Abstract
The authors aimed to compare surrogate markers of atherosclerosis (pulse wave velocity, intima‐media thickness) between adults with and without Down syndrome (DS) and to assess the impact of parathyroid hormone levels and classic cardiovascular risk factors on arterial stiffness. After comparing 51 adults with DS and 51 healthy adults (siblings of DS individuals), the authors found that adults with DS seem to have lower arterial stiffness, as a result of chronic hypotension. Subclinical atherosclerosis parameters do not correlate with traditional cardiovascular risk factors in adults with DS, thus raising the hypothesis that classic predictive models for cardiovascular disease are not valid in this population. Hyperparathyroidism could play an important role in arterial damage in these individuals. The lower than expected prevalence of obesity and dyslipidemia could be explained by better eating habits, with this study being the first to address the anthropometric and clinical profile of a Mediterranean cohort of adults with DS.
Cardiovascular disease (CVD) remains the leading cause of premature death in Europe and accounts for nearly 30% of deaths worldwide, most of which occur in developing countries.1 It is also the main cause of loss of productivity, which is estimated at 150 million disability‐adjusted life years for the year 2020, and generates both direct and indirect economic costs.2, 3 More than three quarters of deaths from CVD are caused by eight risk factors: high blood pressure (BP), excess alcohol consumption, smoking, high body mass index (BMI), high cholesterol, high blood glucose, low fruit and vegetable intake, and physical inactivity.4 CVD is strongly associated with lifestyle, and more than 75% of all related deaths could be prevented with adequate changes in lifestyle. However, genetic factors may also play a role in the disease.
Down syndrome (DS) is caused by complete or partial trisomy of chromosome 21. It is the most common chromosomal abnormality in liveborn infants and the most frequent chromosomal cause of intellectual disability.5 In general, adults with intellectual disabilities have increased cardiovascular risk factors6 Community‐residing adults with DS tend to have a higher prevalence of overweight and obesity, which are probably associated with a sedentary lifestyle and an unhealthy diet, although no firm evidence has been found for differences in lipid profile.7, 8 Furthermore, a higher incidence of type 1 diabetes mellitus has also been observed in individuals with DS.9 In contrast, this group tends to present with a lower prevalence of smoking and hypertension.10
Given the extremely low incidence of cardiovascular events and the mild or nonexistent signs of atherosclerosis at autopsy in this population, individuals with DS have been proposed as an atheroma‐free model.11, 12 Only three cases of acute myocardial infarction have been described in DS patients, all of whom had congenital heart malformations and no atherosclerotic damage.13 The clinical and molecular factors proposed as an explanation for this finding include the decrease in plasminogen activator inhibitor type 1 levels,14 overexpression of cystathionine‐β‐synthase leading to lower levels of homocysteine,15 and altered autonomic regulation in individuals with DS, which results in a reduced sympathetic response to stress.16
The life expectancy of adults with DS has progressively increased in recent decades as a result of significant advances in medical care and social support. While population‐based data from the United States show an increase in median age at death from 25 to 49 years between 1983 and 1997,17 a recent analysis from Australia suggests that adults with DS are expected to have a lifespan similar to that of the general population within the next generation.18 It will therefore be interesting to determine whether this population is really “naturally protected” against atherosclerotic damage and cardiovascular events.
Carotid intima‐media thickness (cIMT) and pulse wave velocity (PWV) are surrogate markers of atherosclerosis, which is associated with cardiovascular risk factors and cardiovascular outcomes.19, 20 Information on subclinical vascular damage in individuals with DS is scarce, and only two studies have attempted to demonstrate a lower frequency of atherosclerosis in this population. Draheim and colleagues21 found lower cIMT values in 52 individuals with DS compared with 52 controls matched for sex and age, while Rodrigues and colleagues22 failed to find any difference in PWV values in adults with DS compared with nontrisomic adults.
To date, research on the burden of atherosclerosis in adults with DS has been limited to small samples. Most prior evidence on assessment of cardiovascular risk in DS has focused on the evaluation of single CVD risk factors, without attempting to explain the relationship between clinical, epidemiological, and analytical variables and surrogate markers of atherosclerosis. Therefore, the aims of this study were as follows: (1) to compare surrogate markers of atherosclerosis between adults with and without DS; (2) to assess the impact of parathyroid hormone (PTH) levels and classic cardiovascular risk factors on arterial stiffness and cIMT in individuals with DS; and (3) to describe the cardiovascular risk profile of individuals with DS living in a Mediterranean setting.
