Abstract
This study aimed to evaluate the effects of glycated hemoglobin (HbA1c) on flow‐mediated dilation, intima‐media thickness, pulse wave velocity, and left ventricular mass index in patients with resistant hypertension (RHTN) comparing RHTN–controlled diabetes mellitus and RHTN–uncontrolled type 2 diabetes mellitus. Two groups were formed: HbA1c <7.0% (RHTN–controlled diabetes mellitus: n = 98) and HbA1c ≥7.0% (RHTN–uncontrolled diabetes mellitus: n = 122). Intima‐media thickness and flow‐mediated dilation were measured by high‐resolution ultrasound, left ventricular mass index by echocardiography, and arterial stiffness by carotid‐femoral pulse wave velocity. No differences in blood pressure levels were found between the groups but body mass index was higher in patients with RHTN–uncontrolled diabetes mellitus. Endothelial dysfunction and arterial stiffness were worse in patients with RHTN–uncontrolled diabetes mellitus. Intima‐media thickness and left ventricular mass index measurements were similar between the groups. After adjustments, multiple linear regression analyses showed that HbA1c was an independent predictor of flow‐mediated dilation and pulse wave velocity in all patients with RHTN. In conclusion, HbA1c may predict the grade of arterial stiffness and endothelial dysfunction in patients with RHTN, and superimposed uncontrolled diabetes mellitus implicates further impairment of vascular function.
Keywords: cardiovascular remodeling, diabetes mellitus, flow‐mediated dilation, pulse wave velocity, resistant hypertension
1. INTRODUCTION
Obesity, type 2 diabetes mellitus (DM), and hypertension are important cardiovascular risk factors that contribute to increased cardiovascular morbidity and mortality.1, 2, 3, 4, 5, 6 The combined presence of obesity and DM leads to overactivation of the sympathetic nervous and renin‐angiotensin‐aldosterone systems, as well as proinflammatory/pro‐oxidative mechanisms, which may cause endothelial dysfunction and increase the arterial stiffness and intima‐media thickness (IMT) found in hypertension.7, 8, 9, 10, 11 Whereas resistant hypertension (RHTN) is clearly associated with obesity and DM12 and with a similar physiopathological basis and structural and functional vascular remodeling, the effect of uncontrolled DM (ucDM) on endothelial function, vascular stiffness, and carotid artery IMT in these patients is uncertain.
Although arbitrary in the number of medications required, RHTN has been largely defined as hypertension that is uncontrolled despite the use of three or more antihypertensive medications, including, if tolerated, a diuretic. The American Heart Association (AHA) Scientific Statement extended the definition to include patients whose blood pressure (BP) was uncontrolled with three medications, but was controlled with the use of four or more medications, ie, “controlled resistant hypertension.”13, 14, 15
In 2010, the American Diabetes Association (ADA) included glycated hemoglobin (HbA1c) ≥6.5% in the diagnosis of DM with this biomarker being used to assess the efficacy of DM treatment.16, 17 Increased pulse wave velocity (PWV), which suggests arterial stiffness and reduced flow‐mediated vasodilation, is used as a marker of endothelial dysfunction and predicts cardiovascular risk in patients with DM.11, 18, 19, 20 The ADA recommends glycemic targets for many adults to include blood glucose levels that should attain an HbA1c level of 7%.21
Left ventricular hypertrophy is a structural and functional alteration that occurs as a compensatory response to chronic pressure and/or volumetric overload. It is an independent cardiovascular risk factor for heart failure and sudden death. There is a direct relationship between the increase in peripheral and central pressure levels and the incidence of left ventricular hypertrophy. Moreover, comorbidities including DM are associated with left ventricular hypertrophy.22, 23
The aim of this study was to compare the effect of HbA1c on endothelial dysfunction, IMT, PWV, and left ventricular mass index in patients with RHTN with controlled DM (cDM) or ucDM. We hypothesized that HbA1c predicts the degree of the vascular triad (endothelium dysfunction, arterial stiffness, and IMT) in patients with RHTN.
2. METHODS
2.1. Study population
A total of 220 patients with RHTN regularly followed up at our specialized tertiary hypertension center (Hospital de Clinicas—University of Campinas, Brazil) were enrolled after screening for RHTN based on the AHA guidelines.13 All patients had complete case histories and underwent physical examinations, electrocardiograms, and laboratory tests. Patients with secondary forms of hypertension, impaired renal function (creatinine clearance <30 mL/min estimated using the Cockcroft‐Gault equation), ischemic heart disease, atrial fibrillation, liver disease, strokes, or peripheral vascular disease were excluded. Patients were followed up and treated for a period of approximately 6 months with appointments scheduled on a regular basis before being considered resistant to treatment. The patients were divided into two groups according to an HbA1c cutoff of 7.0%21: (1) 98 patients with RHTN with cDM (RHTN‐cDM) and (2) 122 patients with RHTN with ucDM (RHTN‐ucDM). The diagnosis of DM was determined by two fasting plasma glucose levels ≥126 mg/dL or a 2‐hour plasma glucose level after a 75‐g oral glucose tolerance test ≥200 mg/dL or HbA1c ≥6.5%.17, 24
This study was approved by the research ethics committee of the Faculty of Medical Sciences, University of Campinas (Campinas, Brazil) and all participants provided signed written consent.
