies have shown that central BP has a stronger association with TOD than with brachial BP.31, 40 However, measurement of central hemodynamics requires a specialized instrument, some technical skills or invasive catheterization. Therefore, the use of brachial pressure is more practical in clinical practice. Third, we used BP values obtained in a single measurement. Twenty‐four‐hour ambulatory BP values may be more valuable.41, 42 Fourth, gender comparison was not reliable in our study due to differences in numbers between genders and small number of enrolled men. Further studies with a large sample size are required for this issue. Finally, our study population is restricted to middle‐aged and elderly patients with high‐risk features requiring invasive CAG. Therefore, generalization of our results to other population needs caution.
5. CONCLUSIONS
In patients undergoing elective invasive CAG, four BP measurements including SBP, DBP, MAP, and PP were differentially associated with several TOD parameters including LVMI, E/e′, eGFR, and CAD. More specifically, SBP and PP had stronger relationships with E/e′ and CAD than DBP and MAP. Targeting SBP and PP rather than DBP and MAP may be more efficient in preventing LV diastolic dysfunction and CAD in this group of patients.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
Kong MG, Kim H‐L, Kim M‐A, et al. Relationships between blood pressure measurements and target organ damage: Data from the Korea women's chest pain registry. J Clin Hypertens. 2018;20:1724–1730. 10.1111/jch.13417
Kong and Kim contributed equally to this work.
REFERENCES
- 1. Prospective Studies Collaboration . Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903‐1913. [DOI] [PubMed] [Google Scholar]
- 2. Benetos A, Thomas F, Bean KE, Guize L. Why cardiovascular mortality is higher in treated hypertensives versus subjects of the same age, in the general population. J Hypertens. 2003;21(9):1635‐1640. [DOI] [PubMed] [Google Scholar]
- 3. Nadar SK, Tayebjee MH, Messerli F, Lip GY. Target organ damage in hypertension: pathophysiology and implications for drug therapy. Curr Pharm Des. 2006;12(13):1581‐1592. [DOI] [PubMed] [Google Scholar]
- 4. Pontremoli R, Ravera M, Bezante GP, et al. Left ventricular geometry and function in patients with essential hypertension and microalbuminuria. J Hypertens. 1999;17(7):993‐1000. [DOI] [PubMed] [Google Scholar]
- 5. Gerdts E, Wachtell K, Omvik P, et al. Left atrial size and risk of major cardiovascular events during antihypertensive treatment losartan intervention for endpoint reduction in hypertension trial. Hypertension. 2007;49(2):311‐316. [DOI] [PubMed] [Google Scholar]
- 6. Jensen JS, Feldt‐Rasmussen B, Strandgaard S, Schroll M, Borch‐Johnsen K. Arterial hypertension, microalbuminuria, and risk of ischemic heart disease. Hypertension. 2000;35:898‐903. [DOI] [PubMed] [Google Scholar]
- 7. Okin PM, Devereux RB, Jern S, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292(19):2343‐2349. [DOI] [PubMed] [Google Scholar]
- 8. Okin PM, Oikarinen L, Viitasalo M, et al. Serial assessment of the electrocardiographic strain pattern for prediction of new‐onset heart failure during antihypertensive treatment: the LIFE study. Eur J Heart Fail. 2011;13(4):384‐391. [DOI] [PubMed] [Google Scholar]
- 9. Korhonen PE, Kautiainen H, Järvenpää S, Kantola I. Target organ damage and cardiovascular risk factors among subjects with previously undiagnosed hypertension. Eur J Prev Cardiol. 2014;21(8):980‐988. [DOI] [PubMed] [Google Scholar]
- 10. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159‐2219. [DOI] [PubMed] [Google Scholar]
- 11. Kannel WB. Elevated systolic blood pressure as a cardiovascular risk factor. Am J Cardiol. 2000;85(2):251‐255. [DOI] [PubMed] [Google Scholar]
- 12. Syrseloudis D, Tsioufis C, Aragiannis D, et al. The dominant role of the systolic component of nondipping status on target‐organ damage in never‐treated hypertensives. Am J Hypertens. 2011;24(3):292‐298. [DOI] [PubMed] [Google Scholar]
- 13. Strandberg TE, Pitkala K. What is the most important component of blood pressure: systolic, diastolic or pulse pressure? Curr Opin Nephrol Hypertens. 2003;12(3):293‐297. [DOI] [PubMed] [Google Scholar]
- 14. Li Y, Wei FF, Wang S, Cheng YB, Wang JG. Cardiovascular risks associated with diastolic blood pressure and isolated diastolic hypertension. Curr Hypertens Rep. 2014;16(11):489. [DOI] [PubMed] [Google Scholar]
- 15. Thooft A, Favory R, Salgado DR, et al. Effects of changes in arterial pressure on organ perfusion during septic shock. Crit Care. 2011;15(5):R222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jhanji S, Stirling S, Patel N, Hinds CJ, Pearse RM. The effect of increasing doses of norepinephrine on tissue oxygenation and microvascular flow in patients with septic shock. Crit Care Med. 2009;37(6):1961‐1966. [DOI] [PubMed] [Google Scholar]
- 17. Zhao L, Song Y, Dong P, Li Z, Yang X, Wang S. Brachial pulse pressure and cardiovascular or all‐cause mortality in the general population: a meta‐analysis of prospective observational studies. J Clin Hypertens. 2014;16(9):678‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Selvaraj S, Steg PG, Elbez Y, et al.Pulse pressure and risk for cardiovascular events in patients with atherothrombosis: from the REACH Registry. J Am Coll Cardiol. 2016;67(4):392‐403. [DOI] [PubMed] [Google Scholar]