Materials and methods
Study design
We conducted a cross‐sectional comparative study of 102 participants (51 adults with DS diagnosed by karyotype and/or phenotype and 51 controls). Adults with DS were consecutively recruited between January 2015 and March 2016 from the Adult Down Syndrome Outpatient Clinic of the Internal Medicine Department, Hospital Universitario de La Princesa in Madrid, Spain. The controls were race‐matched healthy individuals. Family members (mainly siblings of the adults with DS in order to reduce the hypothetical effect of genetic and environmental factors) and legal guardians of adults with DS were invited to volunteer for the study.
Recruitment
Inclusion criteria were age older than 18 years and, for the DS group, a confirmed diagnosis of DS and residence in a community setting. The exclusion criteria included unoperated severe congenital heart disease, atrial fibrillation, severe sensory impairment, inability to provide assent or consent, pregnancy, and, for the control group, an estimated high or very high stratified CVD risk according to the European Society of Hypertension.23
Measurements
The following variables were collected for all study patients:
Epidemiological variables: age, ethnicity, and sex.
Anthropometric variables: height, weight, BMI (calculated as weight [kg]/height [m2]), waist circumference at the umbilicus, and waist‐to‐height ratio. Body weight categories (normal weight, overweight, and obesity) were defined by internationally accepted BMI cutoff values.24 Abdominal obesity was defined according to National Cholesterol Education Program/Adult Treatment Panel III (ATP III) criteria.25
Clinical variables were obtained through systematic review of the medical records as follows: family history of early CVD events, medical history of arterial hypertension, dyslipidemia, diabetes mellitus (based on current guidelines25, 26), comorbidities, pharmacologic treatment, office BP, resting heart rate, and fat/fruit/fiber intake. Smoking status was classified as nonsmoker, former smoker (more than 6 months since quitting), or current smoker (active or less than 6 months since quitting). BP was determined with a validated oscillometric device (Automatic OMRON 711, OMRON Healthcare, Vernon Hills, IL), following the recommendations of the European Society of Cardiology (ESC) guidelines.23 Dietary habits were evaluated using the Block Dietary Fat Screener and the Block Fruit/Vegetable/Fiber Screener.27
Analytical variables: fasting glucose, creatinine, estimated glomerular filtration rate (by the Modification of Diet in Renal Disease‐4 equation), thyroid‐stimulating hormone and free thyroxine, PTH, calcium, phosphorus, 25‐OH‐vitamin D, total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein (LDL) cholesterol, very LDL cholesterol, and triglycerides were recorded using a Roche/Hitachi modular‐D analyzer (Basel, Switzerland). Impaired fasting glucose and diabetes mellitus were diagnosed according to the most recent American Diabetes Association criteria.26 Dyslipidemia was diagnosed according to the most modified ATP III criteria.25
Subclinical atherosclerosis: augmentation index, aortic estimated systolic and diastolic BP, pulse pressure, and PWV were measured using a SphygmoCor Vx Pulse Wave Velocity System device (AtCor Medical, Sydney, Australia) with an applanation tonometer (SPC‐301; Millar Instruments, Houston, TX).28 Carotid‐femoral PWV was measured according to ESC recommendations.25 Ultrasonographic images for carotid mean and maximum cIMT measurements were acquired by a single investigator (PPC) at the far wall of the common carotid artery by B‐mode ultrasound with a standard ultrasound device (SonoSite Micromaxx, SonoSite Inc., Bothell, WA) equipped with a linear 8‐cm transducer operating at 8 MHz. Longitudinal images were recorded at three different angles (0°–30°, 30°–60°, and 60°–90°) over the final centimeter of the right common carotid artery for offline analysis in a computerized image‐analyzing system.29
Ethical aspects
The study was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines, and the local institutional review board approved the study protocol. All participants and family members provided written informed consent before any study procedure was performed. All data were treated in the highest confidentiality according to the most recent Spanish data protection act.
Statistical analysis
All data were processed using SPSS software (SPSS 20.0.0, IBM Corp., Armonk, NY). A descriptive analysis of the sample was made using relative frequencies for qualitative variables and measures of central tendency and dispersion for quantitative variables. A chi‐square test was used to compare qualitative variables between groups. Quantitative variables were analyzed using the t test. Pearson's correlation coefficient was used for univariate analysis of PWV, cIMT, age, and systolic BP. Multiple linear regression analysis was performed to assess the main determinants of PWV and cIMT. Given that most of the control group individuals were older siblings of adults with DS, we adjusted significant differences between groups for age and sex. The significance level was set at P<.05.
Results
The study population comprised 102 participants (51 adults with DS [age, 18–62 years; 61% men] and 51 controls [age, 22–68 years; 39% men]). All participants were Caucasian. Siblings of adults with DS were used as controls in 44 cases (86%), while two controls were parents of adults with DS and five were unrelated healthy participants.
