Abstract
The purpose of this review is to identify, summarize, and critically appraise studies on dietary salt and health outcomes that were published from April to July 2016. The search strategy was adapted from a previous systematic review on dietary salt and health. We have revised our criteria for methodological quality and health outcomes, which are applied to select studies for detailed critical appraisals and written commentary. Overall, 28 studies were identified and are summarized in this review. Four of the 28 studies met criteria for methodological quality and health outcomes and five studies underwent detailed critical appraisals and commentary. Three of these studies found adverse effects of salt on health outcomes (chronic kidney disease and blood pressure) and two were neutral (fracture risk/bone mineral density and cognitive impairment).
Keywords: diet, hypertension, lifestyle modification, nutrition, sodium
1. INTRODUCTION
Meta‐analyses and systematic reviews examining the relationship between dietary salt and health outcomes1, 2 have been the basis for consensus that excess salt (sodium) consumption is associated with multiple adverse health outcomes, including a positive causal relationship with blood pressure (BP).3, 4 This evidence was the basis of the World Health Organization (WHO) dietary salt recommendations, that adults consume <5 g/d of salt (<2000 mg/d of sodium) and children consume lower amounts because of lower energy intakes.1 To prevent and manage noncommunicable diseases associated with excess salt consumption, the WHO set a global target of reducing dietary salt intake by 30% by 2025 and many countries worldwide have implemented salt reduction programs.5
The high profile of dietary salt research has resulted in a rapidly growing literature on the health effects of dietary salt. To keep scientific, clinical, and policy stakeholders up to date with the growing body of literature, regularly updated reviews and critical appraisals of studies relating to health outcomes are published in the Journal, alternating with reviews of studies relating to salt reduction implementation programs.6 The objective of this fourth health outcomes review is to summarize published articles on salt and health outcomes and to highlight and critically appraise the highest‐quality articles that were published between April and July 2016. This article also reports on an updated methodology developed and adopted to ensure an objective review of the most clinically relevant studies.
2. METHODOLOGY
A detailed description of the methodological approach used to identify published articles for this review has been previously reported.6 Briefly, articles were identified on a weekly basis through a MEDLINE search strategy, which was adapted from a previous systematic review used to develop the WHO guideline on dietary sodium intake.1, 2 Table 1 reports on the types of health outcome studies that are included and excluded in this search. This review includes health outcome studies identified during the weeks of April 4 to July 29, 2016 (Figure). Among identified articles, studies were selected to undergo a detailed critical appraisal based on the outcomes examined and methodological quality, as described below. A secondary set of articles was considered for inclusion if judged by the authors to be impactful based on novelty of findings or potential for generating public discourse or scientific controversy or for informing public health policy.
Table 1.
Inclusion and exclusion criteria for health outcome studies identified in weekly reviews
Included:
|
Excluded:
|
Figure 1.
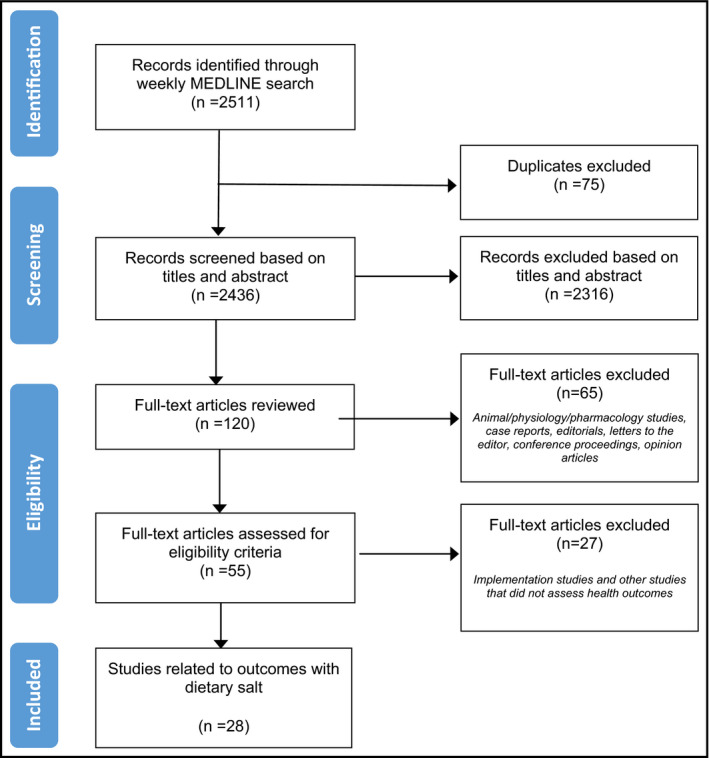
Flow diagram for studies identified from April 2016 to July 2016
Articles were selected for detailed critical appraisal considering a hierarchy of health outcomes, which were those classified based on relevance to patients (Table 2). Mortality (category I) and morbidity (category II) were considered critically important to patients, while symptoms/quality of life/functional status (category III) and the clinical diagnosis, prevention, or treatment of hypertension (category IV) and other clinical surrogate outcomes (category V) were considered important. Studies on physiologic and biomarker surrogate outcomes (category VI) were excluded from eligibility for detailed critical appraisal, as these outcomes are considered less important to patients. However, if an article included a category VI outcome as the primary outcome and category I to V outcomes as secondary outcomes, the study was considered for inclusion as long as it met methodological quality criteria.
