Abstract
Ambulatory blood pressure (BP) and central BP are better predictors for overall cardiovascular risk and mortality than brachial BP. Renal denervation (RDN) has been shown to reduce office brachial and central BP as well as brachial ambulatory BP, but data on central ambulatory BP are limited. Patients (N = 94) with treatment resistant hypertension (TRH) who underwent RDN were included. Ambulatory BP, including central pressures, hemodynamics, and arterial stiffness were measured at baseline and 3, 6, 12 months after RDN by an oscillometric device (MobiloGraph™). At 3, 6, and 12‐month follow‐ups, brachial ambulatory BP was reduced (P for all < .001). Consistently, central ambulatory BP was reduced (P for all < .001). Ambulatory assessed averaged daytime pulse wave velocity improved after RDN (P < .05). Total vascular resistance decreased (P for all < .01). In patients with TRH, RDN improves brachial and central ambulatory BP, arterial stiffness, and total vascular resistance, indicating an improvement of cardiovascular outcome.
Keywords: central ambulatory blood pressure, central hemodynamics, renal denervation, treatment resistant hypertension
1. INTRODUCTION
Arterial hypertension is one of the major modifiable risk factors of cardiovascular (CV) morbidity and mortality.1, 2 Nevertheless, a large proportion of hypertensive patients are not well controlled3 and therefore the management of antihypertensive therapy, particularly in patients with treatment resistant hypertension (TRH), is a remaining problem. Guidelines for management of arterial hypertension recommend ambulatory BP monitoring (ABPM) not only to exclude pseudoresistance, which is of crucial importance in patients with TRH,2 but also to assess CV risk more precisely. The burden of TRH is based on the fact, that patients with TRH have an exaggerated CV risk compared to patients without TRH, who also have an increased risk of CV.3, 4
There is large evidence from meta‐analysis of observational studies as well as pooled individual data that ambulatory BP is superior for prediction of clinical CV outcomes compared to office BP. This prognostical superiority has been consistently documented in both untreated and treated hypertensive patients.2
Moreover, in the attempt for better BP assessment, non‐invasive estimation of central hemodynamics has been put into focus. Pathophysiologically, central pressure in the aorta, which is the perfusion pressure to key organs, (rather than the pressure in the arm) may provide more relevant prognostic information. Indeed, it has been shown in a population‐based study5 and in a hypertension trial6 that the noninvasively measured central BP is superior to brachial BP in predicting CV outcomes. In general, the dissociation between central and brachial BP has been observed to be greater at higher baseline BP levels, regardless of the treatment strategy used.7
Nowadays, central hemodynamics can also routinely be assessed in an ambulatory setting, combining both advantages regarding better CV risk prediction.8, 9 Catheter‐based renal denervation (RDN) has been introduced for BP management in TRH and it was repeatedly shown that RDN results in a clinical substantial BP reduction (office, ABPM, and central),10, 11, 12 but data about central BP under ambulatory conditions are missing.
Hence, the aim of the present prospective observational study was to assess the effect of RDN on central ambulatory BP and hemodynamic parameters under ambulatory conditions.
2. METHODS
2.1. Study cohort and design
This 2‐center observational study included 94 patients in total, with true TRH (office BP ≥ 140/90 mm Hg and confirmation by averaged daytime ambulatory BP ≥ 135/85 mm Hg, while on at least 3 antihypertensive agents including 1 diuretic) on a stable drug regimen (ie, without change in dose or medication for at least 4 weeks prior to baseline) who underwent RDN. In line with the recent position papers of the European Society of Hypertension,13 main exclusion criteria were a renal artery anatomy that is ineligible for treatment and any cause of secondary hypertension (except treated obstructive sleep apnea syndrome). As per protocol, routine follow‐up visits were performed 3, 6, and, 12 months after RDN.
The local ethics committees approved the study protocol (University of Lübeck and University of Erlangen) and the study was performed according to the Declaration of Helsinki and “good clinical practice” (GCP) guidelines. Before enrollment, each patient provided written informed consent.
2.2. Catheter‐based renal denervation
The femoral artery was punctured with standard endovascular technique, and a dedicated radiofrequency catheter was advanced in each renal artery guided by angiography. At least 4 radiofrequency ablations (energy delivery for 120 seconds each), controlled and regulated by a radiofrequency generator, were applied longitudinally and rotationally within the lengths of each renal artery to achieve a full 4‐quadrant ablation. All RDN procedures were performed by experienced operators (≥ 50 RDN procedures), which made the technical success of the procedure most likely.
2.3. Office and 24‐hour ambulatory blood pressure
Office BP was initially measured in both arms after 5 minutes of rest in a sitting position using an oscillometric device with an appropriate cuff size. Subsequent BP measurements were performed on the arm with the higher BP readings, and the average of 3 measurements take at 2‐minute intervals was recorded.