The baseline characteristics for each group are shown in Table 1. None of the adults with DS had a previous diagnosis of arterial hypertension or presented with a systolic BP >140 mm Hg at the office, compared with 11.8% of controls, who were under antihypertensive treatment. Two adults with DS and no members of the control group had diabetes mellitus; 13.7% of controls were smokers (compared with none in the DS group), and none of the DS individuals had relevant prior exposure to secondhand smoke. Adults with DS showed significantly higher BMI values, a higher prevalence of obesity, and a higher waist‐to‐height ratio than controls. No significant differences were found between groups regarding lipid profile or any other laboratory parameter. Adults with DS followed a diet richer in fruit and vegetables than controls.
Table 1.
Baseline Characteristics of the Study Sample
| Adults With DS | Controls | Adjusted Effect | P Value | |
|---|---|---|---|---|
| Epidemiological variables | ||||
| Age, y | 35.8±10.6 | 42.5±12.5 | <.01a | |
| Male sex, % | 60.8 | 39.2 | .03a | |
| Anthropometric variables | ||||
| Obesity, % | 37.3 | 13.7 | – | <.01a |
| Body mass index, kg/m2 | 29.1±4.9 | 24.9±3.8 | 4.6 (2.7–6.4) | <.01a |
| Abdominal obesity, % | 37 | 35 | – | .5 |
| Waist circumference, cm | 93.5±12.1 | 90.0±12.2 | 4.2 (−0.3 to 8.7) | .07 |
| Waist‐to‐height ratio | 0.62±0.1 | 0.54±0.1 | 0.1 (0.07–0.13) | <.01a |
| Eating habits | ||||
| Block dietary fat score >30%, % | 80 | 74.5 | – | .54 |
| Block dietary fruit/vegetables portions per day | 4±1 | 3.5±1 | – | .04a |
| Fasting plasma levels | ||||
| 25‐OH‐vitamin D, ng/mL | 33.6±20.8 | 27.8±11.4 | – | .18 |
| Parathyroid hormone, pg/mL | 45.4±22.7 | 47.8±17.2 | – | .73 |
| Total cholesterol, mg/dL | 201.5±33.6 | 203.8±35.1 | – | .51 |
| HDL cholesterol, mg/dL | 55.7±13.3 | 61.4±14.0 | – | .55 |
| LDL cholesterol, mg/dL | 125.5±31.4 | 123.5±29.6 | – | .77 |
| Triglycerides, mg/dL | 102.8±44.6 | 97.6±56.8 | – | .68 |
| Estimated glomerular filtration rate, mL/min | 86.7±19.4 | 86.4±11.3 | – | .94 |
| Calcium, mEq/dL | 9.3±0.6 | 9.6±0.4 | – | .1 |
| Phosphorus, mg/dL | 3.7±0.5 | 3.5±0.4 | – | .2 |
Abbreviations: DS, Down syndrome; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein. Data are expressed as mean±standard error or as number (percentage).
aSignificant at P<.05 after adjustment for age and sex.
Differences in hemodynamic parameters and surrogate markers of atherosclerosis between the groups are shown in Table 2. Adults with DS had significantly lower values of all peripheral and aortic estimated BP than controls (Figure 1).
Table 2.
Differences Between Groups in Hemodynamic Parameters and Surrogate Markers of Atherosclerosis
| Adults With DS | Controls | Adjusted Effect | P Value | |
|---|---|---|---|---|
| Hemodynamic variables | ||||
| SBP, mm Hg | 113.3±12.9 | 125.4±12.9 | −11.4 [−16.8 to −6.1] | <.01a |
| DBP, mm Hg | 70.3±9.0 | 76.8±9.9 | −5.1 [−8.9 to −1.2] | <.01a |
| AoSBP, mm Hg | 101.3±12.5 | 113.5±14.2 | −8.5 [−13.6 to −3.4] | <.01a |
| AoDBP, mm Hg | 71.0±9.2 | 78.3±10.3 | −5.8 [−9.7 to −1.8] | <.01a |
| Subclinical vascular disease | ||||
| PWV | 5.9±1.3 | 6.9±2.0 | −0.93 [−1.2 to −0.6] | <.01a |
| cIMT | 0.514±0.115 | 0.500±0.09 | 0.04 [−0.0009 to 0.1] | .06 |
Abbreviations: AoDBP, aortic estimated diastolic blood pressure; AoSBP, aortic estimated systolic blood pressure; cIMT, carotid intima‐media thickness; DBP, diastolic blood pressure; DS, Down syndrome; PWV, carotid‐femoral pulse wave velocity; SBP, systolic blood pressure. Data are expressed as mean±standard error or as number (percentage).
aSignificant at P<.05 after adjustment for age and sex.
