Abstract
The purpose of this review was to identify, summarize, and critically appraise studies on dietary salt relating to health outcomes that were published from December 2015 to March 2016. The search strategy was adapted from a previous systematic review on dietary salt and health. Overall, 13 studies were included in the review: one study assessed cardiovascular events, nine studies assessed prevalence or incidence of blood pressure or hypertension, one study assessed kidney disease, and two studies assessed other health outcomes (obesity and nonalcoholic fatty liver disease). Four studies were selected for detailed appraisal and commentary. One study met the minimum methodologic criteria and found an increased risk associated with lower sodium intake in patients with heart failure. All other studies identified in this review demonstrated positive associations between dietary salt and adverse health outcomes.
Keywords: diet/nutrition/hypertension, hypertension—general, outcomes of care, sodium
1. Introduction
Excess salt (sodium) consumption is associated with many adverse health effects, including hypertension and cardiovascular mortality.1, 2 Based on the evidence from systematic reviews assessing dietary sodium reduction, the World Health Organization (WHO) recommends a sodium intake of <2 g/d (equivalent to 5 g/d of salt) in adults, with lower amounts for children based on their energy requirements relative to those of adults.3, 4 All WHO Member States have agreed on a target of reducing dietary sodium intake by 30% by 2025.5
Regularly updated reviews and critical appraisals of identified studies relating to health outcomes are published in the Journal of Clinical Hypertension, alternating with reviews of studies relating to salt reduction implementation programs. The last review of salt and health outcomes covered studies published between August and November 2015.6 The current review identifies and appraises the literature on salt and health outcomes published between December 2015 and March 2016.
2. Methodology
A detailed description of the methodological approach used to identify and evaluate the literature in this review has been previously published.7 In summary, articles were identified on a weekly basis through a MEDLINE search strategy.7 Studies examining the effects of salt on health outcomes, including studies of participants with any comorbidity (with the exception of acute illness), that were published from December 1, 2015 to March 31, 2016, were included in this review.
All included studies were assessed for risk of bias by two independent reviewers. Randomized controlled trials (RCTs) were assessed using the Cochrane risk of bias tool.8 Observational, nonrandomized studies were assessed using a modified Cochrane risk of bias tool.9 For systematic reviews and meta‐analyses, the c tool was applied.10
We identified the subset of included studies that met previously established minimum methodologic criteria for clinical and population studies on dietary salt11 (Box). Detailed appraisals and written commentary were performed for these studies. Other studies were then selected for detailed review based on two independent reviewers identifying them either as potentially high impact, controversial, or important in terms of better understanding the evidence for salt reduction.
Box 1. Minimum methodologic criteria for clinical and population studies on dietary salt.
1.
-
1
For blood pressure outcome:
RCTs or systematic reviews of RCTs;
Minimum intervention period of 4 weeks;
Sodium intake intervention was composed of at least one group receiving decreased sodium intake compared with a control group, with difference of at least 2.3 g of salt (sodium 920 mg or 40 mmol) per day between the intervention and the control; and
Sodium intake measured by 24‐hour urinary excretion.
-
2
For all other outcomes:
Prospective cohort studies, RCTs, prospective cohorts, or systematic reviews evaluating the association between sodium intake and any health outcome other than blood pressure;
At least 1 year duration; and
Sodium intake measured for at least 24 hours using any method.
3. Results
Of 1892 citations identified by the search, 13 studies from eight countries met inclusion criteria (Figure): 11 observational studies12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22 and two meta‐analyses.23, 24 The primary outcomes of these 13 studies included mortality and cardiovascular events,12 blood pressure (BP) or diagnosis of hypertension,13, 14, 15, 16, 17, 18, 19, 20, 23 kidney disease,24 nonalcoholic fatty liver disease21 and obesity.22 Summary descriptions of the 13 included studies are listed in the Table and risk of bias assessments for all included studies are included in Appendix S1.
Figure 1.
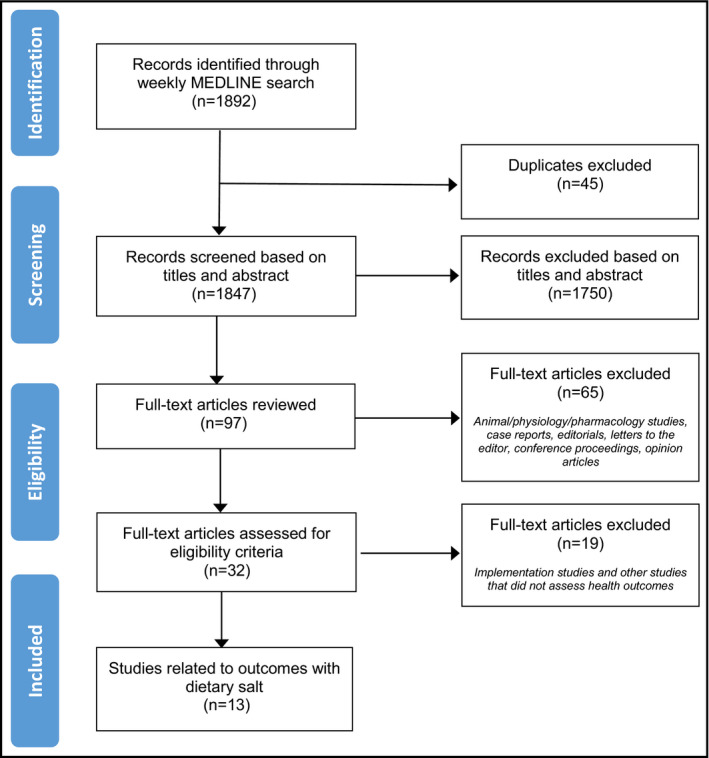
Study flow diagram for studies identified from December 2015 to March 2016
Table 1.