By using a commercially available oscillometric brachial‐cuff based sphygmomanometer with appropriate cuff size, 24‐hour ambulatory BP (central and brachial), as well as vascular parameters (eg, pulse wave velocity [PWV]) under ambulatory conditions, were determined. In brief, after conventional BP and heart rate measurements, the brachial cuff was inflated additionally to the diastolic BP level and held for about 10 seconds to record pulse waves. Subsequently, central pressure curves were obtained through a transfer function. The technology has been validated previously.14, 15, 16
To estimate PWV, the ARC Solver method was applied utilizing several parameters of the pulse wave analysis in a mathematical model, which was validated against intra‐aortic readings,17 magnetic resonance imaging,18 and applanation tonometry.19 Stroke volume and peripheral resistance were derived by a 3‐element Windkessel model, which was fitted into the aortic pulse contour. The method was successfully compared against catheter, impedance cardiography, and Dopper ultrasound.19, 20
Readings were recorded every 15 minutes during the day (8 am‐10 pm) and every 30 minutes at night (10 pm‐8 am), according to current recommendations.21 In the follow‐up, 10 patients (6 months) and 18 patients (12 months), respectively, refused ABPM or data readings were insufficient (eg, < 80% obtained successful recordings).
2.4. Statistical analysis
For all statistical analyses IBM SPSS Statistics for Windows, Version 19.0 was used and graphs were edited with SigmaPlot 8.0 and CorelDraw 11.0.
Normal distribution of data was confirmed by Kolmogorow‐Smirnow tests before further analysis. Data were compared by paired student t‐tests and Wilcoxon tests where appropriate. Multivariate analysis was performed assessing the dependency of the change of PWV from potential cofactors. Data are expressed as mean ± standard deviation (SD) in the text and tables, and mean ± standard error of mean (SEM) or median and interquartile range in the figures. A 2‐sided P‐value of < .05 was considered statistically significant.
3. RESULTS
3.1. Clinical characteristics
In total, 94 patients (34‐88 years) were enrolled into this observational study. The clinical characteristics of the study population are depicted in Table 1. Of note, there was no difference in baseline characteristics between whole study cohort (baseline and 3 months after RDN) and patients with available ABPM data at 6 (n = 84) or 12 months (n = 76) follow‐up after RDN (data not shown). Despite patients being treated with 5.5 ± 1.6 (3‐10) antihypertensive drugs on average, BP was not controlled.
Table 1.
Clinical characteristics
| Parameter | |
|---|---|
| Age (years) | 65.0 ± 11 |
| Gender (m/f) | 60/34 |
| BMI (kg/m2) | 30.1 ± 4.8 |
| Office SBP (mm Hg) | 170 ± 24 |
| Office DBP (mm Hg) | 92 ± 16 |
| Heart rate (beats/min) | 69 ± 13 |
| 24‐h ambulatory SBP (mm Hg) | 153 ± 14 |
| 24‐h ambulatory DBP (mm Hg) | 89 ± 11 |
| Number of antihypertensives (n) | 5.5 ± 1.6 |
| CHD | 51 (54) |
| Diabetes mellitus type 2 | 43 (46) |
| eGFR (mL/min/1.73 m2)a | 66.8 ± 6 |
BMI, body mass index; SBP, systolic blood pressure; DBP diastolic blood pressure; CHD, coronary heart disease; eGFR, estimated glomerular filtration rate.
According to MDRD formula.
Values are mean ± SD or n (%).
Median of full 120‐s ablation points were 5.6 ± 1.4 (left side)/6.0 ± 1.6 (right side; with at least 4 at each side), indicating coverage of a full 4‐quadrant ablation of both renal arteries. No procedural associated severe adverse event was observed.
3.2. Office blood pressure
Three months after RDN, office BP was reduced (systolic: 170 ± 24/92 ± 16 vs 155 ± 22/87 ± 15 mm Hg, both P < .001), with further reduction documented at 6 months (148 ± 21/83 ± 14 mm Hg, both P < .001) and 12 months (146 ± 17/82 ± 13 mm Hg, both P < .001).
3.3. Brachial ambulatory blood pressure
There was a significant reduction in mean 24‐hour brachial ambulatory BP at 3 months by 6 ± 13/4 ± 7 (systolic: 153 ± 13 vs 147 ± 15 mm Hg, P < .001; diastolic: 89 ± 10 vs 85 ± 11 mm Hg, P = .001), at 6 months by 8 ± 15/4 ± 10 (systolic: 153 ± 13 vs 145 ± 15 mm Hg, P < .001; diastolic: 89 ± 11 vs 84 ± 11 mm Hg; P < .001), and at 12 months by 9 ± 16/4 ± 9 mm Hg (systolic: 153 ± 13 vs 144 ± 15 mm Hg, P < .001; diastolic: 88 ± 10 vs 84 ± 11 mm Hg; P < .001); Figure 1 and Table 2. Likewise, also averaged daytime brachial ambulatory BP and nighttime brachial ambulatory BP were reduced at 3, 6, and 12 months after RDN (Table 2).