Figure 1.
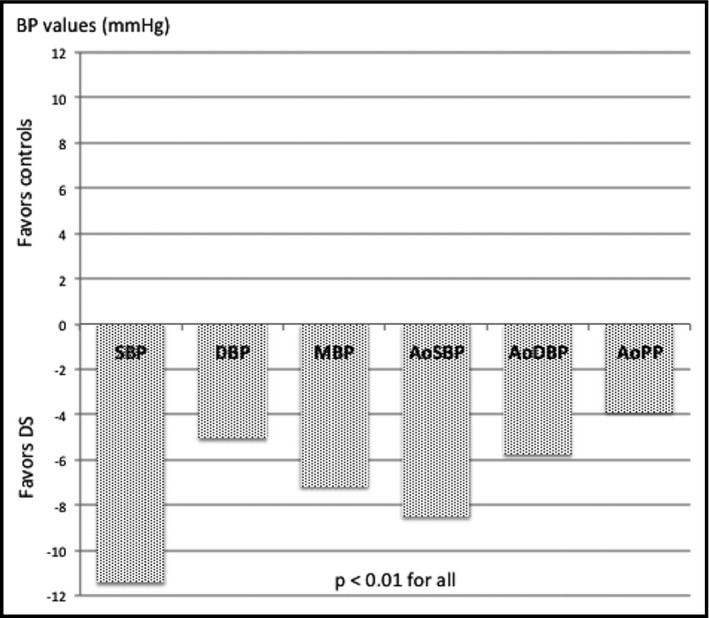
Differences in estimated peripheral and aortic blood pressures between groups adjusted for age and sex. SBP indicates systolic blood pressure; DBP, diastolic blood pressure; MBP, mean blood pressure; AoSBP, estimated aortic systolic blood pressure; AoDBP, estimated aortic diastolic blood pressure; AoPP, aortic pulse pressure.
Multilinear regression analysis (Figure S1 in Supplementary Appendix) demonstrated that age, fasting glucose values, and PTH levels were significantly correlated with PWV in the DS group, whereas age, total cholesterol levels, and PTH levels were significantly related to cIMT in this group. Sex, hypothyroidism, truncular obesity, and the presence of metabolic syndrome were not significantly associated with PWV or cIMT values in the DS group. Regarding the control group, PWV was significantly related to several anthropometric, biochemical, and hemodynamic parameters (age, systolic and diastolic BP, weight, BMI, body fat percentage, total cholesterol levels, and LDL cholesterol); in this group, cIMT values were significantly related to age and BMI only. PWV correlated significantly with cIMT (P=.014).
Adults with DS who had hyperparathyroidism (9.76%), all of which being secondary to vitamin D insufficiency, had significantly higher values of PWV than those with PTH values within normal limits (7.11 m/s vs 5.7 m/s, P=.01); this association was independent of calcium, phosphorus, vitamin D, and BP values (Figure 2).
Figure 2.
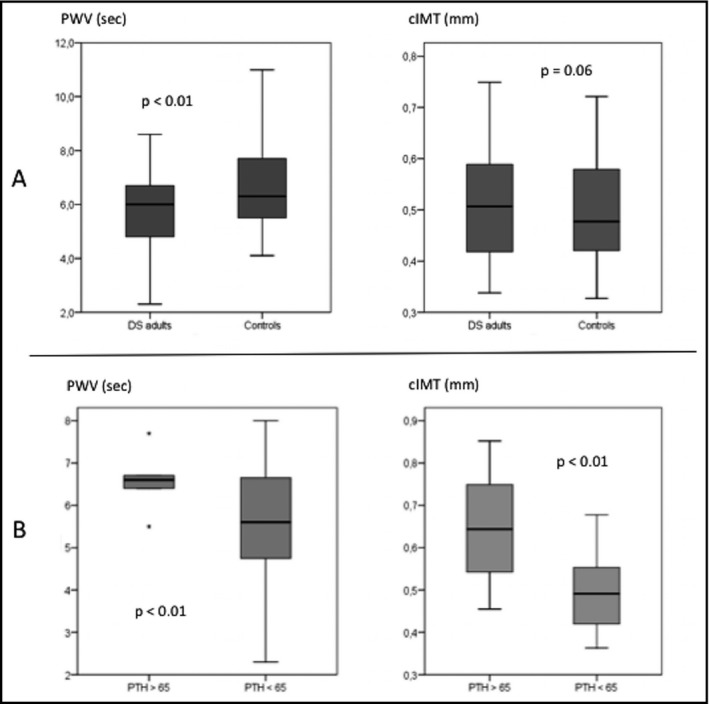
Differences in surrogate markers of atherosclerosis between groups (A). Differences in carotid‐femoral pulse wave velocity (PWV) and carotid intima‐media thickness (cIMT) in adults with Down syndrome (DS) regarding hyperparathyroidism status (B). PTH indicates parathyroid hormone.
