Abstract
Blood pressure variability is an entity that characterizes the continuous and dynamic fluctuations that occur in blood pressure levels throughout a lifetime. This phenomenon has a complex and yet not fully understood physiological background and can be evaluated over time spans ranging from seconds to years. The present paper provides a short overview of methodological aspects, clinical relevance, and potential therapeutic interventions related to the management of blood pressure variability.
Keywords: Blood pressure monitoring, Blood pressure variability, Hypertension, organ damage, vardiovascualar events
1. INTRODUCTION
Blood pressure (BP) is a physiologic parameter characterized by continuous dynamic fluctuations that occur over time spans ranging from seconds to years. These fluctuations are the result of a complex interplay between environmental (eg, seasons, altitude, stress), physical (posture or volemia), and emotional factors inducing BP changes, and cardiovascular regulatory mechanisms aimed at maintaining the so‐called BP “homeostasis.” These mechanisms are intended to ensure a constantly adequate organ perfusion, being able to modify BP levels in response to the changing demands of different organs (eg, BP increase when facing physical or emotional stress and BP reduction during sleep). The size and patterns characterizing these BP variations define the term BP variability (BPV).1 Thus, BPV represents a dynamic and characteristic physiologic feature of the cardiovascular system function, its size being widely different among individual subjects in response to their daily challenges, and also determined by the characterisitc reactivity of their cardiovascular control mechanisms. On the other hand, from a clinical perspective, BPV could be seen as a source of noise that creates difficulties in assessing the individual's “true” BP level. Moreover, evidence is now available to support its role also as an independent predictor of cardiovascular risk.1 Finally, recent studies have suggested that an increased BPV could be a possible target for pharmacological treatment.
2. MEASURING BPV
The term BPV encompasses a wide range of BP variations, occurring over seconds or minutes (very short‐term BPV), along 24 hours (short‐term BPV, usually assessed by ambulatory BP monitoring), and between days (mid‐term or day‐to‐day BPV, assessed with home BP monitoring). Long‐term BPV has also been described, including seasonal BP variations and changes between clinic visits over months or years (visit‐to‐visit BPV; Figure 1).1 Generally, the changes in BP can be divided into those without regular features (random or erratic changes) and those characterized by well‐defined patterns over time, typically related to biological rhythms or behavioral factors (eg, rhythmic fluctuations with periods of 3 seconds, 10 seconds or slower, nocturnal BP fall, siesta dip, morning BP surge, seasonal variations). The former are usually described using simple measures of dispersion (such as standard deviation [SD]) of average values over a given time window or estimates that also take into account the sequence of measurements over time (average real variability [ARV], the time rate of variations; see Table 1). Among more sophisticated methods for BPV assessment, spectral analysis techniques are particularly relevant when describing faster BP changes in beat‐by‐beat recordings, but can also be used for discontinuous 24‐hour BP monitoring. In fact, the so‐called “residual” variability is obtained by removing the slower cyclic components of 24‐hour BP variation using Fourier analysis.
Figure 1.
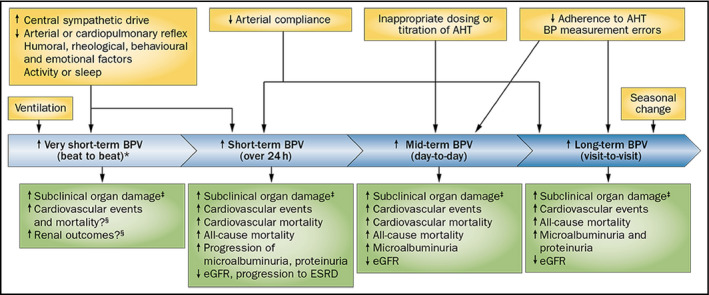
Various types of BPV, their determinants, and prognostic relevance for cardiovascular and renal outcomes. From Parati et al1 by permission. *Assessed in laboratory conditions; ‡cardiac, vascular, and renal subclinical organ damage; § BPV on a beat‐to‐beat basis has not been routinely measured in population studies. Abbreviations: AHT, antihypertensive treatment; BP, blood pressure; BPV, blood‐pressure variability; ESRD, end‐stage renal disease; eGFR, estimated glomerular filtration rate
Table 1.