Description of Included Studies
| Study (Country) | Study Design | Participants | Study Duration | Dietary Salt “Dose” (Actual Mean Intake per d)a | Method of Sodium Intake Measurement | Outcomes | Results |
|---|---|---|---|---|---|---|---|
| Cardiovascular major morbid events | |||||||
| Doukky et al. (US)12 | Prospective cohort | N=833 HF patients overall, 42% male, mean age 63 y; N=260 in propensity‐matched analysis by sodium restriction; 45% male; mean age 64 y | 36 mo |
Overall: 8.3 g salt/d (range: 3.1–39.2 g salt/d). Mean salt intake for sodium‐restricted and sodium‐unrestricted groups not reported |
57‐item FFQ |
Primary outcome: composite of death and HF hospitalization; secondary outcomes: cardiac death and HF hospitalization |
Sodium restriction was associated with an increased risk of death or HF hospitalization (HR 1.85; 95% CI 1.21–2.84) and HF hospitalization (HR, 1.82; 95% CI, 1.11–2.96). |
| Blood Pressure | |||||||
| Correia‐Costa et al. (Portugal)13 | Cross‐sectional | N=298 children aged 8–9 y (sampled from “Generation XXI, Porto, Portugal” cohort); 53% male; mean age 8.8 y | Cross‐sectional |
Total sample: 6.5 g salt/d Boys: 6.8 g salt/d Girls: 6.1 g salt/d |
24‐h urine sodium excretion | 24‐h ABPM | Every 1 g of salt intake was associated with an increase in daytime SBP by 0.56 mm Hg (95% CI, 0.11–1.01) in boys. No significant association in girls. |
| Ito et al. (Japan)14 | Cross‐sectional | N=1501 adults (Shimane CoHRE study); 38% male; age 40–74 y. n=1005 without antihypertensive therapy; n=491 treated with antihypertensive therapy | Cross‐sectional |
Taking diuretics: 10.5 g salt/d Without diuretics: 9.6 g salt/d |
Spot urine sodium, estimated by Tanaka equation | SBP and DBP, using automatic sphygmomanometer | Sodium intake was positively associated with SBP in untreated (β=1.45, 95% CI, 0.93–1.96) and treated patients (β=0.75; 95% CI, 0.21–1.29). Sodium intake was positively associated with pulse pressure. |
| Iuchi et al. (Japan)15 | Nonrandomized intervention study (pre‐/post‐study) | N=10 adults with type 2 diabetes and hypertension not treated with antihypertensive agents (7DACS); 70% male; mean age 60 y | 7 d |
Day 1: 9.8 g salt/d Day 7: 6.8 g salt/d |
Urine sodium from casual urine samples, collected daily for 7 d, estimated using Tanaka equation | BP variability, using ABPM; body weight; plasma glucose and continuous glucose monitoring; ratio of low‐ to high‐frequency power; glycated hemoglobin, cholesterol, creatinine, plasma, and urinary C‐peptide | Short‐term salt restriction was not associated with significant change in SBP variability. There was a reduction in median SBP (–15 mm Hg, IQR −24 to −13 mm Hg). |
| Krupp et al. (Germany)16 | Prospective cohort | N=206 young adults (DONALD study); 52% male; mean age 12 y during data collection at adolescence | Mean follow‐up not reported; study followed participants from infancy to young adulthood (age 18–25 y) |
Boys: 6.7 g salt/d Girls: 6.1 g salt/d |
Three 24‐h urine collections and three 3‐d food records (1 each per annum) | Office BP in young adulthood, using random‐zero or standard mercury sphygmomanometer | Sodium intake was associated with SBP in young adult men only (adjusted β coefficient 0.10 mm Hg per 1 mmol NaCl; 95% CI, 0.03–0.18), and was not associated with DBP. |
| Noh et al. (Korea)17 | Cross‐sectional | N=24 096 adults (KNHANES 2007–2012); high BP group: 66.9% male, mean age 49.9 y; normal BP group: 48.1% male, mean age 40.8 y | Cross‐sectional |
High BP group: 2585 mg sodium/1000 kcal Normal BP group: 2563 mg sodium/1000 kcal |
24‐h dietary recall; high‐ and low‐sodium and potassium intake defined as above and below medians | Office BP, using a mercury sphygmomanometer; hypertension prevalence, defined as SBP ≥140 mm Hg or DBP ≥90 mm Hg | Low‐sodium:low‐potassium intake ratio and high‐sodium:low‐potassium intake ratio were associated with increased risk of high BP compared with low‐sodium:high‐potassium intake ratio (adjusted OR, 1.19; 95% CI, 1.01–1.40 and OR, 1.21; 95% CI, 1.02–1.44, respectively). |
| Thuesen et al. (Denmark)18 | Cross‐sectional | N=3294 adults (Health 2006 study); 44.8% male; mean age 49.4 y | Cross‐sectional | Total sample: 8.99 g/d of salt | Random spot urine sodium, estimated using the Danish model formula | Office BP and fasting serum lipids | Estimated salt intake was positively associated with BP. Association was attenuated by adjustment for obesity (β 0.58 mm Hg/g salt; 95% CI, 0.20–0.97 for SBP, and 0.25 mm Hg/g salt 95% CI, 0.01–0.49 for DBP). |
| Umesawa et al. (Japan)19 | Prospective cohort study | N=889 normotensive adults from Kyowa (CIRCS study); 33% male; mean age 75.3 y | Mean follow‐up: 5.8 y |
Median urine sodium concentration: Quartile 1: 66 mmol/L Quartile 2: 107 mmol/L Quartile 3: 145 mmol/L Quartile 4: 193 mmol/L |
Random spot urine sodium concentration at baseline | BP change from baseline, measured by sphygmomanometer | High urine sodium concentrations were associated with subsequent SBP increases (+7.0 mm Hg in highest quartile; +4.2 mm Hg in lowest quartile, P=.047) in patients with BMI <25, but not overweight patients. |
| Wang et al. (China)23 | Meta‐analysis of quasi‐experimental studies and RCTs |
N=3153 from 6 salt‐restriction studies N=3715 from 4 salt‐restriction spoon studies N=1730 from 4 salt‐substitute studies |
Range: 1–8 wk for salt‐restriction studies Range: 3–12 mo for salt‐restriction spoon studies Range: 12–24 mo for salt‐substitute studies |
For salt‐restriction studies: 1.8–7.7 g salt/d For salt‐restriction spoon studies: 5.3–11.2 g salt/d For salt‐substitute studies: 7.0–10.2 g salt/d |