Figure 1.
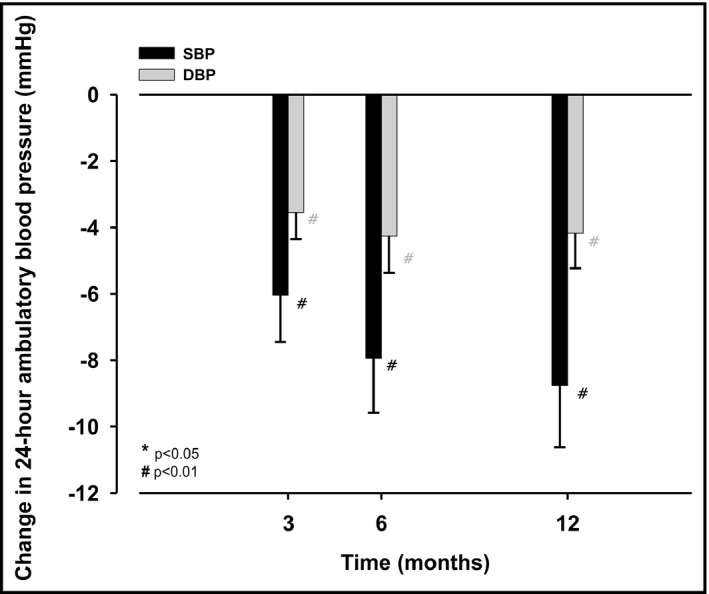
Absolute change in mean 24‐h brachial systolic (black columns) and diastolic (grey columns) ambulatory blood pressure (mm Hg) between before (baseline) and 3, 6, and 12 mo after renal denervation, respectively; values are mean ± SEM; P‐values are comparisons with baseline values
Table 2.
Peripheral and central ambulatory blood pressue before (baseline), 3, 6, and 12 mo after renal denervation
| Baseline | 3 mo | 6 mo | 12 mo | ||||
|---|---|---|---|---|---|---|---|
| (N = 94) | (N = 94) | P‐value | (N = 84) | P‐value | (N = 76) | P‐value | |
| Peripheral | |||||||
| 24‐h | |||||||
| SBP (mm Hg) | 153 ± 13 | 147 ± 15 | <.001 | 145 ± 15 | <.001 | 144 ± 15 | <.001 |
| DBP (mm Hg) | 89 ± 10 | 85 ± 11 | <.001 | 84 ± 11 | <.001 | 84 ± 11 | <.001 |
| Daytime | |||||||
| SBP (mm Hg) | 158 ± 13 | 150 ± 16 | <.001 | 147 ± 15 | <.001 | 147 ± 15 | <.001 |
| DBP (mm Hg) | 93 ± 11 | 88 ± 11 | <.001 | 87 ± 11 | <.001 | 86 ± 11 | <.001 |
| Nighttime | |||||||
| SBP (mm Hg) | 144 ± 18 | 139 ± 18 | .004 | 142 ± 19 | .099 | 139 ± 17 | .003 |
| DBP (mm Hg) | 81 ± 13 | 78 ± 11 | .003 | 80 ± 13 | .362 | 78 ± 13 | .058 |
| Central | |||||||
| 24‐h | |||||||
| SBP (mm Hg) | 138 ± 13 | 133 ± 13 | <.001 | 132 ± 14 | <.001 | 129 ± 13 | <.001 |
| DBP (mm Hg) | 91 ± 11 | 88 ± 11 | <.001 | 87 ± 11 | .002 | 86 ± 11 | <.001 |
| Daytime | |||||||
| SBP (mm Hg) | 143 ± 13 | 135 ± 14 | <.001 | 133 ± 13 | <.001 | 132 ± 13 | <.001 |
| DBP (mm Hg) | 95 ± 11 | 91 ± 12 | <.001 | 89 ± 12 | <.001 | 88 ± 12 | <.001 |
| Nighttime | |||||||
| SBP (mm Hg) | 130 ± 17 | 126 ± 16 | .039 | 129 ± 18 | .517 | 125 ± 16 | .015 |
| DBP (mm Hg) | 83 ± 13 | 79 ± 11 | .005 | 82 ± 13 | .382 | 81 ± 14 | .034 |
DBP, diastolic blood pressure; SBP, systolic blood pressure.