2.2. Office BP and ABPM
The office BP was used for diagnosis and follow‐up employing the guidelines of the AHA statement with 24‐hour ambulatory BP monitoring (ABPM) being used to exclude pseudoresistance and the white‐coat effect.25
The average of two office BP readings was calculated with hypertension being defined as a systolic BP (SBP) ≥140 mm Hg and⁄or a diastolic BP (DBP) ≥90 mm Hg on at least three occasions at 2‐week intervals. ABPM was performed with an automatic oscillometric device (Spacelabs 90207, Spacelabs Inc). BP was measured automatically at 20‐minute intervals during an entire 24‐hour period with patients engaged in their normal daily activities. The following parameters were calculated: the average 24‐hour systolic, diastolic, and pulse (difference between systolic and diastolic) BPs.
2.3. Flow‐mediated dilation
Endothelial dysfunction in hypertension was determined using the flow‐mediated dilation (FMD) technique described by Celermajer and colleagues.26 High‐resolution ultrasound was used to evaluate the endothelium‐dependent function of a medium‐caliber artery27 after applying the compression/decompression test (occlusion for 5 minutes). This method has been validated and is standardized according to the International Brachial Artery Reactivity Task Force Guidelines for the ultrasound assessment of endothelial‐dependent vasodilation of the brachial artery published in 2002 by Corretti and colleagues.27 Dilation was measured using a linear vascular transducer (7–12 MHz, Vivid S6; GE Medical System) coupled to computer‐assisted analysis software and an automated brachial analyzer software device (MIA, LLC). All scanned images were stored on a compact disc for future analysis by two independent observers. The variability between the arterial diameter measurements should be <2%; intraobserver differences <1%, as were seen in this study.
The patients rested quietly for 15 minutes before the first scan and remained in the supine position throughout the study. The brachial artery was scanned longitudinally 5 to 10 cm above the elbow. When a satisfactory position was found, a special probe holder was fixed around the arm to secure the ultrasound transducer in one position for all of the measurements.18 A polyurethane cuff (Hokanson, Inc) placed around the arm above the transducer was inflated to a pressure of 250 mm Hg for 5 minutes and then rapidly deflated. The maximum dilatation was recorded from 45 to 120 seconds after cuff deflation with this being used to calculate the FMD. Percentage changes in the brachial artery diameter compared with the baseline diameter (100%) were calculated according to the formula: FMD = ([diameter after decompression − baseline diameter]/baseline diameter) × 100.
2.4. Pulse wave velocity
Vascular stiffness was determined using the SphygmoCor CPV (AtCor Medical) device, which, when synchronized with the electrocardiogram, allows a calculation of PWV between the carotid and femoral arteries.28 Based on PWV data from direct tape measure distances, using the more accurate 80% direct tape measure distance, the cutoff value should be adapted according to 10 m/s.
PWV measurement is calculated by dividing the distance between the two arteries by the time of the pulse transit between the two sites of interest.19 The more rigid the “arterial tree” under study, the less time is necessary for the pulse to travel between the two recording sites and, therefore, the greater the speed of the wave. Arterial stiffness is identified by a PWV >10 m/s.29
2.5. Intima‐media thickness
IMT was assessed by high‐resolution ultrasound using a linear vascular transducer (7 to 12 MHz, Vivid S6, GE Medical System) equipment, following the manufacturer's instructions. Assessment of the distal segment of the common carotid artery was standardized to a minimum of 100 measurement points or a longitudinal length of at least 1.0 cm of the artery excluding the carotid bulb.30
This method is established and standardized by the report of the 34th Bethesda Conference Task Force #3 Noninvasive Measurement of Atherosclerosis.31 The brightness of the examination room was controlled, and the room temperature was set at 24°C. The variability between the IMT measurements should be <2%, as was seen in this study.
2.6. Biochemical examinations
Blood samples were collected in the morning, after 12 hours of fasting, and after 30 minutes of resting. Fasting glucose levels, HbA1c, total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, triglycerides, and creatinine clearance were measured by conventional methods in the Physiology Laboratory of Hospital de Clinicas, Unicamp, SP, Brazil (Table 1).
Table 1.