Summary of principal indices of blood pressure variability
| Type of Index | Type of BPV assessed |
|---|---|
Frequency:
|
|
Dispersion:
|
|
Sequence:
|
|
Instability:
|
|
| Specific patterns of BPV | |
|
|
From 22, by permission.
Assessment of Short term BPV only.
Not for assessment of Short term BPV.
The few studies directly comparing the prognostic value of different estimates of BPV did not provide clear indications as to which index should be preferred. At present, a reasonable choice could be to use the indices supported by the strongest outcome evidence, at least until better solutions are found. Based on a recent meta‐analysis,2 the preferred indices might include SD for the clinic (visit‐to‐visit) and home BPV, and ARV, or SD (specifically, the “weighted” SD mentioned below) for 24‐hour BPV. It is also important to consider that that these estimates of BPV are directly correlated with mean BP levels, and therefore it is important to adjust them for average BP. In research, this can be achieved with statistical methods, while in individual patients, a mathematical correction can be made by calculating the coefficient of variation (CV = SD*100/mean) or the variation independent of the mean (VIM).
In regards to 24‐hour BPV, one should consider that 24‐hour SD is confounded by the contribution of nocturnal BP fall and generally should not be used for cardiovascular risk assessment.3 Instead, indices unaffected by day‐to‐night changes should be preferred, such as ARV or weighted 24‐hour SD (ie, the average of daytime and nighttime SD corrected for the respective duration of day and night). Daytime and nighttime SD, used separately, may also be applied, but it is unclear which should be preferred. Nocturnal BPV appeared superior to daytime BPV in 2 studies, but this finding should be further confirmed.4, 5 The methodology for assessing specific BP patterns (ie, nocturnal dipping, morning surge, etc.) is not discussed in this article.
3. CLINICAL RELEVANCE
Over the years, some studies have demonstrated an independent relationship between the different components of BPV and organ damage or cardiovascular events persisting even after adjustment to average BP levels. Concerning 24‐hour BPV, although a collaborative analysis of population data from different countries (each of them, however, applying somewhat different methods for 24‐hour BP monitoring) concluded that the independent prognostic value of 24‐hour BPV might be limited,6 this was not confirmed in other populations.7 A recent study formally demonstrated that BPV (especially nighttime SD) might have significant potential to reclassify cardiovascular risk.4 More recently, the prognostic role of day‐by‐day home BPV and clinic‐within‐visit and visit‐to‐visit BPV was also addressed by outcome studies, indicating their possible role in risk stratification. The results of the available studies were formally summarized in recent systematic reviews and meta‐analyses.2, 8, 9 In particular, the paper by Stevens et al considered all 3 principal types of BPV (24‐hour, home, and clinic), and for all of them, was able to show a significant impact on the risk of events and/or mortality. Although only a few studies fulfilled the formal requirements for inclusion in this meta‐analysis, the results indicate that all the BPV indices considered were independently associated with outcome, with the respective hazard ratios being quite similar.2 This is not to say that these indices are to be considered interchangeable; in fact, the correlation between them is quite poor.10 Moreover, the relationship with outcomes may differ depending on the choice of BPV estimate. Generally, most studies indicate systolic BPV as being more closely related to outcome, but as far as 24‐hour BPV is considered, diastolic BPV seems more closely related to events, at least in adults (Figure 2), while a more prominent role of SBP variability is suggested in the elderly.6, 7 The pathophysiological meaning of these differences is unclear, but it should be emphasized that BPV (in particular systolic BPV) correlates with arterial stiffness,11 which in turn is related to aging and increasing SBP (but not DBP) levels. In fact, a recent large study on the relationship between BPV and chronic kidney disease (where arterial stiffness is also relevant) showed that with worsening renal function, an increase occurs in systolic, but not in diastolic, BPV.12 This might imply that systolic BPV reflects primarily vascular stiffness and aging, while diastolic BPV has a different pathophysiological background such as, for instance, impaired autonomic function with increased sympathetic activity, and endothelial dysfunction.13
Figure 2.