3.4. Central ambulatory blood pressure
RDN resulted in a reduction of mean 24‐hour central ambulatory BP at 3 months by 6 ± 12/3 ± 8 (systolic: 138 ± 13 vs 133 ± 13 mm Hg, P < .001; diastolic: 91 ± 11 vs 88 ± 11 mm Hg, P < .001), at 6 months by 7 ± 15/4 ± 9 (systolic: 138 ± 13 vs 132 ± 14 mm Hg, P < .001; diastolic: 91 ± 11 vs 87 ± 11 mm Hg; P < .001), and at 12 months by 9 ± 15/5 ± 9 mm Hg (systolic: 138 ± 13 vs 129 ± 13 mm Hg, P < .001; diastolic: 90 ± 11 vs 85 ± 11 mm Hg; P < .001; Figure 2 and Table 2. In accordance, also averaged daytime central ambulatory BP and nighttime central ambulatory BP were reduced at 3, 6, and 12 months after RDN (Table 2).
Figure 2.
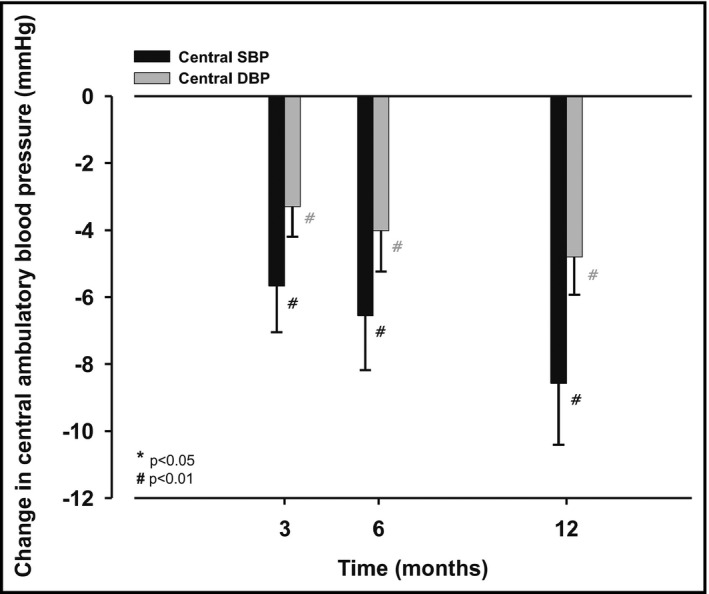
Absolute change in mean 24‐h central systolic (black columns) and diastolic (grey columns) ambulatory blood pressure (mm Hg) between before (baseline) and 3, 6, and 12 mo after renal denervation, respectively; values are mean ± SEM; P‐values are comparisons with baseline values
3.5. Pulse wave velocity under ambulatory conditions
There was a numerically reduction of averaged 24‐hour PWV assessed under ambulatory conditions at 3 months (10.0 ± 1.7 vs 9.8 ± 1.7 m/s, P = .037), at 6 months (10.0 ± 1.6 vs 9.9 ± 1.7 m/s, P = .154), and at 12 months (10.0 ± 1.6 vs 9.9 ± 1.7 m/s, P = .104) after RDN. However, averaged daytime PWV assessed under ambulatory conditions was significantly reduced at 3 months (10.1 ± 1.7 vs 9.9 ± 1.6 m/s, P = .003), at 6 months (10.2 ± 9.9 vs 9.9 ± 1.7 m/s, P = .03), and at 12 months (10.2 ± 1.6 vs 10.0 ± 1.7 m/s, P = .018) after RDN (Figure 3), but no improvements in nighttime values were observed.
Figure 3.
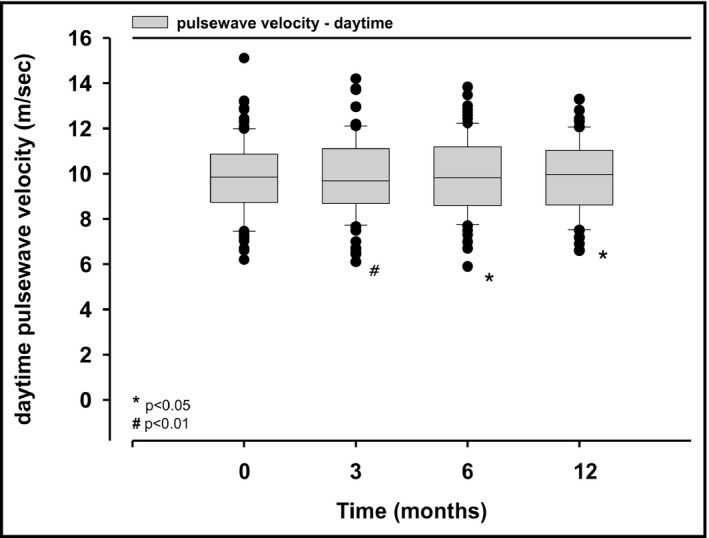
Averaged daytime pulse wave velocity assessed under ambulatory conditions before (baseline), 3, 6, and 12 mo after renal denervation; values are median and interquartile range; P‐values are comparisons with baseline values
Multivariate analysis revealed that the significantly lowered PWV was independent of HR and MAP during follow up (multivariate tests: Wilks's Lambda > 0.05).