Clinical characteristics of RHTN groups
| cDM(n = 98) | ucDM(n = 122) | |
|---|---|---|
| Age, y | 57.52 ± 11.18 | 59.75 ± 10.42 |
| Female sex, % | 65 | 68 |
| BMI, kg/m² | 30.08 ± 4.60 | 31.70 ± 4.48* |
| ABPM SBP, mm Hg | 151.0 ± 20.1 | 151.1 ± 14.14 |
| ABPM DBP, mm Hg | 89.06 ± 12.18 | 87.72 ± 10.92 |
| ABPM PP, mm Hg | 62.20 ± 16.77 | 63.42 ± 14.32 |
| Glycated hemoglobin, % | 6.13 ± 0.35 | 8.08 ± 0.96≠ |
| Duration of RHTN + DM | 6.42 ± 3.94 | 7.44 ± 4.71 |
| Office SBP, mm Hg | 162.2 ± 17.9 | 153.6. ± 13.7 |
| Office DBP, mm Hg | 89.1 ± 21.1 | 87.2 ± 11.2 |
| Heart rate, beats per min | 71.2 ± 10.6 | 72.1 ± 12.1 |
| Total cholesterol, mg/dL | 204.61 ± 43.11 | 202.92 ± 43.11 |
| Current smokers, % | 5 | 9 |
Data are expressed as mean ± standard deviation. ABPM, ambulatory blood pressure monitoring; BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; PP, pulse pressure; RHTN, resistant hypertension; SBP, systolic blood pressure; ucDM, uncontrolled diabetes mellitus. *P = .0095 vs controlled diabetes mellitus (cDM). ≠ P < .0001 vs cDM.
2.7. Statistical analyses
Descriptive statistics are shown as mean and standard deviation according to normal distribution. Student t test was used to evaluate differences in clinical and biochemical variables between the RHTN‐cDM and RHTN‐ucDM groups. Any associations between HbA1c, PWV, and FMD were expressed by Pearson's correlation. Multiple linear regression analyses were performed to determine the effect of the different variables (age, sex, body mass index [BMI], SBP, DBP, and mean BP) on endothelial function (FMD) and arterial stiffness (PWV). Analyses were performed using SigmaPlot software version 12.0 (Systat Software, Inc) and the GraphPad Prism 5 program (GraphPad Prism Inc, 2010). A P value <.05 indicated statistical significance.
3. RESULTS
There were no differences between the RHTN‐cDM and RHTN‐ucDM groups in respect to age and BP levels (office BP and ABPM) (Table 1). There were more women in both the RHTN‐cDM (65%) and the RHTN‐ucDM (68%) groups. BMI was higher in the RHTN‐ucDM group compared with the RHTN‐cDM group (Table 1). The patients took a mean of 4.4 antihypertensive medications daily. The most frequently prescribed medications were diuretics (97.2%), angiotensin II receptor blockers or angiotensin‐converting enzyme inhibitors (100.0%), calcium channel blockers (84.6%), aldosterone receptor antagonists (79.5%), and β‐blockers (53.8%). All patients with DM took oral glucose‐lowering medications and 46% were on insulin therapy (Table 2).
Table 2.
Multiple linear regression for the presence of vascular damage
| β‐Coefficient (SE) | P value | |
|---|---|---|
| FMD, % | ||
| Age, y | 0.04001 (0.0209) | .0594 |
| BMI, kg/m² | −0.00796 (0.05001) | .8730 |
| Sex (male/female) | 1.1782 (1.7520) | .5032 |
| SBP, mmHg | −0.01731 (0.01300) | .1869 |
| DBP, mmHg | 0.01697 (0.02302) | .4632 |
| Heart rate, beats per min | −0.001700 (0.01802) | .9251 |
| Glycated hemoglobin, % | −0.8580 (0.2698) | .0021* |
| Total cholesterol, mg/dL | −0.000720 (0.04420) | .8709 |
| Duration of RHTN + DM | 0.05428 (0.04585) | .2400 |
| Smoking | −0.1396 (0.6711) | .8358 |
| PWV, m/s | ||
| Age, y | −0.01739 (0.01824) | .3432 |
| BMI, kg/m² | −0.00008 (0.04360) | .9984 |
| Sex (male/female) | −1.8247 (1.5274) | .2358 |
| SBP, mmHg | 0.03206 (0.01133) | .0059* |
| DBP, mmHg | −0.00180 (0.02007) | .9285 |
| Heart rate, beats per min | −0.01125 (0.01571) | .4760 |
| Glycated hemoglobin, % | 0.9010 (0.2352) | .0003* |
| Total cholesterol, mg/dL | −0.00428 (0.00385) | .2691 |
| Time duration RHTN + DM | −0.03083 (0.03997) | .4428 |
| Smoking | 0.3924 (0.5851) | .5044 |
BMI, body mass index; DBP, diastolic blood pressure; DM, diabetes mellitus; FMD, flow‐mediated dilatation; PWV, pulse wave velocity; RHTN, resistant hypertension; SBP, systolic blood pressure; SE, standard error.
*P value <.05.
Figure 1 shows that FMD (RHTN‐cDM: 8.10 ± 2.03% and RHTN‐ucDM: 6.96 ± 1.54%) and arterial stiffness (PWV–RHTN‐cDM: 10.96 ± 1.79 m/s and RHTN‐ucDM: 12.07 ± 1.73 m/s) were more impaired in patients with RHTN‐ucDM (P <.0001). IMT and left ventricular mass index measurements did not differ significantly between the RHTN‐cDM and RHTN‐ucDM groups (IMT–RHTN‐cDM: 0.99 ± 0.21 mm and RHTN‐ucDM: 1.03 ± 0.19 mm [P = not significant] and left ventricular mass index–RHTN‐cDM: 183.70 ± 54.77 g/m2; RHTN‐ucDM: 197.40 ± 57.71 g/m2 [P = not significant]).