Adults with DS had a higher prevalence of hypothyroidism (66%). The condition was subclinical in 41% and did not account for differences in lipid profile or subclinical vascular disease parameters.
The use of medication is shown in Table S1 in the Supplementary Appendix. Adults with DS received significantly more antidepressants (selective serotonin uptake inhibitors in all cases), neuroleptic drugs, vitamin D and B supplements, and levothyroxine, while antihypertensive drugs were prescribed only in the control group.
Discussion
Our study showed that carotid‐femoral PWV significantly decreased by 1 m/s in the DS group than in the control group because of lower systolic BP in the DS group. Thus, after adjustment for systolic BP, age, and sex, we failed to demonstrate any differences for surrogate markers of atherosclerosis (PWV and cIMT) after multivariate analysis. These findings are in accordance with the only two previous studies assessing subclinical atherosclerosis in individuals with DS based on cIMT and PWV. Consistent with Rodrigues and colleagues,22 we found that chronic low BP values could play a key role in reducing arterial stiffness in adults with DS. These findings confirm the importance of BP as the main determinant of arterial stiffness, both in adults with DS and in adults without DS, although genetic differences in arterial wall architecture and function, which affect endothelial function and elasticity, are also important. All BPs (peripheral [systolic, diastolic, and mean] and estimated aortic pressures [systolic, diastolic, and pulse pressure]) were significantly lower in the DS group.
Surrogate markers of atherosclerosis correlate with anthropometric, hemodynamic, and lipid parameters in the general population,19 as we found in the control group in our study. However, they do not seem to behave in the same way in adults with DS, as carotid‐femoral PWV depended on age, fasting glucose levels, and PTH levels; cIMT depended on age, total cholesterol, and PTH levels. None of the anthropometric variables showed a statistical association with arterial stiffness in this population.
On the whole, traditional CVD risk factors correlated poorly with PWV and cIMT values in adults with DS, suggesting that atherosclerosis and arterial stiffening in this population are not related to the same conditions as in adults without DS. Furthermore, this hypothesis could imply that traditional cardiovascular risk prediction tools may not be valid in adults with DS.
Epigenetic mechanisms are crucial in vascular damage and in characterizing the phenotype in DS individuals. Firstly, overexpression of genes located in trisomic chromosome 21 regulating DNA methylation machinery (such as DYRK1A, CHAF1B, and HMGN1) are likely to alter gene expression. Secondly, oxidative stress from overexpression of a variety of chromosome 21 genes could also induce DNA methylation.30
The association between hyperparathyroidism and CVD risk is well established.31, 32 Moreover, the influence of high PTH levels on arterial stiffness has recently been described by several authors, not only in individuals with primary hyperparathyroidism33 but also in individuals with vitamin D insufficiency34; however, firm evidence is lacking, as this association has not been found in other studies.35 Pathophysiological mechanisms for a possible association have not been clearly elucidated. The correlation between PTH levels and BP regulation has been largely studied, with a great variety of mechanisms underlying such association: (1) increased serum and intracellular calcium raises vascular tone in smooth muscles, and thus peripheral arterial resistance36; (2) activation of the renin‐angiotensin‐aldosterone leads to vasoconstriction37; (3) the upregulation of the sympathetic nervous system and the impaired endotelial vasodilatory function result in increased arterial siffness38; and (4) hyperparathyroidism‐induced renal damage, in part due to an increase in intracellular calcium in renal proximal tubule cells.39 Schlüter and colleagues,40 on the other hand, described a direct effect of PTH on myocytes through activation of protein kinase C, which resulted in increased cellular protein mass. The association between PTH and higher cIMT values has also been described,41 although not in individuals with DS.
In our study, PTH values were an independent determinant of both PWV and cIMT in the DS group. For the whole sample, secondary hyperparathyroidism was associated with a significant increase of 1.4 m/s in PWV values compared with individuals with normal PTH levels. This correlation was independent of calcium, phosphorus, and vitamin D levels, as well as of any traditional cardiovascular risk factor, including systolic BP. The fact that these deleterious effects of elevated plasma PTH levels may also be present in adults with DS is especially important, since vitamin D insufficiency seems to be a prevalent condition in this population.
We found no statistical association between the presence of hypothyroidism and PWV or cIMT values.