3.6. Total vascular resistance under ambulatory conditions
RDN resulted in a significant decrement of averaged 24‐hour total vascular resistance (TVR) at 3 months by 4.0% (1.67 ± 0.2 vs 1.60 ± 0.2 mm Hg/min/mL, P = .005), at 6 months by 5.5% (1.67 ± 0.2 vs 1.58 ± 0.2 mm Hg/min/mL, P < .001), and after 12 months by 6.7% (1.68 ± 0.2 vs 1.56 ± 0.2 mm Hg/min/mL, P < .001; Figure 4). Consistently, TVR was significantly reduced at all pre‐specified time‐points, irrespective whether analyzed for daytime or nighttime data, separately (data not shown).
Figure 4.
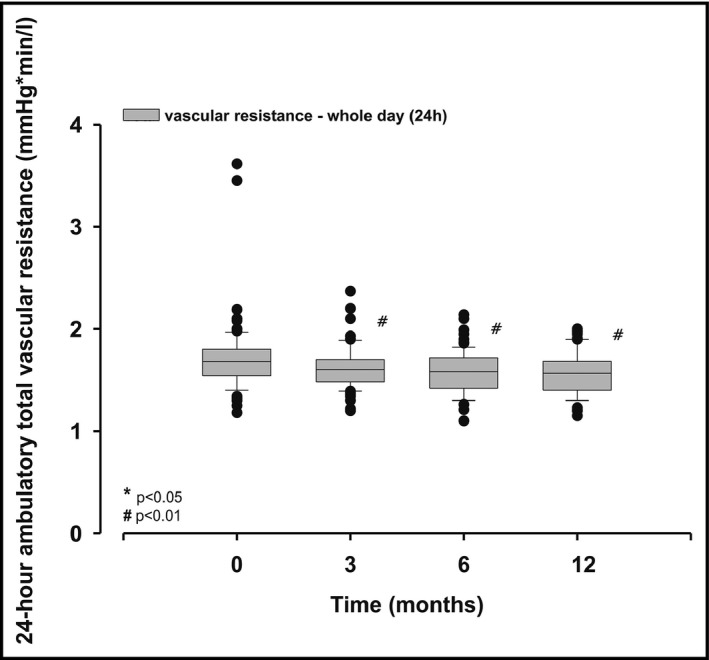
Averaged mean 24‐h total vascular resistance assessed under ambulatory conditions before (baseline), 3, 6, and 12 mo after renal denervation; values are median and interquartile range; P‐values are comparisons with baseline values
3.7. Cardiac output under ambulatory conditions
Overall, 24‐hour cardiac output remained stable for all follow‐up time‐points (Figure 5). Also, there was no change in cardiac output, irrespective whether analyzed for daytime or nighttime data, separately (data not shown).
Figure 5.
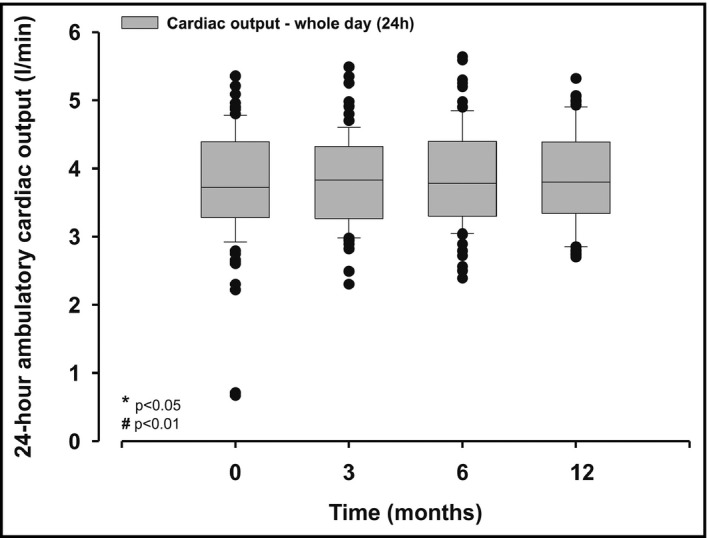
Averaged 24‐h cardiac output assessed under ambulatory conditions before (baseline), 3, 6, and 12 mo after renal denervation; values are median and interquartile range; P‐values are comparisons with baseline values
4. DISCUSSION
It is well accepted that both ambulatory BP and central BP are better predictors of CV events compared to office BP.2, 5, 6 Further technological developments offer the opportunity for simultaneous assessment of conventional ambulatory BP, but also for estimation of central hemodynamics under ambulatory conditions.8, 9 Recent studies have shown that RDN is effective in both the short and long‐term to lower office, ambulatory brachial, and central BP,10, 11, 12 which we were able to confirm with our 2‐center, at least mid‐term, follow‐up study. However, more importantly, we were able to show for the first time, that RDN also results in a clinically significant reduction of central BP assessed under ambulatory conditions, hence, expanding the knowledge of BP reduction after RDN. Moreover, it has to be mentioned that findings of central hemodynamics are not based on single (office) measurements, but rather on multiple (ie, up to 76) repetitive measurements under ambulatory conditions at each time‐point of follow‐up.