24‐h urine sodium excretion for salt‐restriction studies 24‐h urine sodium or salt weighing for salt‐restriction spoon studies First morning urine collection for salt‐substitute studies |
BP; salt intake |
Salt restriction was associated with 0.94 mm Hg/0.62 mm Hg reduction per 1 g of dietary salt restriction in hypertensive individuals. Use of salt‐restriction spoons with education was associated with a 1.46 g/d reduction in salt intake. Use of salt‐substitute reduced BP (–4.2/–0.6 mm Hg) in hypertensive individuals. |
| Yokokawa et al. (Thailand)20 | Cross‐sectional | N=793 adults at high risk for cardiovascular disease (baseline data from RESIP‐CVD study); 51.8% male; mean age 66.5 y | Cross‐sectional |
Total sample: 9.9 g salt/d Low salt intake (<10 g/d salt group): 8.3 g salt/d High salt intake (≥10.0 g/d salt group): 11.9 g salt/d |
Overnight urine sodium (averaged over 3 consecutive d) | BP, measured with oscillometric device; presence of cardiovascular risk factors | Higher salt intake was associated with greater use of antihypertensive medications, family history of hypertension, and less awareness of high salt intake, compared with lower salt intake. |
| Kidney disease | |||||||
| Liu et al. (China)24 | Meta‐analysis of observational studies | N=5638 from 9 studies (6 prospective, 3 cross‐sectional) | Range: 11 mo to 10 y for prospective studies; N/A for cross‐sectional studies | Only highest category of salt intake was extracted and this was variably reported | Various: food intake questionnaire, 24‐h recall, 24‐h urine sodium | CKD (defined as eGFR <60 mL/min/1.73 m2 or eGFR ≥60 mL/min/1.73 m2 with albuminuria) | Compared with the lowest sodium intake, highest sodium intake level was associated with an increased risk of CKD (pooled relative risk, 1.09; 95% CI, 1.01–1.19). |
| Other health outcomes | |||||||
| Huh et al. (Korea)21 | Cross‐sectional | N=27 433 from South Korea (KNHANES 2008–2010); 42.9% male; mean age 51.5 y | Cross‐sectional |
Lowest tertile: 6.1 g salt/d Middle tertile: 8.2 g salt/d Highest tertile: 10.8 g salt/d |
Random spot urine sodium, estimated using Tanaka equation |
NAFLD, assessed by HSI and FLI prediction scores; Hepatic fibrosis, assessed by BARD and FIB‐4 score in patients with FLI ≥60 |
High sodium intake was associated with an increased risk of NAFLD (adjusted OR, 1.39; 95% CI, 1.26–1.55 for HSI; OR, 1.75; 95% CI, 1.39–2.20 for FLI). |
| Lee et al. (Korea)22 | Cross‐sectional | N=1467 children (KNHANES 2010–2011); 57.4% male; mean age 13.4 y | Cross‐sectional |
Overall: 10.8 g salt/d Boys: 12.0 g salt/d Girls: 9.1 g salt/d |
24‐h dietary recall; UNa/Cr measured by single spot urine |
Overweight/obesity, by BMI; Central adiposity, by waist circumference; % body fat, by dual‐energy x‐ray absorptiometry |
Sodium intake by recall was associated with BMI (OR for obesity 2.79; 95% CI, 1.66–4.68) and central adiposity (OR, 2.14; 95% CI, 1.25–3.67), but not % body fat. UNa/Cr was associated with obesity, central adiposity, and % body fat. |
Abbreviations: ABPM, ambulatory blood pressure monitoring; BMI, body mass index; BP, blood pressure; CIRCS, Circulatory Risk in Communities Study; CKD, chronic kidney disease; CI, confidence interval; DBP, diastolic blood pressure; DONALD, Dortmund Nutritional and Anthropometric Longitudinally Designed Study; FFQ, food frequency questionnaire; FIB‐4, Fibrosis‐4 index; FLI, fatty liver index; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, hazard ratio; HSI, hepatic steatosis index; IQR, interquartile range; KNHANES, Korea National Health and Nutrition Examination Survey; NaCl, sodium chloride; N/A, not applicable; NAFLD, nonalcoholic fatty liver disease; RCT, randomized controlled trial; RESIP‐CVD, Reducing Salt Intake for Prevention of Cardiovascular Diseases in High‐Risk Patients by Advanced Health Education Intervention study; SBP, systolic blood pressure; Shimane CoHRE, Center for the Community‐based Health Research and Education of Shimane University; UNa/Cr, urine sodium‐to‐creatinine ratio; US, United States; 7DACS, 7‐day Ambulatory Blood Pressure Monitoring and Continuous Glucose Monitoring Study.
Unless otherwise stated, units are in g per day of salt (sodium chloride) intake. To convert to mg per day of sodium, multiply by 400. To convert to mmol per day of sodium, multiply by 17.4.
Only one of the 13 studies met the minimum methodological criteria for clinical and population studies on dietary salt for detailed review: Doukky and colleagues12 performed a propensity‐matched cohort study assessing the effect of sodium intake on death and heart failure (HF) hospitalizations. Krupp and colleagues,16 which assessed the effect of sodium intake in adolescence on BP in young adulthood, did not meet minimum methodologic criteria due to its observational study design, but was selected for review because of the paucity of studies in this population. The two meta‐analyses (Wang and colleagues,23 a meta‐analysis of RCTs and quasi‐experimental studies assessing the effect of dietary salt reduction strategies on BP in Chinese adults, and Liu and colleagues24 a meta‐analysis of observational studies assessing the association between salt intake and risk of chronic kidney disease [CKD]) did not meet the minimum methodologic criteria due to their inclusion of studies with cross‐sectional design or spot urine to estimate sodium intake, but were also selected for detailed review because of the importance of being able to interpret their contribution to the evidence base.