Prevalence of obesity seems to be higher in community‐residing individuals with DS than in those living in more supervised settings, such as special institutions for intellectually disabled persons.42 In our study, the prevalence of obesity was almost three‐fold higher in the DS group after adjustment for age and sex, and BMI was a mean 4.57 kg/m2 higher in adults with DS. We did not find any statistical difference in abdominal obesity between the groups; this discordance between BMI and abdominal obesity (kappa index, 0.24) could probably be the result of the fact that individuals with DS are much shorter than those without DS (21 cm in our study, P<.001); therefore, classic cutoff values of 88 cm and 102 cm for abdominal obesity in women and men, respectively, are probably not valid for individuals with DS.
We failed to identify any significant differences regarding lipid profile between the groups. Although available data addressing dyslipidemia in persons with DS are plentiful, contradictory results prevent firm conclusions from being drawn. Worsening of lipid profile has been described in individuals with hypothyroidism43 and in those taking antidepressants and antipsychotic drugs.44, 45 In our population, dyslipidemia was 1.6‐fold higher in hypothyroid persons than in euthyroid persons (P=.01), although a poor relationship was found between the prevalence of dyslipidemia and medical treatment, probably because of the low proportion of persons taking antidepressants and antipsychotics (27% and 10%, respectively, for adults with DS).
All the adults with DS in our study lived in a community setting. The results for the Block Dietary Fat Screener and Block Fruit/Vegetable/Fiber Screener questionnaires show that individuals with DS followed a Mediterranean diet more closely than controls. Thus, differences in BMI and waist‐to‐height ratio seem not to be associated with dietary habits. As far as we know, this is the first study to assess dietary habits in a Mediterranean cohort. However, we believe a Mediterranean diet could have accounted for the lower prevalence of overweight and obesity in our cohort than in populations not following a Mediterranean diet46 and for the lower prevalence of dyslipidemia in individuals with DS than in previous studies in non‐Mediterranean settings.47
Study Strengths and Limitations
Our study has several strengths. To our knowledge, it is the first to describe a comprehensive CVD profile in a population of adults with DS in a Mediterranean setting, including a thorough evaluation of surrogate markers of atherosclerosis such as PWV and cIMT. Moreover, a substantial number of controls were siblings of individuals with DS, thus reducing the hypothetical effect of genetic and environmental confounding factors. Finally, we defined broad inclusion criteria for the DS group in order to enable the application of our results to clinical practice. Nevertheless, our study is subject to a series of limitations. Firstly, the sample size was limited to 51 adults with DS and 51 controls, although it is still the largest sample among studies assessing surrogate markers of atherosclerosis in adults with DS. Secondly, we decided to measure cIMT over the distal centimeter of the common carotid artery owing to the specific anthropometric characteristics of adults with DS (higher cIMT and shorter neck), although it is known that the common carotid artery is generally less affected by systemic atherosclerosis than the carotid bifurcation or the internal carotid artery.
Conclusions
Our analysis of surrogate markers of atherosclerosis indicates that premature aging in adults with DS does not affect the arterial tree. Low BP values seem to play an essential role in cardiovascular protection in this population. While no correlation was observed between PWV and cIMT and obesity and lipid parameters in adults with DS (in contrast with the healthy population), elevated PTH values were significantly correlated with both markers in this group. We also found a lower prevalence of obesity and dyslipidemia than in previous data, suggesting a hypothetical impact of a Mediterranean diet. Our findings indicate that the use of surrogate markers of atherosclerosis such as PWV and cIMT could be very useful when evaluating CVD risk in adults with DS, in whom CVD risk assessment tools do not seem to be reliable. Further information is required to establish the exact mechanisms for CVD protection and the particular role of subclinical vascular disease in adults with DS.
Conflict of Interest/Funding
The authors declare no conflicts of interest or external financial support.
Supporting information
Figure S1. Multilinear regression analysis between pulse wave velocity (PWV) (left), carotid intima‐media thickness (cIMT) (right), and significant variables in the Down syndrome group.
Table S1. Medication use by group, No. (%).
Acknowledgment
We would like to thank the participants and care providers for their participation and assistance in the research project.
J Clin Hypertens (Greenwich). 2017;19:205–211. DOI: 10.1111/jch.12980. © 2016 Wiley Periodicals, Inc.
References
- 1. Perk J, De Backer G, Gohlke H, et al. European guidelines on cardiovascular disease prevention in clinical practice. The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635–1701. [DOI] [PubMed] [Google Scholar]
- 2. SIGN (Scottish Intercollegiate Guidelines Network) . Risk Estimation and the Prevention of Cardiovascular Disease. A National Clinical Guideline, 2007. Report No. 97. Available at http://www.sign.ac.uk/pdf/sign97.pdf.