However, the underlying mechanism of RDN attributing to BP reduction is still controversially debated. RDN leads not only to a decrease of renal efferent sympathetic activity, but also the afferent sensory signaling, arising from the kidneys to the central nervous system and, hence, towards key organs, including the vasculature, in particular, the small resistance vessels.22, 23 Therefore, it can be assumed that changes in TVR following RDN are related to a reduction of sympathetic nerve activity (SNA). Indeed, it was shown that (at least in part) BP reduction due to RDN is associated with reduction in sympathetic nerve activity (assessed by multiple SNA).24 Previously, in a single‐center study (N = 30) from Ewen et al, it was shown that RDN significantly reduces (office) measured TVR, however, independently of changes in cardiac output.25 Therefore, our data of a significant reduction of TVR being stable over the follow‐up, which may have been changed, amongst others, by peripheral vasodilation and improvement of endothelial dysfunction, are confirmatory. Consistently, as seen in latter study25 the reduction of TVR was independently of changes of cardiac output, which remained stable over the 12 months follow‐up after RDN.
Increment of TVR due to arterial remodeling and endothelial dysfunction add to the burden of cardiac workload by increasing central afterload (eg, increase of central systolic pressure and stiffer aorta indicated by increased central pulse pressure). However, central aortic pressure parameters and left ventricular (LV) load are determined not only by cardiac output and peripheral vascular resistance, but also by the stiffness of conduit arteries and the timing and magnitude of pressure wave reflections.26, 27 Interestingly, arterial stiffness in general and aortic stiffness, in particular, are known to be markedly increased in patients with TRH,28 and BP response to RDN is diminished in patients with proposed enhanced arterial stiffness.29, 30 Even more, it was recently proposed that arterial stiffness may be used as selection criteria for RDN.31 This concept is further supported by an animal model, which showed that thoracic sympathetic denervation improved both structural and functional remodeling of the aortic wall.32
The gold‐standard of arterial stiffness,33 and an acknowledged parameter of target organ damage (TOD),2 is PWV, which was shown to be an independent prognostic indicator of CV events.34 Patients suffering from TRH have an increased risk for TOD compared to well controlled hypertensives.35 As long as studies of RDN on hard end points are underway, but results are still missing, the meaning of intermediate end points like PWV are of crucial importance. It was repeatedly shown that RDN has beneficial effects on TOD, like LV hypertrophy36 and albuminuria.37 In contrast, data on arterial stiffness (eg, PWV) are conflicting.38, 39, 40
The improvement of the PWV assessed under ambulatory conditions, which could be observed for the daytime measurements during the whole follow‐up, reflects a reversible level of arterial stiffness. Acute changes of PWV are BP‐dependent. However, it is reasonable that in the long‐term improvement of PWV may also be BP‐independent, based on reversible mechanisms attributed by RDN like reduction of peripheral vascoconstriction. Hence, we adjusted PWV to BP and to heart rate (HR).41 By doing so, multivariate analysis revealed that the significantly lowered PWV was independent of HR and mean arterial pressure during follow up, suggesting at least a synergistic action (BP‐reduction per se) as well as further modi of action on PWV due to RDN.
One might hypothesize that RDN may predominantly reduce daytime values since sympathetic tone is dominant here. Indeed, our data unequivocally show that central hemodynamics assessed under ambulatory conditions is improved, especially during the daytime. Furthermore, our results might suggest that the main effect of RDN is reduction of sympathetic drive.