- 19. Cho KI, Shim WJ, Park SM, et al. Association of depression with coronary artery disease and QTc interval prolongation in women with chest pain: data from the KoRean wOmen'S chest pain rEgistry (KoROSE) study. Physiol Behav. 2015;143:45‐50. [DOI] [PubMed] [Google Scholar]
- 20. Kim HL, Kim MA, Shim WJ, et al. Sex difference in the association between brachial pulse pressure and coronary artery disease: the Korean Women's Chest Pain Registry (KoROSE). J Clin Hypertens. 2017;19(1):38‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee CS, Cha RH, Lim YH, et al. Ethnic coefficients for glomerular filtration rate estimation by the Modification of Diet in Renal Disease study equations in the Korean population. J Korean Med Sci. 2010;25(11):1616‐1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1‐39.e14. [DOI] [PubMed] [Google Scholar]
- 23. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277‐314. [DOI] [PubMed] [Google Scholar]
- 24. Missault LH, De Buyzere ML, De Bacquer DD, Duprez DD, Clement DL. Relationship between left ventricular mass and blood pressure in treated hypertension. J Hum Hypertens. 2002;16(1):61‐66. [DOI] [PubMed] [Google Scholar]
- 25. Lorber R, Gidding SS, Daviglus ML, Colangelo LA, Liu K, Gardin JM. Influence of systolic blood pressure and body mass index on left ventricular structure in healthy African‐American and white young adults: the CARDIA study. J Am Coll Cardiol. 2003;41(6):955‐960. [DOI] [PubMed] [Google Scholar]
- 26. Report of the Joint National Committee on Detection . Evaluation, and treatment of high blood pressure. A cooperative study. JAMA. 1977;237(3):255‐261. [PubMed] [Google Scholar]
- 27. Poblete PF, Kyle MC, Pipberger HV, Freis ED. Effect of treatment on morbidity in hypertension. Veterans Administration Cooperative Study on Antihypertensive Agents. Effect on the electrocardiogram. Circulation. 1973;48(3):481‐490. [DOI] [PubMed] [Google Scholar]
- 28. Rutan GH, McDonald RH, Kuller LH. A historical perspective of elevated systolic vs diastolic blood pressure from an epidemiological and clinical trial viewpoint. J Clin Epidemiol. 1989;42(7):663‐673. [DOI] [PubMed] [Google Scholar]
- 29. Kannel WB. Epidemiology of essential hypertension: the Framingham experience. Proc R Coll Phys Edinb. 1991;21:273‐287. [Google Scholar]
- 30. Chen CH, Ting CT, Lin SJ, et al. Which arterial and cardiac parameters best predict left ventricular mass? Circulation. 1998;98(5):422‐428. [DOI] [PubMed] [Google Scholar]
- 31. Lekakis JP, Zakopoulos NA, Protogerou AD, et al. Cardiac hypertrophy in hypertension: relation to 24‐h blood pressure profile and arterial stiffness. Int J Cardiol. 2004;97(1):29‐33. [DOI] [PubMed] [Google Scholar]
- 32. Jokiniitty JM, Majahalme SK, Kähönen MA, Tuomisto MT, Turjanmaa VM. Pulse pressure is the best predictor of future left ventricular mass and change in left ventricular mass: 10 years of follow‐up. J Hypertens. 2001;19(11):2047‐2054. [DOI] [PubMed] [Google Scholar]
- 33. Abhayaratna WP, Srikusalanukul W, Budge MM. Aortic stiffness for the detection of preclinical left ventricular diastolic dysfunction: pulse wave velocity versus pulse pressure. J Hypertens. 2008;26(4):758‐764. [DOI] [PubMed] [Google Scholar]
- 34. Cirillo M, Stellato D, Laurenzi M, Panarelli W, Zanchetti A, De Santo NG. Pulse pressure and isolated systolic hypertension: association with microalbuminuria. The GUBBIO Study Collaborative Research Group. Kidney Int. 2000;58(3):1211‐1218. [DOI] [PubMed] [Google Scholar]
- 35. Hanratty R, Chonchol M, Havranek EP, et al. Relationship between blood pressure and incident chronic kidney disease in hypertensive patients. Clin J Am Soc Nephrol. 2011;6(11):2605‐2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khattar RS, Acharya DU, Kinsey C, Senior R, Lahiri A. Longitudinal association of ambulatory pulse pressure with left ventricular mass and vascular hypertrophy in essential hypertension. J Hypertens. 1997;15(7):737‐743. [DOI] [PubMed] [Google Scholar]
- 37. Dart AM, Kingwell BA. Pulse pressure—a review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001;37(4):975‐984. [DOI] [PubMed] [Google Scholar]
- 38. Benetos A, Safar M, Rudnichi A, et al. Pulse pressure: a predictor of long‐term cardiovascular mortality in a French male population. Hypertension. 1997;30(6):1410‐1415. [DOI] [PubMed] [Google Scholar]
- 39. Mitchell GF, Moyé LA, Braunwald E, et al. Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. SAVE investigators. Survival and Ventricular Enlargement. Circulation. 1997;96(12):4254‐4260. [DOI] [PubMed] [Google Scholar]
- 40. Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50(1):197‐203. [DOI] [PubMed] [Google Scholar]
- 41. Mancia G, Zanchetti A, Agabiti‐Rosei E, et al. Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment‐induced regression of left ventricular hypertrophy. SAMPLE Study Group. Study on Ambulatory Monitoring of Blood Pressure and Lisinopril Evaluation. Circulation. 1997;95(6):1464‐1470. [DOI] [PubMed] [Google Scholar]
- 42. Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46(1):156‐161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