Figure 1.
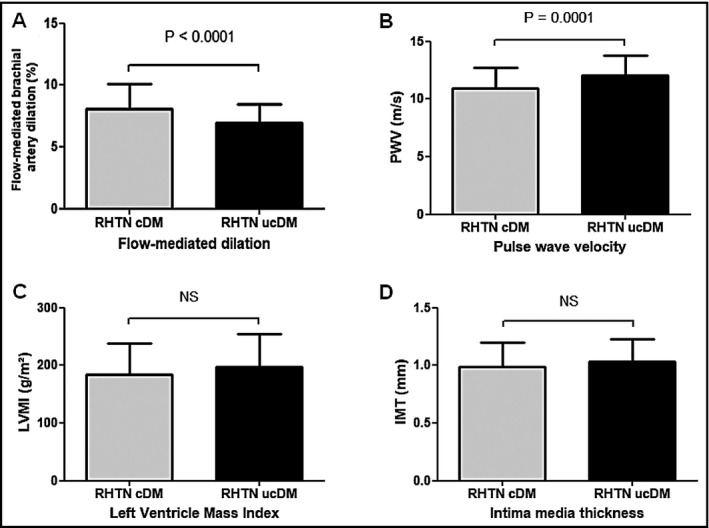
A, Flow‐mediated dilation (resistant hypertension–controlled diabetes mellitus [RHTN‐cDM]: 8.10 ± 2.03% and RHTN–uncontrolled diabetes mellitus [ucDM]: 6.97 ± 1.54%; P value <.0001). B, Pulse wave velocity (10.96 ± 1.79; m/s RHTN‐ucDM: 12.07 ± 1.73 m/s; P value <.0001). C, Intima‐media thickness (RHTN‐cDM: 0.99 ± 0.21 mm and RHTN‐ucDM: 1.03 ± 0.19 mm; P value = .159). D, Left ventricular mass index (RHTN‐cDM: 183.70 ± 54.77 g/m2 and RHTN‐ucDM: 197.40 ± 57.71 g/m2; P value = .075)
Simple linear regression showed that HbA1c was correlated with FMD (panel A: r = −.34; P <.001) and PWV (panel B: r = .24; P = .0006), but not with left ventricular mass index (panel C: r = .045; P = .498) or IMT (panel D: r = .024; P = .731) in all patients with RHTN (cDM + ucDM) (Figure 2). Finally, after adjustments for age, BMI, sex, SBP, DBP, heart rate, duration of RHTN and T2DM, total cholesterol, and smoking (measured during the FMD and PWV examinations), a multiple linear regression analysis identified HbA1c as an independent predictor for FMD (β‐coefficient = −0.8580; P = .0021) and PWV (β‐coefficient = 0.9010; P = .0003) in the totality of patients with RHTN.
Figure 2.
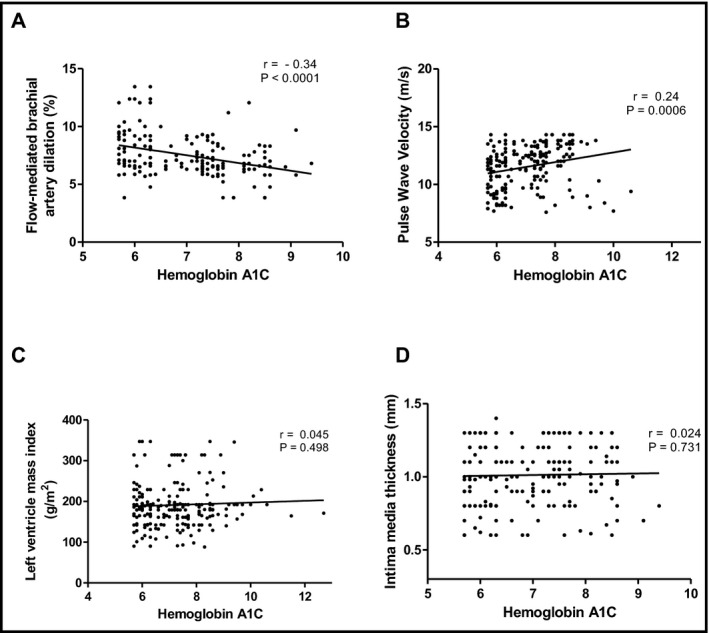
Simple linear regression plotting glycated hemoglobin (hemoglobin A1C) vs flow‐mediated dilation (A, r = −.34; P value <.001) and pulse wave velocity (B, r = .25; P value = .0006), left ventricular mass index (C, r = .045; P value = .498), and intima‐media thickness (D, r = 0.024; P value = .731) in all resistant hypertension (controlled diabetes mellitus plus uncontrolled diabetes mellitus were plotted together)
4. DISCUSSION
This study found that the degree of vascular damage was higher in patients with RHTN‐ucDM compared with patients with RHTN‐cDM. Moreover, HbA1c can indicate the degrees of arterial stiffness and endothelial dysfunction in patients with RHTN (cDM and ucDM) independently of age, BMI, sex, SBP, DBP levels, heart rate, duration of RHTN and T2DM, total cholesterol, and smoking. These findings suggest that HbA1c might be useful as a biomarker of structural and functional vascular damage in patients with RHTN.