Table 2.
Hierarchy of outcomes used to select studies for a detailed critical appraisal
| Type of outcome | Examples |
|---|---|
|
Category I Mortality reduction |
|
|
Category II Morbidity reduction |
|
|
Category III Symptoms/quality of life/functional status |
|
|
Category IV Clinical surrogate outcomes |
|
|
Category V Other clinical surrogate outcomes |
|
|
Category VI Physiologic/biomarker surrogate outcomes |
|
Methodological quality criteria, adapted from the systematic review used to develop the WHO sodium guidelines,3 were also considered when selecting articles for detailed critical appraisal and commentary (Table 3). Included in the appraised articles were randomized controlled trials (RCTs) that allocated at least one group of participants to reduced sodium intake and one group to higher sodium intake (control group), achieved an intake difference of ≥2.3 g salt (920 mg sodium) between intervention and control, and measured sodium intake with a 24‐hour urine collection. Studies with concomitant interventions (ie, antihypertensive drugs or other dietary interventions) were not appraised because the impact of sodium intake could not be independently assessed. Cohort studies included in the detailed appraisals had a prospective design; included 400 or more participants (continuous outcomes) or events (dichotomous outcomes); and measured sodium intake with 24‐hour urine collection, food record, 24‐hour food recall, or semiquantitative food frequency questionnaire (FFQ). Excluded from detailed critical appraisal were studies with cross‐sectional designs and those that included only sodium to potassium ratio as an exposure variable or related variables such as salty food preference (ie, the exposure must include sodium intake or excretion alone). Studies with a primary outcome of BP and hypertension (category IV) were only eligible for detailed critical appraisal if they were RCTs or systematic reviews of RCTs meeting the minimum methodological criteria listed above and were ≥4 weeks' duration. RCTs and cohort studies assessing category I, II, or V outcomes were eligible for appraisal if they had a duration of ≥1 year. RCTs and cohort studies assessing category III outcomes were eligible if they had a duration of ≥4 weeks. The quality of meta‐analyses and systematic reviews, which can include a heterogeneous combination of studies from high to low methodological quality, were judged by two independent reviewers for inclusion. In summary, articles included for detailed critical appraisals focused exclusively on RCTs, systematic reviews of RCTs, and prospective observational studies of clinically important patient outcomes (categories I to V) (Table 2).7
Table 3.