3.1. Studies that met the minimum methodological criteria
1. What is the association between sodium intake and mortality and cardiovascular outcomes in patients with heart failure?
Doukky R, Avery E, Mangla A, et al. Impact of dietary sodium restriction on heart failure outcomes. JACC Heart Fail. 2016:4:24–35.
Design: Longitudinal cohort study.
Setting: Secondary analysis of RCT data (Heart Failure Adherence and Retention Trial [HART]) that evaluated the effects of a self‐care management behavioral intervention vs an education control group on clinical outcomes among heart failure (HF) patients recruited from 10 centers in Chicago, Illinois.
Median follow‐up: 36 months.
Participants: The overall cohort included 833 patients with reduced or preserved ejection fraction (n=145 with sodium intake <6.2 g salt per day [<2500 mg sodium per day], and n=688 with sodium intake ≥6.2 g salt per day [≥2500 mg sodium per day]). Exclusion criteria: uncertain 12‐month prognosis, asymptomatic patients unlikely to have a primary end point, logistical issues (ie, language), unstable cardiovascular disease, patients unlikely to undergo or benefit from behavioral treatment (ie, psychological disorders), symptoms that may be eliminated by surgery, and patients who were unwilling to make lifestyle changes. The propensity‐matched cohort included 260 patients who were matched according to dietary sodium intake.
Exposure: Sodium intake as measured by the average of four measures (at baseline and at years 1, 2, and 3) of a 57‐item food frequency questionnaire (FFQ) that assessed sources of dietary sodium in the past week. Sodium intake was calculated based on FFQ responses, plus an additional 3.1 g salt per day (1250 mg sodium per day), which the authors considered the amount derived from food items commonly found in the American diet.
Outcomes: The primary outcome was a composite of death or HF hospitalization. Secondary outcomes were cardiac death and HF hospitalization. Outcomes were determined by a blinded adjudication committee using predefined criteria.
Risk of bias:
Sampling: High risk.
Representativeness: High risk.
Reliability/validity of exposure: High risk.
Reliability/validity of outcome: Low risk.
Blinding of outcome assessment: Low risk.
Risk of selective outcome reporting: Low risk.
Confounding: Low risk.
Sources of funding: National Institutes of Health.
Summary of results: For the entire cohort, mean salt intake was 8.3 g/d (3336 mg/d sodium; range: 1250–15 678 mg/d). In the propensity‐matched cohort, lower sodium intake was associated with an increased risk of death or HF hospitalization (hazard ratio [HR], 1.85; 95% confidence interval [CI], 1.21–2.84) and HF hospitalization (HR, 1.82; 95% CI, 1.11–2.96) compared with higher sodium intake. There was no association between dietary sodium and all‐cause or cardiac death. Findings were similar for the entire cohort after adjustment for propensity scores. In patients who were not taking an angiotensin‐converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB), lower sodium intake was associated with a significantly higher risk of death or HF hospitalization (HR, 5.78; 95% CI, 1.93–17.27), but there was no increase in risk in the subgroup treated with ACEIs/ARBs.
Comment: There have been conflicting findings among observational studies and RCTs assessing the association between dietary sodium and clinical outcomes in HF patients. A strength of this study was the use of longitudinal data using propensity matching. Even after controlling for variables that had a >10% absolute difference after matching, the association between sodium intake and outcomes did not change. However, there are several limitations to consider. First, the study population was highly selected, which limits generalizability of the findings. For example, the patients were chosen to participate in a 1‐year behavioral intervention RCT that included 18 two‐hour small group meetings. The authors noted that those without sodium intake data, and excluded from the analysis, had low adherence to HF therapies and had poor outcomes. Second, the observational design cannot prove causality and there is also a risk of reverse causality (ie, lower sodium intake may reflect unmeasured factors that increase risk of death or hospitalization). Despite propensity matching, there may have still been imbalance in diabetes, lung disease, and stroke between groups. Finally, dietary sodium was estimated from a 57‐item FFQ administered at several time points. This FFQ included an assumed sodium intake of 3.1 g/d of salt (1250 mg/d of sodium) for each participant, regardless of actual food intake. This underlying assumption may have been a source of error. In general, FFQs generally underestimate dietary sodium25, 26, 27, 28 and there are no known studies that show the FFQ used produces a valid estimate of sodium. These limitations may have resulted in misclassification of patients into high‐ and low‐sodium intake groups. Overreporting or underreporting of dietary intakes were also not assessed, which resulted in the inclusion of a very wide range of salt intake. As noted by the authors, several covariates were not captured, including energy intake.
3.2. Studies that did not meet minimum methodological criteria
1. What is the association between salt intake during adolescence and blood pressure in young adulthood?
Krupp D, Shi L, Egert S, et al. Prospective relevance of fruit and vegetable consumption and salt intake during adolescence for blood pressure in young adulthood. Eur J Nutr. 2015;54:1269–1279.
Design: Longitudinal cohort study.
Setting: Dortmund Nutritional and Anthropometric Longitudinally Designed Study (DONALD), Germany.
Median follow‐up: Not reported; duration from infancy up to 18 to 25 years.
Participants: 206 young adults, recruited as healthy babies (age 3–6 months), with available sodium intake data during adolescence and BP/anthropometric data during young adulthood.Exclusion criteria: Preterm birth, missing or implausible data for BP, urine samples, dietary records, birth weight, or gestational age.
Exposure: Sodium intake as measured by the average of three or more annual assessments of 24‐hour urinary sodium excretion (single measure) and three or more annual assessments of nutrient and food intake estimates obtained from a 3‐day food record, between 11 and 16 years of age.
Outcomes: Systolic and diastolic BP in young adulthood, age 18 to 25 years (mean of two seated BP readings at each assessment, which occurred every 2 years).
Risk of bias:
Sampling: High risk.
Representativeness: High risk.
Reliability/validity of exposure: Low risk.
Reliability/validity of outcome: Low risk.
Blinding of outcome assessment: Low risk.
Risk of selective outcome reporting: Low risk.