As previously mentioned, DM is frequently found in patients with RHTN as the two diseases have etiological aspects in common such as obesity, inflammation, oxidative stress, insulin resistance, and factors associated with increased microvascular and macrovascular impairment. Treatment of type 2 DM by reducing HbA1c levels using novel oral hypoglycemic agents such as sodium‐glucose cotransporter inhibitors have shown promising results with significant reductions in BP, weight, endothelial dysfunction, arterial stiffness, and microalbuminuria in patients with hypertension and those with DM. Thus, pleiotropic effects of these drugs, which are conceivably BP‐ and glucose‐independent, could represent the mechanism of cardiovascular protection in this drug class.32
RHTN leads to more severe arterial vasculopathy characterized by increased endothelial dysfunction,18, 33, 34, 35 IMT36, 37 and vascular stiffness33 compared with controlled hypertension. We have demonstrated that increased PWV and impaired FMD are directly correlated with BP levels.33 In addition, RHTN associated with the presence of DM may contribute to the overall atherosclerosis process.
Hyperglycemia causes progression of DM with complications caused by endothelial dysfunction through a phenomenon called metabolic memory.38 It was well demonstrated by Tacito and colleagues39 in patients with type 1 DM that endothelial dysfunction, characterized by reduced FMD, appears within the first few years after the onset of type 1 DM and is thus an early marker of vascular involvement. This complex mechanism increases the production of advanced glycation end products and expression of its receptor (RAGE), as well as enhances oxidative stress by anion superoxide formation and promotes mitochondrial DNA damage.40, 41 It is known that superoxide anions inactivate endothelium‐derived nitric oxide, the most important endogenous vasodilator, thereby promoting endothelial dysfunction and vasoconstriction.34, 42 In addition, oxidative stress causes inflammation, hypertrophy, apoptosis, cell migration, fibrosis, and angiogenesis, leading to vascular remodeling.35 Thus, the development of the DM complications related to vessels increases cardiovascular morbidity and mortality in patients with hypertension, even when they are receiving the combined treatment of glucose‐lowering drugs and antihypertensive agents.20
It has previously been demonstrated that DM increases arterial stiffness and constitutes a strong risk factor for early mortality.43 Arterial wall stiffness is exacerbated by DM and hypertension, and is even more severe in patients with RHTN.33 The development of vascular stiffness depends on the balance between two predominant proteins in the vascular wall, collagen and elastin. An abnormal overproduction of collagen, to the detriment of elastin, contributes to arterial stiffness.44, 45, 46 However, immunohistochemical and ultrastructural studies show that arterial stiffness is not only influenced by the amount and density of stiff wall material but mainly by the spatial organization of these proteins.43
Increased arterial stiffness causes a premature return of reflected waves in late systole, increasing SBP and decreasing the DBP, thereby increasing the central pulse pressure. High SBP is associated with left ventricular hypertrophy and increased myocardial oxygen demand with high risk of coronary events. Moreover, high arterial stiffness can increase the risk of stroke through several mechanisms, including an increase in central pulse pressure causing arterial remodeling of both the extracranial and intracranial arteries. This thickening of the carotid wall and the development of stenosis and plaques increase the likelihood of plaque rupture and the prevalence and severity of cerebral white matter lesions.19
In patients with DM, in addition to target organ damage and cardiovascular remodeling, it is known that high levels of intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 are markers of pathogenic events of endothelial dysfunction and potential development of cardiac, vascular, and DM diseases. Studies suggest that these molecules, induced in response to inflammatory cytokines, are strong predictors of cardiovascular events in patients with DM.47
Another biomarker related to cardiovascular risks is monocyte chemoattractant protein 1. Data show that serum levels of this chemokine are higher in patients with hypertension and endothelial dysfunction compared with patients with normotension.48
Adiponectin, secreted exclusively by adipocytes, has antidiabetic and antiatherogenic activity. However, an inverse correlation between circulating adiponectin levels and BP has been reported in healthy patients and those with DM.49
A recent meta‐analysis on the association of two polymorphisms in the adiponectin‐encoding gene related to hypertension and the changes of circulating adiponectin and BP showed that although no significant association between the ADIPOQ T45G or G276T polymorphism and hypertension was found, the heterozygous G276T mutation was responsible for increased circulating adiponectin levels and increased BP values in patients with hypertension.50
Weight reduction and better glycemic control are associated with reduced PWV in young adults. The SAVE (Slow the Adverse Effects of Vascular Aging) study51 has shown that weight reduction combined with improved insulin sensitivity improves arterial stiffness, a change related to regression of the vascular remodeling process by proliferation and migration of vascular smooth muscle cells. It is also known that poor glycemic control is associated with lower circulating levels of endothelial progenitor cells and higher PWV values. On the other hand, pleiotropic effects of new hypoglycemic agents such as sodium‐glucose co‐transporter 2 inhibitors can modulate some molecular and biochemical pathways, reducing oxidative stress and markers of inflammation and fibrosis (nuclear factor‐κβ and the expression of collagen IV). Hence, new studies are needed to confirm all of these possible mechanisms.52
Recently, the work of Lambadiari and colleagues53 in patients with DM using liraglutide showed that 6 months of treatment resulted in significant improvement of endothelial function, arterial stiffness, left ventricular strain with reduction of the NT‐proBNP marker, and oxidative stress. As previously discussed, treatment of type 2 DM with new oral hypoglycemic agents such as sodium‐glucose co‐transporter 2 inhibitors showed promising results with significant reduction in BP, weight, endothelial dysfunction, arterial stiffness, and microalbuminuria in patients with hypertension and those with DM.54
Patients in this study did not benefit from these drugs. The hypoglycemic therapy of patients in this study included glibenclamide 5 mg/d or glicazide 60 mg/d associated with metformin 2.0 g/d and insulin as needed.