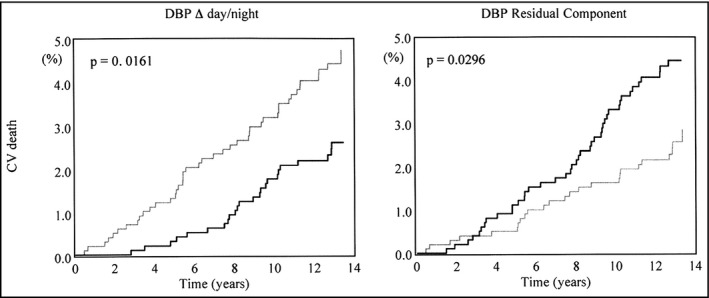
Opposite impact on cardiovascular mortality of day–night change in diastolic blood pressure (DBP; left) and of “erratic” residual DBP variability (right). Kaplan‐Meier curves are shown for subjects with values above (black lines) and below (gray lines) the population median. From Mancia et al7 used by permission
4. TREATMENT EFFECTS ON BPV
BPV is known to decrease along with the decrease in average BP induced by antihypertensive drug treatment. It is less clear whether some antihypertensive drug classes might show a more pronounced ability to reduce BPV, compared with others. Undoubtedly, long‐acting dihydropyridine calcium antagonists seem the most promising drugs in this regard. This class of antihypertensive agents was indeed found to be was more effective in reducing BPV and the related organ damage in experimental animals.14 Currently, several clinical studies have also consistently supported the potential advantage of these drugs in reducing ambulatory, home, and clinic BPV, but these papers mainly included post hoc analyses of trials, while ad hoc studies are few.15, 16 Apart from the use of specific drugs, it seems fundamental that iatrogenic increase in BPV, induced for example by short‐acting antihypertensive drugs, should be avoided. In fact, the smoothness index, an index that includes information on the homogeneity of antihypertensive drug effects over 24 hours, correlates with both a reduction in 24 hour BPV and the regression of organ damage in hypertension.17 Also, the combination of long‐acting drugs seems useful to buffer excessive BP fluctuations over a 24 hour period, with their administration being characterized by the highest rating in the smoothness index.18, 19, 20 In the long‐term management of hypertensive patients (concerning clinic BPV) adherence to prescribed treatment appears to also be an important factor.21
5. CLINICAL CONSIDERATIONS
The possible clinical significance of BPV is not yet fully established, but 3 aspects should be considered. (1) BPV by definition introduces uncertainty in assessing subject's BP status, especially when spot clinic measurements are used. (2) Assessment of BPV might be useful in improving cardiovascular risk stratification although the size of its actual independent contribution in this regard remains to be better documented. (3) Increased BPV may be a target for treatment, aiming at improved outcome possibly without generating additional costs. The choice of long‐acting drugs, in particular, dihydropyridine calcium antagonists and the combination of long‐lasting compounds, might be indicated in individuals with elevated BPV, although the possible clinical benefits from such an approach have not yet been fully demonstrated.
CONFLICT OF INTEREST
None.
Parati G, Stergiou GS, Dolan E, Bilo G. Blood pressure variability: clinical relevance and application. J Clin Hypertens. 2018;20:1133–1137. 10.1111/jch.13304
REFERENCES
- 1. Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood‐pressure variability. Nat Rev Cardiol. 2013;10:143‐155. [DOI] [PubMed] [Google Scholar]
- 2. Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta‐analysis. BMJ. 2016;354:i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bilo G, Giglio A, Styczkiewicz K, et al. A new method for assessing 24‐h blood pressure variability after excluding the contribution of nocturnal blood pressure fall. J Hypertens. 2007;25:2058‐2066. [DOI] [PubMed] [Google Scholar]
- 4. Palatini P, Reboldi G, Beilin LJ, et al. Added predictive value of nighttime blood pressure variability for cardiovascular events and mortality: the ambulatory blood pressure‐international study. Hypertension. 2014;64:487‐493. [DOI] [PubMed] [Google Scholar]
- 5. Pringle E, Phillips C, Thijs L, et al. Syst‐Eur investigators. Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J Hypertens. 2003;21:2251‐2257. [DOI] [PubMed] [Google Scholar]