Confounding: High risk.
Sources of funding: Ministry of Science and Research of North Rhine Westphalia, Germany; Federal Agency for Agriculture and Food.
Summary of results: Higher salt intake during adolescence was related to higher systolic BP in young adulthood in men (adjusted β coefficient 0.10 mm Hg per 1 mmol NaCl; 95% CI, 0.03–0.18), but not in women. Among boys, there was a 7.5‐mm Hg difference in BP between the lowest and highest quartiles. Adjustment for body mass index did not attenuate the relationship between sodium and BP. Higher fruit and vegetable intake was related to lower systolic BP in young adult women (100 g/d higher fruit and vegetable consumption was associated with 0.9 mm Hg lower systolic BP; 95% CI, 0.1–1.7) but not in men.
Comment: In addition to the longitudinal study design, a strength of this study was repeated measures of dietary sodium consumption using 24‐hour urine collections. However, only 48% of participants provided dietary and urinary data during the follow‐up period in young adulthood, which may have produced biased findings and limited their generalizability. Other aspects of the study may further limit generalizability. For example, infants recruited into the DONALD study belonged to families who had greater educational attainment and higher socioeconomic status as compared with the general German population.29 In addition, the negative BP standard deviation scores demonstrated that adolescent BP values were lower in the DONALD cohort compared with the German reference population. As the authors noted, physical activity was not included in the regression models.
2. What is the impact of dietary salt reduction and salt substitution strategies on blood pressure in Chinese adults?
Wang M, Moran AE, Liu J, et al. A meta‐analysis of effect of dietary salt restriction on blood pressure in Chinese adults. Glob Heart. 2015;10:291–299.e6.
Design: Meta‐analyses of quasi‐experimental (preintervention and postintervention) studies and RCTs.
Methods:
Data sources: MEDLINE and China National Knowledge Infrastructure (to July 2014), international conference reports, and reference lists from identified articles.
Study selection and assessment: RCTs or quasi‐experimental studies of at least 1 week's duration conducted in Chinese adults 35 years and older, evaluating the effect of: (1) salt restriction on BP; (2) the use of cooking salt‐restriction spoons (standard‐sized spoons to be used in adding salt to food during preparation, coupled with education on salt reduction) on salt intake; and (3) the use of salt substitutes on BP. Six salt restriction studies (n=3153; all preintervention/postintervention studies; duration 1–8 weeks), four salt‐restriction spoon studies (n=3715; two pre‐/post‐studies, two RCTs; duration 3–12 months), and four salt substitute studies (n=1730; all RCTs; duration 12–24 months) met inclusion criteria. Risk of bias assessments were conducted according to study population, blinding, use of a control group, and method used to estimate salt intake. Potential sources of heterogeneity were explored using meta‐regression.
Method of sodium intake measurement: Studies of salt restriction used 24‐hour urinary sodium excretion; studies evaluating salt‐restriction spoons used the weighing of salt before cooking or 24‐hour urinary sodium excretion; salt substitute studies used first morning urine samples.
Outcomes: BP and salt intake.
Subgroup analyses: Hypertensive status (hypertensive vs combination of hypertensive and normotensive participants), age, and sex.
-
Risk of bias:
-
○
A priori design: No.
-
○
Duplicate study selection/data extraction: Yes.
-
○
Comprehensive literature search: Yes.
-
○
Status of publication used as an inclusion criterion: No.
-
○
List of studies included: Yes.
-
○
Characteristics of included studies provided: Yes.
-
○
Quality of studies assessed and documented: Yes.
-
○
Quality of included studies used appropriately in formulating conclusions: No.
-
○
Methods to combine findings appropriate: Yes.
-
○
Publication bias assessed: Yes.
-
○
Conflict of interest: None declared.
-
○
Summary of results: This meta‐analysis found that for each 1‐g reduction in salt intake, there was an overall reduction of 0.58 mm Hg (95% CI, 0.55–0.60 mm Hg) for systolic BP, and a reduction of 0.30 mm Hg (95% CI, 0.27–0.31 mm Hg) for diastolic BP. In the subgroup with hypertension, there was a reduction of 0.94 mm Hg (95% CI, 0.69–1.03 mm Hg) for systolic BP and a reduction of 0.62 mm Hg (0.38–0.71 mm Hg) for diastolic BP. Interventions examining the use of salt‐restriction spoons were associated with a reduction in salt intake by 1.46 g (95% CI, 0.52–2.40 g) per day after 3 to 12 months of follow‐up. The use of salt substitutes reduced systolic BP by 4.2 mm Hg (95% CI, 1.3–7.0 mm Hg) in individuals with hypertension, but there was no statistically significant difference in diastolic BP.
Comment: This meta‐analysis by Wang and colleagues is consistent with and extends the findings of previous studies that demonstrate BP reduction with salt restriction and salt substitution in Chinese adults, particularly among those with hypertension. Overall, this study's search strategy appears to have likely included all relevant studies to date. Study selection and data extraction were performed in duplicate. A large variety of interventions and salt reduction strategies were examined. Notably, many of the included studies addressed culturally tailored interventions (eg, use of salt‐restriction spoons) that may be especially relevant to Chinese populations where, in contrast to many Western societies, a large source of dietary salt is from home cooking. Still, several weaknesses should be acknowledged. The number of published Chinese studies are relatively few. Consequently, the authors were unable to examine the dose‐response relationship between salt reduction and BP lowering. Further, this meta‐analysis may have been underpowered to detect a significant difference in BP pressure with the use of salt substitutes in normotensive populations. The majority of studies that assessed the effect of dietary salt reduction on BP were only 1 week in duration and all were pre‐/post‐studies with no independent control group. The corresponding meta‐analysis included studies that contributed to more than one point estimate. For example, data from 487 patients in the GenSalt study were included twice: at the initial study and again at the follow‐up study 4.5 years later.30 The authors did not account for these correlated comparisons. Also, noted by the authors, some studies evaluating salt substitutes estimated salt intake with spot urine collection (as opposed to multiple 24‐hour urine collections), and many other studies examining salt‐restriction spoons did not have independent control groups. Thus, unmeasured confounding may have been present.