As increased arterial stiffness is associated with higher mortality in patients with end‐stage renal disease, who essentially have DM and hypertension, strategies aimed at restoring functional and structural damage may prevent the progression of the disease. Thus, evaluation of arterial stiffness has provided strong evidence that PWV improves the prediction of risk for cardiovascular diseases and mortality in the general population and particularly in patients with hypertensive DM. Specifically, subgroups of patients with end‐stage renal failure who have elevated PWV have shown a 48% increased risk of cardiovascular events and thus PWV can be used as an appropriate target for new risk reduction strategies55, 56 while FMD, because it is a functional marker, is used as an auxiliary method to evaluate vascular impairment.
No difference was observed in the IMT measurements between the study groups. Previous research in our laboratory showed that no differences are found when comparing groups of patients with severe and long‐term hypertension and DM. However, both atherosclerotic plaques and increased IMT values can be detected in these individuals.57, 58 Thus, uncompensated type 2 DM results in greater impairment of vascular function in patients with RHTN, leading to more severe atherosclerosis.
5. STUDY LIMITATIONS
The limitations of this study include its cross‐sectional design, as a cause‐effect relationship cannot be inferred. Although severe cardiorenal impairment is a common condition in individuals with diabetic RHTN, these patients were not included in this study to avoid potential bias.
6. CONCLUSIONS
Superimposed uncontrolled DM implicates greater impairment of vascular function (endothelial dysfunction and arterial rigidity) in patients with RHTN. Particular care is mandatory for the early detection of abnormal levels of HbA1c in patients with RHTN to reduce further vascular dysfunction in this complex hypertensive syndrome. Patients must also be followed up by a multidisciplinary team in specialized clinics for RHTN.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Moreno B, de Faria AP, Ritter AMV, et al. Glycated hemoglobin correlates with arterial stiffness and endothelial dysfunction in patients with resistant hypertension and uncontrolled diabetes mellitus. J Clin Hypertens. 2018;20:910–917. 10.1111/jch.13293
Funding informationThis study was supported by the State of São Paulo Research Foundation (FAPESP), the National Council for Scientific and Technological Development (CNPq), and Coordination for the Improvement of Higher Education Personnel (CAPES), Brazil.