- 3. Leal J, Luengo‐Fernandez R, Gray A, et al. Economic burden of cardiovascular diseases in the enlarged European Union. Eur Heart J. 2006;27:1610–1619. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . Global health risks: mortality and burden of disease attributable to selected major risks. WHO Library Cataloguing‐in‐Publication Data, 2009:10. Available at http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf.
- 5. Frid C, Drott P, Lundell B, et al. Mortality in Down's syndrome in relation to congenital malformations. J Intell Disabil Res. 1999;43:234–241. [DOI] [PubMed] [Google Scholar]
- 6. Beange H, McElduff A, Baker W. Medical disorders of adults with mental retardation: a population study. Am J Ment Retard. 1995;99:595–604. [PubMed] [Google Scholar]
- 7. Melville CA, Cooper SA, McGrother CW, et al. Obesity in adults with Down syndrome: a case‐control study. J Intell Disabil Res. 2005;49:125–133. [DOI] [PubMed] [Google Scholar]
- 8. Pueschel SM, Craig WY, Haddow JE. Lipids and lipoproteins in persons with Down's syndrome. J Intell Disabil Res. 1992;36:365–369. [DOI] [PubMed] [Google Scholar]
- 9. Bergholdt R, Eising S, Nerup J, Pociot F. Increased prevalence of Down's syndrome in individuals with type 1 diabetes in Denmark: a nationwide population‐based study. Diabetologia. 2006;49:1179–1182. [DOI] [PubMed] [Google Scholar]
- 10. Richards BW, Enver F. Blood pressure in Down's syndrome. J Ment Defic Res. 1979;23:123–135. [DOI] [PubMed] [Google Scholar]
- 11. Murdoch JC, Rodger JC, Rao SS, et al. Down's syndrome: an atheroma‐free model? BMJ. 1977;2:226–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ylä‐Herttuala S, Luoma J, Nikkari T, Kivimäki T. Down's syndrome and atherosclerosis. Atherosclerosis. 1989;76:269–272. [DOI] [PubMed] [Google Scholar]
- 13. Raina T, McGrath E, Gunn J. Myocardial infarction in a patient with down syndrome: a case report and review of the literature. Clin Cardiol. 2011;34:87–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hopkins WE, Fukagawa NK, Sobel BE, Schneider DJ. Plasminogen activator inhibitor type 1 in adults with Down syndrome and protection against macrovascular disease. Am J Cardiol. 2000;85:784–786. [DOI] [PubMed] [Google Scholar]
- 15. Ait Yahya‐Graison E, Aubert J, Dauphinot L, et al. Classification of human chromosome 21 gene‐expression variations in Down syndrome: impact on disease phenotypes. Am J Hum Genet. 2007;81:475–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fernhall B, Otterstetter M. Attenuated responses to sympathoexcitation in individuals with Down syndrome. J Appl Physiol. 2003;94:2158–2165. [DOI] [PubMed] [Google Scholar]
- 17. Yang Q, Rasmussen SA, Friedman JM. Mortality associated with Down's syndrome in the USA from 1983 to 1997: a population‐based study. Lancet. 2002;359:1019–1025. [DOI] [PubMed] [Google Scholar]
- 18. Bittles AH, Glasson EJ. Clinical, social and ethical implications of changing life expectancy in Down syndrome. Dev Med Child Neurol. 2004;46:282–286. [DOI] [PubMed] [Google Scholar]
- 19. Lorenz MW, Markus HS, Bots ML, et al. Prediction of clinical cardiovascular events with carotid intima‐media thickness: a systematic review and meta‐analysis. Circulation. 2007;115:459–467. [DOI] [PubMed] [Google Scholar]
- 20. Willum‐Hansen T, Staessen JA, Torp‐Pedersen C, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. [DOI] [PubMed] [Google Scholar]
- 21. Draheim CC, Geijer JR, Dengel DR. Comparison of intima‐media thickness of the carotid artery and cardiovascular disease risk factors in adults with versus without the Down syndrome. Am J Cardiol. 2010;106:1512–1516. [DOI] [PubMed] [Google Scholar]
- 22. Rodrigues AN, Coelho LC, Goncalves WL, et al. Stiffness of the large arteries in individuals with and without Down syndrome. Vasc Health Risk Manag. 2011;7:375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mancia G, De Backer G, Management of arterial hypertension of the European Society of Hypertension , European Society of Cardiology . Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;2007:1105–1187. [DOI] [PubMed] [Google Scholar]