- 6. Hansen TW, Thijs L, Li Y, et al. Prognostic value of reading‐to‐reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55:1049‐1057. [DOI] [PubMed] [Google Scholar]
- 7. Mancia G, Bombelli M, Facchetti R, et al. Long‐term prognostic value of blood pressure variability in the general population: results of the Pressioni Arteriose Monitorate e Loro Associazioni Study. Hypertension. 2007;49:1265‐1270. [DOI] [PubMed] [Google Scholar]
- 8. Mena LJ, Felix VG, Melgarejo JD, Maestre GE. 24‐hour blood pressure variability assessed by average real variability: a systematic review and meta‐analysis. J Am Heart Assoc. 2017;6:e006895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang J, Shi X, Ma C, et al. Visit‐to‐visit blood pressure variability is a risk factor for all‐cause mortality and cardiovascular disease. J Hypertens. 2017;35:10‐17. [DOI] [PubMed] [Google Scholar]
- 10. Abellán‐Huerta J, Prieto‐Valiente L, Montoro‐García S, Abellán‐Alemán J, Soria‐Arcos F. Correlation of blood pressure variability as measured by clinic, self‐measurement at Home, and Ambulatory Blood Pressure Monitoring. Am J Hypertens. 2018;31:305‐312. [DOI] [PubMed] [Google Scholar]
- 11. Schillaci G, Bilo G, Pucci G, et al. Relationship between short‐term blood pressure variability and large‐Artery stiffness in human hypertension: findings from 2 large databases. Hypertension. 2012;60:369‐377. [DOI] [PubMed] [Google Scholar]
- 12. Sarafidis PA, Ruilope LM, Loutradis C, et al. Blood pressure variability increases with advancing chronic kidney disease stage. J Hypertens. 2018;36:1076‐1085. [DOI] [PubMed] [Google Scholar]
- 13. Bilo G, Parati G. Blood pressure variability and kidney disease. J Hypertens. 2018;36:1019‐1021. [DOI] [PubMed] [Google Scholar]
- 14. Liu J‐G, Xu L‐P, Chu Z‐X, Miao C‐Y, Su D‐F. Contribution of blood pressure variability to the effect of nitrendipine on end‐organ damage in spontaneously hypertensive rats. J Hypertens. 2003;21:1961‐1967. [DOI] [PubMed] [Google Scholar]
- 15. Eguchi K. Effects of antihypertensive therapy on blood pressure variability. Curr Hypertens Rep. 2016;18:75. [DOI] [PubMed] [Google Scholar]
- 16. Kollias A, Stergiou GS, Kyriakoulis KG, Bilo G, Parati G. Treating visit‐to‐visit blood pressure variability to improve prognosis. Hypertension. 2017;70:862‐866. [DOI] [PubMed] [Google Scholar]
- 17. Parati G, Omboni S, Rizzoni D, Agabiti‐Rosei E, Mancia G. The smoothness index: a new, reproducible and clinically relevant measure of the homogeneity of the blood pressure reduction with treatment for hypertension. J Hypertens. 1998;16:1685‐1691. [DOI] [PubMed] [Google Scholar]
- 18. Parati G, Schumacher H, Bilo G, Mancia G. Evaluating 24‐h antihypertensive efficacy by the smoothness index: a meta‐analysis of an ambulatory blood pressure monitoring database. J Hypertens. 2010;28:2177‐2183. [DOI] [PubMed] [Google Scholar]
- 19. Parati G, Schumacher H. Blood pressure variability over 24 h: prognostic implications and treatment perspectives. An assessment using the smoothness index with telmisartan–amlodipine monotherapy and combination. Hypertens Res. 2014;37:187‐193. [DOI] [PubMed] [Google Scholar]
- 20. Parati G, Dolan E, Ley L, Schumacher H. Impact of antihypertensive combination and monotreatments on blood pressure variability: assessment by old and new indices. Data from a large ambulatory blood pressure monitoring database. J Hypertens. 2014;32:1326‐1333. [DOI] [PubMed] [Google Scholar]
- 21. Kronish IM, Lynch AI, Oparil S, et al. The association between antihypertensive medication nonadherence and visit‐to‐visit variability of blood pressure: findings from the antithpertensive and lipid‐lowering treatment to prevent heart attack trial. Hypertension. 2016;68:39‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parati G, Ochoa JE. Chapter 3: Blood pressure variability and blood pressure load. In: Dorobantu M, Mancia G, Grassi G, Voicu V, eds. Hypertension and Heart Failure‐ Epidemiology, Mechanisms and Treatment. Cham, Switzerland: Springer International Publishing AG; 2018. In press. [Google Scholar]