3. What is the association between sodium intake and risk of chronic kidney disease?
Liu N, Sun W, Xing Z, et al. Association between sodium intakes with the risk of chronic kidney disease: evidence from a meta‐analysis. Int J Clin Exp Med. 2015;8:20939–20945.
Design: Meta‐analysis of observational studies.
Methods:
Data sources: PubMed (MEDLINE) and Web of Science, through December 31, 2014, and reference lists from retrieved articles.
Study selection and assessment: Prospective cohort, case‐control or cross‐sectional studies assessing the association between sodium intake and CKD and reporting adjusted relative risk with 95% CI. Nine studies (6 prospective, 3 cross‐sectional) met inclusion criteria (N=40 934 adults from seven countries, mean age range 39–68 years). Follow‐up in prospective studies ranged from 11 months to 10 years. Studies were pooled using random‐effects models. Study quality was assessed with a numerical score that was not described in the publication. Meta‐regression and subgroup analyses were conducted to explore heterogeneity.
Method of sodium intake measurement: 24‐hour urine sodium (7 studies); 24‐hour dietary recall (2 studies).
Outcomes: CKD, defined as estimated glomerular filtration rate <60 mL/min/1.73 m2 or estimated glomerular filtration rate ≥60 mL/min/1.73 m2 with albuminuria.
Subgroup analyses: Study design, geographic location, measures of sodium intake.
-
Risk of bias:
-
○
A priori design: No.
-
○
Duplicate study selection/data extraction: Yes.
-
○
Comprehensive literature search: Yes.
-
○
Status of publication used as an inclusion criterion: No.
-
○
List of studies included: Yes for included studies; No for excluded studies.
-
○
Characteristics of included studies provided: Yes.
-
○
Quality of studies assessed and documented: Yes, but quality score was not described.
-
○
Quality of included studies used appropriately in formulating conclusions: Yes.
-
○
Methods to combine findings appropriate: Yes.
-
○
Publication bias assessed: Yes.
-
○
Conflict of interest: None declared.
-
○
Summary of results: The pooled results of the meta‐analysis demonstrated that the highest sodium intake level vs the lowest level was significantly associated with risk of CKD (pooled relative risk, 1.088; 95% CI, 1.009–1.193). There was significant heterogeneity between studies (I 2=78.1%), although meta‐regression did not reveal the cause of heterogeneity. Subgroup analysis by study design showed a statistically significant effect in prospective studies but not in cross‐sectional studies and in Europe but not in the United States.
Comment: The meta‐analysis included a large number of patients, and for each study, data were extracted from the models adjusting for the most potential confounders. However, it is possible that some studies did not control for potential important confounders, such as ACEI/ARB use. The meta‐analysis compared highest vs lowest sodium intake but could not quantify a dose‐response relationship because of limited data. Between‐study heterogeneity persisted in the meta‐regression with publication year, country, study design, measures of sodium intake, and number of CKD cases as covariates. However, the authors did not account for patient demographics in the meta‐regression, which likely contributed to heterogeneity in effect size. For example, all patients in the included study by Fan and colleagues31 had CKD at baseline (mean estimated glomerular filtration rate 32.5 mL/min/1.73 m2). Furthermore, this study assessed the outcome of kidney failure requiring dialysis or transplantation.31 Because the study by Fan and colleagues differed in patient population and outcome definition from the other studies, it should not have been included in the meta‐analysis. Variations in the ranges of highest and lowest categories of sodium intake between studies also likely contributed to heterogeneity. Although most studies used 24‐hour urine sodium to quantify salt intake, two studies used 24‐hour dietary recall, a method subject to recall bias and underreporting. These two studies, which also used a cross‐sectional design and were performed in the United States, did not demonstrate a significant relative risk in contrast to the other studies. These differences are likely a reflection of study methodology and design, rather than differences between United States and Europe.
4. Discussion
This review identified 13 studies assessing the association of dietary sodium and health outcomes. One study met minimum methodological criteria; this study found an increased risk associated with lower sodium intake in patients with HF.12 All other studies included in this review demonstrated positive associations between dietary salt and adverse health outcomes including BP outcomes, kidney disease, obesity, and nonalcoholic fatty liver disease.
The minimum methodological criteria were established to ensure that only robust research studies are used to inform evidence‐based guidelines for sodium reduction. Like previous reviews, the majority of studies in this review did not meet the standards. While this means they should not be used to inform national or international guidelines, they still add to the evidence on the benefits for salt reduction programs. The only study that did meet the minimum methodologic standards was for HF patients and showed an increased risk associated with sodium restriction. In the past two annual reviews of studies that met minimum methodological criteria, all studies found health benefits with salt reduction.11 Dietary sodium restriction in HF is the primary nutritional recommendation to control the signs and symptoms associated with hypervolemia caused by sodium retention.32, 33 However, studies examining dietary sodium in the HF setting have demonstrated conflicting results, which may be based on variation in research design and methods. RCTs examining clinical outcomes in the HF population exhibit confounding effects from cointerventions, including high‐dose loop diuretics and strict fluid restriction, making the independent effects of sodium restriction impossible to examine.34, 35, 36 By design, observational studies cannot prove causality and may be prone to reverse causality, and many of these studies may be underpowered. The method of assessing exposure to dietary sodium should also be considered, as urinary sodium excretion is strongly influenced by the use of diuretics and the presence of renal impairment,37 and some survey methods such as the FFQ used by Doukky and colleagues may not sufficiently characterize sodium intake because of their limited precision in estimating sodium intake.25, 26, 27, 28 Small well‐designed, randomized controlled physiologic studies have identified short‐term neurohormonal activation caused by sodium restriction38, 39; however, it is unknown how these surrogate outcome findings relate to clinical outcomes. Sodium and water balance in people with HF is usually maintained using loop diuretics, which are likely to have a much more substantive impact on vascular volume than efforts to reduce dietary sodium. Hence, in achieving euvolemia, there may be more benefit in maintaining a consistent day‐to‐day level of sodium intake vs lowering dietary sodium to levels where patients may not be able to consistently adhere. As noted by Doukky and colleagues, high‐quality RCTs are needed to elucidate the impact of dietary sodium on clinical outcomes in HF, for which two known trials are currently recruiting (the Study of Dietary Intervention Under 100 mmol in Heart Failure [SODIUM‐HF] and the Geriatric Out of Hospital Randomized Meal Trial in Heart Failure [GOURMET‐HF]).40, 41
The longitudinal cohort study by Krupp and colleagues16 demonstrated that high sodium intake during adolescence was associated with significantly higher BP levels by age 18 to 25 years in men, but not women, and suggested that the effect of adolescent dietary habits on early adulthood BP may be sex‐specific. Although this study has limited generalizability because of a small sample size, especially with respect to the subgroup analysis on sex, the validity of the study is strengthened by the use of annual 24‐hour urine samples to assess sodium intake.