REFERENCES
- 1. Isomaa B, Almgren P, Tuomi T, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683‐689. [DOI] [PubMed] [Google Scholar]
- 2. Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world––a growing challenge. N Engl J Med. 2007;356:213‐215. [DOI] [PubMed] [Google Scholar]
- 3. Mohamedali B, Yost G, Bhat G. Obesity as a risk factor for consideration for left ventricular assist devices. J Card Fail. 2015;21:800‐805. [DOI] [PubMed] [Google Scholar]
- 4. Zethelius B, Gudbjörnsdottir S, Eliasson B, Eeg‐Olofsson K, Svensson AM, Cederholm J. Risk factors for atrial fibrillation in type 2 diabetes: report from the Swedish National Diabetes Register (NDR). Diabetologia. 2015;58:2259‐2268. [DOI] [PubMed] [Google Scholar]
- 5. Destro M, Dognini GP, Pozzi A, et al. 5c.06: hypertension and cardiovascular risk factors: a shot on northern Italy population in real life setting. J Hypertens. 2015;33(suppl 1):e69. [Google Scholar]
- 6. Greenland P, Fuster V. Cardiovascular risk factor control for all. JAMA. 2017;318:130‐131. [DOI] [PubMed] [Google Scholar]
- 7. Manrique C, Lastra G, Gardner M, Sowers JR. The renin angiotensin aldosterone system in hypertension: roles of insulin resistance and oxidative stress. Med Clin North Am. 2009;93:569‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stiefel P, Vallejo‐Vaz AJ, García Morillo S, Villar J. Role of the renin‐angiotensin system and aldosterone on cardiometabolic syndrome. Int J Hypertens. 2011;2011:685238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aroor AR, Demarco VG, Jia G, et al. The role of tissue renin‐angiotensin‐aldosterone system in the development of endothelial dysfunction and arterial stiffness. Front Endocrinol. 2013;4:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rao A, Pandya V, Whaley‐Connell A. Obesity and insulin resistance in resistant hypertension: implications for the kidney. Adv Chronic Kidney Dis. 2015;22:211‐217. [DOI] [PubMed] [Google Scholar]
- 11. Jia G, Aroor AR, DeMarco VG, Martinez‐Lemus LA, Meininger GA, Sowers JR. Vascular stiffness in insulin resistance and obesity. Front Physiol. 2015;6:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Martins LC, Figueiredo VN, Quinaglia T, et al. Characteristics of resistant hypertension: ageing, body mass index, hyperaldosteronism, cardiac hypertrophy and vascular stiffness. J Hum Hypertens. 2011;25:532‐538. [DOI] [PubMed] [Google Scholar]
- 13. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403‐1419. [DOI] [PubMed] [Google Scholar]
- 14. Calhoun DA. Refractory and resistant hypertension: antihypertensive treatment failure versus treatment resistance. Korean Circ J. 2016;46:593‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siddiqui M, Dudenbostel T, Calhoun DA. Resistant and refractory hypertension: antihypertensive treatment resistance vs treatment failure. Can J Cardiol. 2016;32:603‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. American Diabetes Association . Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(suppl 1):S11‐S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Diabetes Association . 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(suppl 1):S11‐S24. [DOI] [PubMed] [Google Scholar]
- 18. Yugar‐Toledo JC, Tanus‐Santos JE, Sabha M, et al. Uncontrolled hypertension, uncompensated type II diabetes, and smoking have different patterns of vascular dysfunction. Chest. 2004;125:823‐830. [DOI] [PubMed] [Google Scholar]
- 19. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588‐2605. [DOI] [PubMed] [Google Scholar]
- 20. Prattichizzo F, Giuliani A, Ceka A, et al. Epigenetic mechanisms of endothelial dysfunction in type 2 diabetes. Clin Epigenetics. 2015;7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American Diabetes Association . 6. Glycemic targets. Diabetes Care. 2017;40(suppl 1):S48‐S56. [DOI] [PubMed] [Google Scholar]
- 22. Cuspidi C, Vaccarella A, Negri F, Sala C. Resistant hypertension and left ventricular hypertrophy: an overview. J Am Soc Hypertens. 2010;4:319‐324. [DOI] [PubMed] [Google Scholar]
- 23. Muiesan ML, Salvetti M, Rizzoni D, et al. Resistant hypertension and target organ damage. Hypertens Res. 2013;36:485‐491. [DOI] [PubMed] [Google Scholar]
- 24. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):S62‐S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898‐902. [DOI] [PubMed] [Google Scholar]
- 26. Celermajer DS, Sorensen KE, Gooch VM, et al. Non‐invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111‐1115. [DOI] [PubMed] [Google Scholar]
- 27. Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial‐dependent flow‐mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257‐265. [DOI] [PubMed] [Google Scholar]
- 28. Palatini P, Casiglia E, Gąsowski J, et al. Arterial stiffness, central hemodynamics, and cardiovascular risk in hypertension. Vasc Health Risk Manag. 2011;7:725‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159‐2219. [DOI] [PubMed] [Google Scholar]
- 30. Simon A, Gariepy J, Chironi G, Megnien JL, Levenson J. Intima‐media thickness: a new tool for diagnosis and treatment of cardiovascular risk. J Hypertens. 2002;20:159‐169. [DOI] [PubMed] [Google Scholar]
- 31. Redberg RF, Vogel RA, Criqui MH, Herrington DM, Lima JA, Roman MJ. 34th Bethesda Conference: task force #3––What is the spectrum of current and emerging techniques for the noninvasive measurement of atherosclerosis? J Am Coll Cardiol. 2003;41:1886‐1898. [DOI] [PubMed] [Google Scholar]