Criteria for methodological quality used to select studies for detailed critical appraisal
| Study design | Study quality criteria for inclusion |
|---|---|
| Randomized controlled trials |
|
| Cohort studies |
|
Two independent reviewers assessed articles for inclusion in the detailed critical appraisal, which included a risk of bias assessment and a written commentary. Risk of bias assessments were also conducted by two independent reviewers. RCTs were assessed using the Cochrane risk of bias tool.8 Observational, nonrandomized studies were assessed using a modified Cochrane risk of bias tool.9 For meta‐analyses, the AMSTAR tool was applied.10
3. RESULTS
The weekly searches identified 2511 citations, of which 93 possibly relevant health outcome studies met the criteria for full review (Figure). A total of 28 dietary salt studies met the inclusion criteria set for outcomes and research design: two meta‐analyses, three RCTs, five prospective cohort studies, five nonrandomized or uncontrolled trials, 11 cross‐sectional studies, and two case‐control studies. These studies are summarized in Table S1. The outcomes examined were diverse: one study assessed mortality outcomes (category I),11 two studies assessed morbidity outcomes (category II),12, 13 two studies assessed outcomes related to symptoms/quality of life/functional status (category III),14, 15 six studies assessed BP outcomes (category IV),16, 17, 18, 19, 20, 21 seven studies assessed other clinically relevant surrogate outcomes (category V),22, 23, 24, 25, 26, 27, 28 and 10 studies assessed physiologic outcomes (category VI).29, 30, 31, 32, 33, 34, 35, 36, 37, 38
Of 28 identified studies, four met the inclusion criteria for outcomes examined and methodological quality, and thus were included in the detailed risk of bias assessments and critical appraisals. One of these studies was an RCT and three were prospective cohort studies. An additional meta‐analysis that assessed the association between salt intake and hypertension in urban and rural populations in low‐ and middle‐income countries did not meet methodological quality criteria but was included because of the high burden of disease attributable to excess salt consumption and the subsequent importance of public policies on salt reduction in these regions.39 The studies found that: salt intake does not significantly impact fracture risk or bone mineral density (BMD) in postmenopausal women,13 high salt intake is associated with chronic kidney disease (CKD) progression and all‐cause mortality,12 salt intake does not modify the association between hypertension and cognitive decline in postmenopausal women,14 the association between salt intake and prevalence of hypertension is highest in urban populations compared with rural populations,19 and salt reduction results in clinically relevant BP reductions in people with diabetes mellitus.20 The risk of bias assessments for these five studies are included in Table S2 (a–e), a summary of study characteristics and results are shown in Table 4, and the written critical appraisals and commentary are below. No primary research studies in the physiologic category (category VI) met the minimum methodological criteria. A range of outcomes were captured by the studies considered to be of lower quality, including all‐cause mortality,11 headaches/migranes,15 BP,16, 17, 18, 21 cognitive function,22, 25 acne,23 obesity and ghrelin,24, 38 multiple sclerosis,26 BMD,27 gastric cancer,28 flow‐mediated dilatation,29, 35 pulse wave velocity,36 markers of the renin‐angiotensin‐aldosterone system,21 heart rate,31 insulin resistance and the metabolic syndrome,30, 37 brain tissue white matter hyperintensity,32 uric acid,33 and urinary albumin.34 Most of these studies found adverse effects of dietary salt on health, except for three that were neutral22, 25, 26 and one that found an inverse relationship between salt intake and headaches/migranes.15
Table 4.
Summary of studies that qualified for a detailed critical appraisal and commentary
| Study (country) | Study design | Participants | Study Duration | Dietary salt “dose” (actual mean intake per d) | Method of sodium intake measurement | Outcomes | Results |
|---|---|---|---|---|---|---|---|
| Category II: morbidity outcomes | |||||||
| Carbone et al (United States)13 | Prospective cohort | N=4426 postmenopausal women (Women's Health Initiative), mean age: 63.6 y (observational study) and 63.3 y (RCT study) | Mean follow‐up: 11.4 y |
Salt intake from FFQ: 6.1 g/d salt Calibrated salt intake: 7.5 g/d salt |
FFQ at baseline. Biomarker‐calibrated estimates obtained using 24‐h urine excretion |
Fracture risk and changes in BMD at the hip (total and femoral neck) and lumbar spine, measured with dual x‐ray absorptiometry; incident clinical fractures, measured by self‐report | There was no significant association between sodium intake and changes in BMD at the lumbar spine or hip, but high sodium intake was associated with reduced hip fractures (HR, 0.81; 95% CI, 0.67–0.97) |