While they did not meet the minimum methodological criteria, the two meta‐analyses were also assessed in this review. One demonstrated beneficial effects of salt restriction on BP23 and the other showed that higher sodium intake was associated with greater risk of CKD.24 Although meta‐analyses are generally considered to provide stronger levels of evidence compared with primary studies, both of these meta‐analyses had major methodological weaknesses that increased their risk of bias. In the meta‐analysis by Wang and colleagues, which focused on adults in China, one of the important identified limitations was the weak study design for included studies (short‐term self‐controlled studies for trials assessing dietary salt reduction on BP). Even so, the overall study provides evidence consistent with other studies: that salt restriction and the use of culturally tailored salt reduction strategies are associated with significant reductions in BP, particularly for individuals with hypertension. The meta‐analysis by Liu and colleagues of observational studies lacked detailed descriptions of included studies and reasons for excluding studies, and some studies should not have been pooled due to differences in study design and patient population. While this meta‐analysis suggests that high salt intake may be associated with a greater risk of CKD, its clinical applicability is limited because a dose‐response relationship could not be assessed.
5. Conclusions
The findings from this review complement previous reviews that support the need to reduce salt. Twelve of 13 identified studies showed benefits associated with salt reduction. One study met the minimum methodological criteria and showed harm with salt reduction, although this study was in HF patients, with limited generalizability. The review further strengthens the evidence in support of existing national and international recommendations for national population‐wide programs to reduce salt as well as highlights the importance of better designed trials to understand salt restriction in HF patients.
Conflict of Interest
NC is a member of World Action on Salt and Health (a dietary salt reduction organization) but has no financial interests to declare. JA and AAL have no conflicts of interest to declare. MMYW is a research consultant with Arbor Research Collaborative for Health. JW is the Director of the World Health Organization (WHO) Collaborating Centre on Population Salt Reduction.
Supporting information
Acknowledgments
The process to provide regular updates on the science of sodium is supported by the World Hypertension League, the WHO Collaborating Centre on Population Salt Reduction (George Institute for Global Health), Pan American Health Organization/WHO Technical Advisory Group on Cardiovascular Disease Prevention through Dietary Sodium, and World Action on Salt and Health and the HSF CIHR Chair in Hypertension Prevention and Control.
Wong MMY, Arcand JA, Leung AA, Thout RS, Campbell NRC, Webster J. The science of salt: A regularly updated systematic review of salt and health outcomes (December 2015–March 2016). J Clin Hypertens. 2017. 19:322‐332. doi: 10.1111/jch.12970.
References
- 1. Mozaffarian D, Fahimi S, Singh GM, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624–634. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . A global brief on hypertension. 2013. http://ish-world.com/downloads/pdf/global_brief_hypertension.pdf. Accessed October 31, 2016.
- 3. Aburto NJ, Ziolkovska A, Hooper L, et al. Effect of lower sodium intake on health: systematic review and meta‐analyses. BMJ. 2013;346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Guideline: Sodium intake for adults and children. 2012. http://www.who.int/nutrition/publications/guidelines/sodium_intake_printversion.pdf. Accessed October 31, 2016. [PubMed]
- 5. World Health Organization . Report of the Formal Meeting of Member States to conclude the work on the comprehensive global monitoring framework, including indicators, and a set of voluntary global targets for the prevention and control of noncommunicable diseases. Report, 1–6. 2012. Geneva, Switzerland, World Health Organization.