- 24. Tsigos C, Hainer V, Basdevant A, et al. Obesity Management Task Force of the European Association for the Study of Obesity. Management of obesity in adults: European Clinical Practice Guidelines. Obes Facts. 2008;1:106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 26. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35:S64–S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Block G, Gillespie C, Rosenbaum EH, Jenson C. A rapid food screener to assess fat and fruit and vegetable intake. Am J Prev Med. 2000;18:284–288. [DOI] [PubMed] [Google Scholar]
- 28. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 29. de Groot E, Hovingh GK, Wiegman A, et al. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation. 2004;109:III33–III38. [DOI] [PubMed] [Google Scholar]
- 30. Gopalakrishnan S, Van Emburgh BO, Robertson KD. DNA methylation in development and human disease. Mutat Res. 2008;2:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rostland SG, Drueke TB. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int. 1999;56:383–392. [DOI] [PubMed] [Google Scholar]
- 32. Andersson P, Rydberg E, Willenheimer R. Primary hyperparathyroidism and heart disease—a review. Eur Heart J. 2004;25:1776–1787. [DOI] [PubMed] [Google Scholar]
- 33. Rubin M, Maurer M, McMahon D, et al. Arterial stiffness in mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2005;90:3326–3330. [DOI] [PubMed] [Google Scholar]
- 34. Casado J, Parra P, Vega L, Suárez C. Relation between parathyroid hormone and cardiovascular risk in patients with vitamin D deficiency. Med Clin. 2013;141:292–294. [DOI] [PubMed] [Google Scholar]
- 35. Barletta G, De Feo ML, Del Bene R, et al. Cardiovascular effects of parathyroid hormone: a study in healthy subjects and normotensive patients with mild primary hyperparathyroidism. J Clin Endocrinol Metab. 2000;85:1815–1821. [DOI] [PubMed] [Google Scholar]
- 36. Chopra S, Cherian D, Jacob JJ. The thyroid hormone, parathyroid hormone and vitamin D associated hypertension. Indian J Endocrinol Metab. 2011;15(Suppl4):S354–S360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gennari C, Nami R, Gonnell S. Hypertension and primary hyperparathyroidism: the role of adrenergic and renin‐angiotensin‐aldosterone systems. Mineral Electroly Metab. 1995;21:77–81. [PubMed] [Google Scholar]
- 38. Nilsson IL, Åberg J, Rastad J, Lind L. Endothelial vasodilatory dysfunction in primary hyperparathyroidism is reversed after parathyroidectomy. Surgery. 1999;126:1049–1055. [DOI] [PubMed] [Google Scholar]
- 39. Hedbäck GM, Odén AS. Cardiovascular disease, hypertension and renal function in primary hyperparathyroidism. J Intern Med. 2002;251:476–483. [DOI] [PubMed] [Google Scholar]
- 40. Schlüter K, Wingender E, Piper HM. Parathyroid hormone‐related protein antagonizes the action of parathyroid hormone on adult cardiomyocytes. J Biol Chem. 1996;271:3074–3078. [DOI] [PubMed] [Google Scholar]
- 41. Nuzzo V, Tauchmanova L, Fonderico F, et al. Increased intima‐media thickness of the carotid artery wall, normal blood pressure profile and normal left ventricular mass in subjects with primary hyperparathyroidism. Eur J Endocrinol. 2002;147:453–459. [DOI] [PubMed] [Google Scholar]
- 42. Rimmer JH, Yamaki K. Obesity and intellectual disability. Ment Retard Dev Disabil Res Rev. 2006;12:22–27. [DOI] [PubMed] [Google Scholar]
- 43. Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician. 2012;86:244–251. [PubMed] [Google Scholar]
- 44. Colotto M, Vinci F, Vo Hong N, et al. Effect of treatment with selective serotonin reuptake inhibitors on lipid profile: state of the art. Clin Ter. 2012;163:e41–e45. [PubMed] [Google Scholar]
- 45. Newcomer JW. Second‐generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19:1–93. [DOI] [PubMed] [Google Scholar]
- 46. Bulló M, Garcia‐Aloy M, Martínez‐González MA, et al. Association between a healthy lifestyle and general obesity and abdominal obesity in an elderly population at high cardiovascular risk. Prev Med. 2011;53:155–161. [DOI] [PubMed] [Google Scholar]
- 47. de Winter CF, Bastiaanse LP, Hilgenkamp TI, et al. Cardiovascular risk factors (diabetes, hypertension, hypercholesterolemia and metabolic syndrome) in older people with intellectual disability: results of the HA‐ID study. Res Dev Disabil. 2012;33:1722–1731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Multilinear regression analysis between pulse wave velocity (PWV) (left), carotid intima‐media thickness (cIMT) (right), and significant variables in the Down syndrome group.
Table S1. Medication use by group, No. (%).