Our study has several limitations. First, central hemodynamics were non‐invasively assessed, but the validity of the device used has been tested against invasive measurements16 and reproducibility has been shown in routine ambulatory settings.9 Second, our study lacks a control group, but it is still of high quality since we used blind end point evaluation, patients had true TRH and ambulatory BP was measured. Third, RDN was done with a mono‐electrode radiofrequency catheter. However, interventions were performed in 2 very experienced centers. Moreover, rigorous efforts were done to cover a full 4‐quadrant ablation of both renal arteries. Fourth, TVR and cardiac output was not assessed invasively with right heart catheterization. However, used approach showed good agreement with measurements of a non‐invasive impedance‐cardiograph as well as assessment with Thermodilution method using a pulmonary catheter.42 In general, bias may be diminished by comparing intra‐individual (same patients) changes.
In summary, our data suggests that RDN improves both peripheral and central BP, as well as aortic stiffness and TVR in 24‐hour measurements under ambulatory conditions. Hence, RDN may improve CV prognosis of patients with true TRH.
CONFLICT OF INTEREST
JW has received speaker honoraria from Medtronic; RES has received travel support, speaker and consultancy fees and institution grants from Medtronic Inc. For all other authors none were declared.
ACKNOWLEDGMENTS
We highly appreciate the work of the interventionalists and study nurses in both centers.
Ott C, Franzen KF, Graf T, et al. Renal denervation improves 24‐hour central and peripheral blood pressures, arterial stiffness, and peripheral resistance. J Clin Hypertens. 2018;20:366–372. 10.1111/jch.13193
Christian Ott, Klaas F. Franzen, Michael Reppel and Kai Mortensen contributed equally to the manuscript.
REFERENCES
- 1. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217‐223. [DOI] [PubMed] [Google Scholar]
- 2. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159‐2219. [DOI] [PubMed] [Google Scholar]
- 3. Judd E, Calhoun DA. Apparent and true resistant hypertension: definition, prevalence, and outcomes. J Hum Hypertens. 2014;28:463‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635‐1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197‐203. [DOI] [PubMed] [Google Scholar]
- 6. Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure‐lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213‐1225. [DOI] [PubMed] [Google Scholar]
- 7. Williams B. Evaluating interventions to reduce central aortic pressure, arterial stiffness, and morbidity–mortality. J Hypertens. 2012;30(Suppl):S13‐S18. [DOI] [PubMed] [Google Scholar]
- 8. Papaioannou TG, Argyris A, Protogerou AD, et al. Non‐invasive 24 hour ambulatory monitoring of aortic wave reflection and arterial stiffness by a novel oscillometric device: the first feasibility and reproducibility study. Int J Cardiol. 2013;169:57‐61. [DOI] [PubMed] [Google Scholar]
- 9. Protogerou AD, Argyris A, Nasothimiou E, et al. Feasibility and reproducibility of noninvasive 24‐h ambulatory aortic blood pressure monitoring with a brachial cuff‐based oscillometric device. Am J Hypertens. 2012;25:876‐882. [DOI] [PubMed] [Google Scholar]
- 10. Mahfoud F, Ukena C, Schmieder RE, et al. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation. 2013;128:132‐140. [DOI] [PubMed] [Google Scholar]
- 11. Azizi M, Sapoval M, Gosse P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open‐label, randomised controlled trial. Lancet. 2015;385:1957‐1965. [DOI] [PubMed] [Google Scholar]
- 12. Symplicity HTNI, Esler MD, Krum H, et al. Renal sympathetic denervation in patients with treatment‐resistant hypertension (The Symplicity HTN‐2 Trial): a randomised controlled trial. Lancet. 2010;376:1903‐1909. [DOI] [PubMed] [Google Scholar]
- 13. Schmieder RE, Redon J, Grassi G, et al. Updated ESH position paper on interventional therapy of resistant hypertension. EuroIntervention. 2013;9(Suppl R):R58‐R66. [DOI] [PubMed] [Google Scholar]
- 14. Wassertheurer S, Kropf J, Weber T, et al. A new oscillometric method for pulse wave analysis: comparison with a common tonometric method. J Hum Hypertens. 2010;24:498‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakagomi A, Okada S, Shoji T, Kobayashi Y. Crucial effect of calibration methods on the association between central pulsatile indices and coronary atherosclerosis. Am J Hypertens. 2017;30:24‐27. [DOI] [PubMed] [Google Scholar]
- 16. Weber T, Wassertheurer S, Rammer M, et al. Validation of a brachial cuff‐based method for estimating central systolic blood pressure. Hypertension 2011;58:825‐832. [DOI] [PubMed] [Google Scholar]