- 6. Wong MMY, Arcand J, Leung AA, et al. The science of salt: a regularly updated systematic review of salt and health outcomes (August to November 2015). J Clin Hypertens (Greenwich). 2015;2016:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arcand J, Webster J, Johnson C, et al. Announcing “up to date in the science of sodium.” J Clin Hypertens (Greenwich). 2016;18:85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011: http://www.cochrane-handbook.org. [Google Scholar]
- 9. McLaren L, Sumar N, Barberio AM, et al. Population‐level interventions in government jurisdictions for dietary sodium reduction. Cochrane Database Syst Rev. 2016;(9):CD010166. DOI: 10.1002/14651858.CD010166.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson C, Raj TS, Trieu K, et al. The science of salt: a systematic review of quality clinical salt studies 2014 to 2015. J Clin Hypertens (Greenwich). 2016;18:832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doukky R, Avery E, Mangla A, et al. Impact of dietary sodium restriction on heart failure outcomes. JACC Heart Fail. 2016;4:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Correia‐Costa L, Cosme D, Nogueira‐Silva L, et al. Gender and obesity modify the impact of salt intake on blood pressure in children. Pediatr Nephrol. 2016;31:279–288. [DOI] [PubMed] [Google Scholar]
- 14. Ito T, Takeda M, Hamano T, et al. Effect of salt intake on blood pressure in patients receiving antihypertensive therapy: Shimane CoHRE Study. Eur J Int Med. 2016;28:70–73. [DOI] [PubMed] [Google Scholar]
- 15. Iuchi H, Sakamoto M, Suzuki H, et al. Effect of one‐week salt restriction on blood pressure variability in hypertensive patients with type 2 diabetes. PLoS One. 2016;11:e0144921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krupp D, Shi L, Egert S, et al. Prospective relevance of fruit and vegetable consumption and salt intake during adolescence for blood pressure in young adulthood. Eur J Nutr. 2015;54:1269–1279. [DOI] [PubMed] [Google Scholar]
- 17. Noh H‐M, Park S‐Y, Lee H‐S, et al. Association between high blood pressure and intakes of sodium and potassium among Korean adults: Korean National Health and Nutrition Examination Survey, 2007‐2012. J Acad Nutr Diet. 2015;115:1950–1957. [DOI] [PubMed] [Google Scholar]
- 18. Thuesen BH, Toft U, Buhelt LP, et al. Estimated daily salt intake in relation to blood pressure and blood lipids: the role of obesity. Eur J Prev Cardiol. 2015;22:1567–1574. [DOI] [PubMed] [Google Scholar]
- 19. Umesawa M, Yamagishi K, Noda H, et al. The relationship between sodium concentrations in spot urine and blood pressure increases: a prospective study of Japanese general population: the Circulatory Risk in Communities Study (CIRCS). BMC Cardiovasc Disord. 2016;16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yokokawa H, Yuasa M, Nedsuwan S, et al. Daily salt intake estimated by overnight urine collections indicates a high cardiovascular disease risk in Thailand. Asia Pac J Clin Nutr. 2016;25:39–45. [DOI] [PubMed] [Google Scholar]
- 21. Huh JH, Lee KJ, Lim JS, et al. High dietary sodium intake assessed by estimated 24‐h urinary sodium excretion is associated with NAFLD and hepatic fibrosis. PLoS One. 2015;10:e0143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee SK, Kim MK. Relationship of sodium intake with obesity in Korean children and adolescents: Korea National Health and Nutrition Examination Survey. Br J Nutr. 2016;115:834–841. [DOI] [PubMed] [Google Scholar]
- 23. Wang M, Moran AE, Liu J, et al. A meta‐analysis of effect of dietary salt restriction on blood pressure in chinese adults. Glob Heart. 2015;10:291–299.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu N, Sun W, Xing Z, et al. Association between sodium intakes with the risk of chronic kidney disease: evidence from a meta‐analysis. Int J Clin Exp Med. 2015;8:20939–20945. [PMC free article] [PubMed] [Google Scholar]
- 25. Block G, Woods M, Potosky A, et al. Validation of a self‐administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–1335. [DOI] [PubMed] [Google Scholar]
- 26. Ishihara J, Inoue M, Kobayashi M, et al. Impact of the revision of a nutrient database on the validity of a self‐administered food frequency questionnaire (FFQ). J Epidemiol. 2006;16:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Date C, Fukui M, Yamamoto A, et al. Reproducibility and validity of a self‐administered food frequency questionnaire used in the JACC study. J Epidemiol. 2005;15(suppl 1):S9–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154:1089–1099. [DOI] [PubMed] [Google Scholar]
- 29. Kroke A, Manz F, Kersting M, et al. The DONALD Study. History, current status and future perspectives. Eur J Nutr. 2004;43:45–54. [DOI] [PubMed] [Google Scholar]
- 30. Gu D, Zhao Q, Chen J, et al. Reproducibility of blood pressure responses to dietary sodium and potassium interventions: the GenSalt study. Hypertension. 2013;62:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fan L, Tighiouart H, Levey AS, et al. Urinary sodium excretion and kidney failure in nondiabetic chronic kidney disease. Kidney Int. 2014;86:582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810–1852. [DOI] [PubMed] [Google Scholar]
- 33. Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1–e194. [DOI] [PubMed] [Google Scholar]
- 34. Paterna S, Gaspare P, Fasullo S, et al. Normal‐sodium diet compared with low‐sodium diet in compensated congestive heart failure: is sodium an old enemy or a new friend? Clin Sci (Lond). 2008;114:221–230. [DOI] [PubMed] [Google Scholar]
- 35. Paterna S, Parrinello G, Cannizzaro S, et al. Medium term effects of different dosage of diuretic, sodium, and fluid administration on neurohormonal and clinical outcome in patients with recently compensated heart failure. Am J Cardiol. 2009;103:93–102. [DOI] [PubMed] [Google Scholar]
- 36. Parrinello G, Di PP, Licata G, et al. Long‐term effects of dietary sodium intake on cytokines and neurohormonal activation in patients with recently compensated congestive heart failure. J Card Fail. 2009;15:864–873. [DOI] [PubMed] [Google Scholar]
- 37. Arcand J, Floras JS, Azevedo E, et al. Evaluation of 2 methods for sodium intake assessment in cardiac patients with and without heart failure: the confounding effect of loop diuretics. Am J Clin Nutr. 2011;93:535–541. [DOI] [PubMed] [Google Scholar]
- 38. Alvelos M, Ferreira A, Bettencourt P, et al. The effect of dietary sodium restriction on neurohumoral activity and renal dopaminergic response in patients with heart failure. Eur J Heart Fail. 2004;6:593–599. [DOI] [PubMed] [Google Scholar]
- 39. Damgaard M, Norsk P, Gustafsson F, et al. Hemodynamic and neuroendocrine responses to changes in sodium intake in compensated heart failure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1294–R1301. [DOI] [PubMed] [Google Scholar]
- 40. Colin‐Ramirez E, McAlister FA, Zheng Y, et al. The long‐term effects of dietary sodium restriction on clinical outcomes in patients with heart failure. The SODIUM‐HF (Study of Dietary Intervention Under 100 mmol in Heart Failure): a pilot study. Am Heart J. 2015;169:274–281. e1. [DOI] [PubMed] [Google Scholar]
- 41. Wessler JD, Maurer MS, Hummel SL. Evaluating the safety and efficacy of sodium‐restricted/Dietary Approaches to Stop Hypertension diet after acute decompensated heart failure hospitalization: design and rationale for the Geriatric OUt of hospital Randomized MEal Trial in Heart Failure (GOURMET‐HF). Am Heart J. 2015;169:342–348. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