- 32. Solini A, Giannini L, Seghieri M, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Figueiredo VN, Yugar‐Toledo JC, Martins LC, et al. Vascular stiffness and endothelial dysfunction: correlations at different levels of blood pressure. Blood Press. 2012;21:31‐38. [DOI] [PubMed] [Google Scholar]
- 34. de Faria AP, Fontana V, Modolo R, et al. Plasma 8‐isoprostane levels are associated with endothelial dysfunction in resistant hypertension. Clin Chim Acta. 2014;433:179‐183. [DOI] [PubMed] [Google Scholar]
- 35. Sinha N, Dabla PK. Oxidative stress and antioxidants in hypertension––a current review. Curr Hypertens Rev. 2015;11:132‐142. [DOI] [PubMed] [Google Scholar]
- 36. Takiuchi S, Kamide K, Miwa Y, et al. Diagnostic value of carotid intima‐media thickness and plaque score for predicting target organ damage in patients with essential hypertension. J Hum Hypertens. 2003;18:17. [DOI] [PubMed] [Google Scholar]
- 37. Geraci G, Mulè G, Costanza G, Mogavero M, Geraci C, Cottone S. Relationship between carotid atherosclerosis and pulse pressure with renal hemodynamics in hypertensive patients. Am J Hypertens. 2016;29:519‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Testa R, Bonfigli AR, Prattichizzo F, La Sala L, De Nigris V, Ceriello A. The “Metabolic Memory” theory and the early treatment of hyperglycemia in prevention of diabetic complications. Nutrients. 2017;9:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tacito LH, Pires AC, Yugar‐Toledo JC. Impaired flow‐mediated dilation response and carotid intima‐media thickness in patients with type 1 diabetes mellitus with a mean disease duration of 4.1 years. Arch Endocrinol Metab. 2017;61:542‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ceriello A. The emerging challenge in diabetes: the “metabolic memory”. Vascul Pharmacol. 2012;57:133‐138. [DOI] [PubMed] [Google Scholar]
- 41. Voronova V, Zhudenkov K, Helmlinger G, Peskov K. Interpretation of metabolic memory phenomenon using a physiological systems model: what drives oxidative stress following glucose normalization? PLoS ONE. 2017;12:e0171781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zicha J, Dobesova Z, Kunes J. Relative deficiency of nitric oxide‐dependent vasodilation in salt‐hypertensive Dahl rats: the possible role of superoxide anions. J Hypertens. 2001;19:247‐254. [DOI] [PubMed] [Google Scholar]
- 43. Laurent S, Boutouyrie P, Lacolley P. Structural and genetic bases of arterial stiffness. Hypertension. 2005;45:1050‐1055. [DOI] [PubMed] [Google Scholar]
- 44. Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wagenseil JE, Mecham RP. Elastin in large artery stiffness and hypertension. Cardiovasc Transl Res. 2012;5:264‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schiffrin EL, Tedgui A, Lehoux S. Mechanical stress and the arterial wall. In: Safar ME, O'Rourke MF, Frohlich ED, eds. Blood Pressure and Arterial Wall Mechanics in Cardiovascular Diseases. London, UK: Springer London; 2014:97‐106. [Google Scholar]
- 47. Cheung BM, Li C. Diabetes and hypertension: is there a common metabolic pathway? Curr Atheroscler Rep. 2012;14:160‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Panee J. Monocyte Chemoattractant Protein 1 (MCP‐1) in obesity and diabetes. Cytokine. 2012;60:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ghadge AA, Khaire AA, Kuvalekar AA. Adiponectin: a potential therapeutic target for metabolic syndrome. Cytokine Growth Factor Rev. 2018;39:151‐158. [DOI] [PubMed] [Google Scholar]
- 50. Wu J, Xu G, Cai W, et al. The association of two polymorphisms in adiponectin‐encoding gene with hypertension risk and the changes of circulating adiponectin and blood pressure: a meta‐analysis. Oncotarget. 2017;8:14636‐14645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hughes TM, Althouse AD, Niemczyk NA, Hawkins MS, Kuipers AL, Sutton‐Tyrrell K. Effects of weight loss and insulin reduction on arterial stiffness in the SAVE trial. Cardiovasc Diabetol. 2012;11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Osorio H, Coronel I, Arellano A, et al. Sodium‐glucose cotransporter inhibition prevents oxidative stress in the kidney of diabetic rats. Oxid Med Cell Longev. 2012;2012:542042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lambadiari V, Pavlidis G, Kousathana F, et al. Effects of 6‐month treatment with the glucagon like peptide‐1 analogue liraglutide on arterial stiffness, left ventricular myocardial deformation and oxidative stress in subjects with newly diagnosed type 2 diabetes. Cardiovasc Diabetol. 2018;17:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Maliha G, Townsend RR. SGLT2 inhibitors: their potential reduction in blood pressure. J Am Soc Hypertens. 2015;9:48‐53. [DOI] [PubMed] [Google Scholar]
- 55. Blacher J, Safar ME, Guerin AP, Pannier B, Marchais SJ, London GM. Aortic pulse wave velocity index and mortality in end‐stage renal disease. Kidney Int. 2003;63:1852‐1860. [DOI] [PubMed] [Google Scholar]
- 56. Diaz A, Tringler M, Wray S, Ramirez AJ, Cabrera Fischer EI. The effects of age on pulse wave velocity in untreated hypertension. J Clin Hypertens (Greenwich). 2018;20:258‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Naqvi TZ, Lee MS. Carotid intima‐media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7:1025‐1038. [DOI] [PubMed] [Google Scholar]
- 58. Baroncini LA, de Castro Sylvestre L, Filho RP. Carotid intima‐media thickness and carotid plaque represent different adaptive responses to traditional cardiovascular risk factors. Int J Cardiol Heart Vasc. 2015;9:48‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]