- 17. Weber T, Wassertheurer S, Hametner B, Parragh S, Eber B. Noninvasive methods to assess pulse wave velocity: comparison with the invasive gold standard and relationship with organ damage. J Hypertens. 2015;33:1023‐1031. [DOI] [PubMed] [Google Scholar]
- 18. Feistritzer HJ, Klug G, Reinstadler SJ, et al. Oscillometric analysis compared with cardiac magnetic resonance for the assessment of aortic pulse wave velocity in patients with myocardial infarction. J Hypertens. 2016;34:1746‐1751. [DOI] [PubMed] [Google Scholar]
- 19. Hametner B, Parragh S, Mayer C, et al. Assessment of model based (input) impedance, pulse wave velocity, and wave reflection in the Asklepios cohort. PLoS ONE. 2015;10:e0141656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wassertheurer S, Mayer C, Breitenecker F. Modeling arterial and left ventricular coupling for non‐invasive measurements. Simul Model Pract Th. 2008;16:988‐997. [Google Scholar]
- 21. O'Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731‐1768. [DOI] [PubMed] [Google Scholar]
- 22. Stella A, Zanchetti A. Functional role of renal afferents. Physiol Rev. 1991;71:659‐682. [DOI] [PubMed] [Google Scholar]
- 23. Katholi RE. Renal nerves in the pathogenesis of hypertension in experimental animals and humans. Am J Physiol. 1983;245:F1‐F14. [DOI] [PubMed] [Google Scholar]
- 24. Hering D, Marusic P, Walton AS, et al. Sustained sympathetic and blood pressure reduction 1 year after renal denervation in patients with resistant hypertension. Hypertension. 2014;64:118‐124. [DOI] [PubMed] [Google Scholar]
- 25. Ewen S, Cremers B, Meyer MR, et al. Blood pressure changes after catheter‐based renal denervation are related to reductions in total peripheral resistance. J Hypertens. 2015;33:2519‐2525. [DOI] [PubMed] [Google Scholar]
- 26. O'Rourke M. Mechanical principles in arterial disease. Hypertension. 1995;26:2‐9. [DOI] [PubMed] [Google Scholar]
- 27. Izzo JL Jr. Arterial stiffness and the systolic hypertension syndrome. Curr Opin Cardiol. 2004;19:341‐352. [DOI] [PubMed] [Google Scholar]
- 28. Pickering TG. Arterial stiffness as a cause of resistant hypertension? J Clin Hypertens. 2007;9:390‐395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ewen S, Ukena C, Linz D, et al. Reduced effect of percutaneous renal denervation on blood pressure in patients with isolated systolic hypertension. Hypertension. 2015;65:193‐199. [DOI] [PubMed] [Google Scholar]
- 30. Ott C, Schmid A, Toennes SW, et al. Central pulse pressure predicts BP reduction after renal denervation in patients with treatment‐resistant hypertension. EuroIntervention. 2015;11:110‐116. [DOI] [PubMed] [Google Scholar]
- 31. Okon T, Rohnert K, Stiermaier T, et al. Invasive aortic pulse wave velocity as a marker for arterial stiffness predicts outcome of renal sympathetic denervation. EuroIntervention. 2016;12:e684‐e692. [DOI] [PubMed] [Google Scholar]
- 32. Angouras DC, Dosios TJ, Dimitriou CA, et al. Surgical thoracic sympathectomy induces structural and biomechanical remodeling of the thoracic aorta in a porcine model. J Surg Res. 2012;172:68‐76. [DOI] [PubMed] [Google Scholar]
- 33. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588‐2605. [DOI] [PubMed] [Google Scholar]
- 34. Ben‐Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muiesan ML, Salvetti M, Rizzoni D, et al. Resistant hypertension and target organ damage. Hypertens Res. 2013;36:485‐491. [DOI] [PubMed] [Google Scholar]
- 36. Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59:901‐909. [DOI] [PubMed] [Google Scholar]
- 37. Ott C, Mahfoud F, Schmid A, et al. Improvement of albuminuria after renal denervation. Int J Cardiol. 2014;173:311‐315. [DOI] [PubMed] [Google Scholar]
- 38. Brandt MC, Reda S, Mahfoud F, Lenski M, Bohm M, Hoppe UC. Effects of renal sympathetic denervation on arterial stiffness and central hemodynamics in patients with resistant hypertension. J Am Coll Cardiol. 2012;60:1956‐1965. [DOI] [PubMed] [Google Scholar]
- 39. Mortensen K, Franzen K, Himmel F, et al. Catheter‐based renal sympathetic denervation improves central hemodynamics and arterial stiffness: a pilot study. J Clin Hypertens. 2012;14:861‐870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Verloop WL, Vink EE, Spiering W, et al. Effects of renal denervation on end organ damage in hypertensive patients. Eur J Prev Cardiol. 2015;22:558‐567. [DOI] [PubMed] [Google Scholar]
- 41. Reference Values for Arterial Stiffness Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: establishing normal and reference values. Eur Heart J. 2010;31:2338‐2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wassertheurer S, Mayer C, Breitenecker C. Modeling arterial and left ventricular coupling for non‐invasive measurements. Simulat Model Pract Theor. 2008;16:988‐997. [Google Scholar]