| He et al (United States)12 | Prospective cohort | N=3757 patients with CKD (Chronic Renal Insufficiency Cohort Study), 56% men, age 21–74 y | Mean follow‐up period was not reported |
Total sample: 9.2 g/d salt Described by quartiles of sodium excretion: quartile 1: 5.2 g/d salt quartile 2: 7.8 g/d salt quartile 3: 10.0 g/d salt quartile 4: 14.0 g/d salt |
Average of three 24‐h urine sodium measurements | Primary outcome: CKD progression (defined as incident ESRD or halving of eGFR). Other outcomes were all‐cause mortality and composite outcome of CKD progression and mortality | High urinary sodium excretion was associated with increased risk of CKD progression (adjusted HR, 1.54; 95% CI, 1.23–1.92), mortality (HR, 1.45; 95% CI, 1.08–1.95), and the composite outcome of CKD progression and all‐cause mortality (HR, 1.43; 95% CI, 1.18–1.73) |
| Category III: Symptoms/quality of life/functional status outcomes | |||||||
| Haring et al (United States)14 | Prospective cohort | N=6426 postmenopausal women (Women's Health Initiative Memory Study), age 65–79 y | Median follow‐up: 9.1 y | Actual sodium intake was not reported. Data on sodium intake was used to classify respondents into three subgroups | FFQ at baseline. 24‐h urine excretion measurement performed in a subset to correct dietary self‐report for potential measurement errors | Incidence of cognitive decline (identified by the incidence of mild cognitive impairment or probable dementia, diagnosed based on the criteria by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) | Women with hypertension had a higher risk of cognitive decline compared with women without hypertension (HR, 1.20; 95% CI, 1.04–1.39), as did women with antihypertensive treatment and uncontrolled BP (HR, 1.30; 95% CI, 1.05–1.60). No significant interaction with sodium intake |
| Category IV: Clinical surrogate outcomes (BP) | |||||||
| Subasinghe et al (Australia)19 | Meta‐analysis of observational studies (longitudinal, case‐control, cross‐sectional) | N=134 916 from 18 studies conducted in low‐and middle‐income countries (11 in a rural population, 6 in an urban population, 1 in both) | Not reported | For the 9 studies that reported salt intake: 6.9 g/d to 42.3 g/d salt | Highly variable: household questionnaire, food records, FFQ, 24‐h recall | Prevalence of hypertension (defined as systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg and/or prescription of antihypertensive medication; and systolic BP ≥130 mm Hg and/or diastolic BP ≥85 mm Hg) | Association between salt intake and hypertension was greater in urban populations (pooled OR, 1.42; 95% CI, 1.19–1.69 per 1 g greater salt intake) than in rural populations (pooled OR, 1.07; 95% CI, 1.04–1.10) |
| Suckling et al (United Kingdom)20 | Double‐blind, crossover RCT | N=46 patients with diet‐controlled type 2 diabetes mellitus or impaired glucose tolerance and untreated normal or high normal BP, 52% men, mean age: 58 y | 12 wk (two 6‐wk periods) |
Baseline salt intake: 8.0 g/d salt Reduced salt (placebo) group: 6.8 g/d salt High salt (salt tablet) group: 9.7 g/d salt |
Average of two 24‐h urine sodium measurements | Clinic BP (oscillometric); 24‐h ambulatory BP monitoring; urinary albumin excretion; carotid‐femoral pulse wave velocity; endothelial function (digital volume pulse analysis) | Compared with the high salt group, the low salt group had reduced BP (systolic BP: –4.2 mm Hg, P<.001; diastolic BP: –1.7 mm Hg, P=.055) and lower urine ACR (median 0.64 vs 0.73, P<.05) |
Abbreviations: ACR, albumin/creatinine ratio; BMD, bone mineral density; BP, blood pressure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; FFQ, food frequency questionnaire; FMD, flow‐mediated dilatation; HR, hazard ratio; OR, odds ratio; RCT, randomized controlled trial.
Detailed critical appraisals of selected studies:
3.1. Is there an association between dietary sodium and BMD or fracture risk?
Carbone L, Johnson KC, Huang Y, et al. Sodium intake and osteoporosis. Findings from the Women's Health Initiative. J Clin Endocrinol Metab. 2016;101:1414–1421.
Design: Prospective observational study.
Setting: 40 US clinical centers participating in the Women's Health Initiative Study (WHI). Specifically, data from participants in the WHI‐Observational Study and the WHI‐Dietary Modification Trial was used.
Participants: N=69 735 postmenopausal women aged 50 to 79 years; median follow‐up: 11.4 years.
Exposure: Sodium intake, estimated by the semiquantitative Block FFQ. Biomarker‐derived calibration equations were used to correct for measurement error associated with self‐reported intake and adjusted for factors that impact BMD or fracture risk.
Outcomes: Fracture risk and BMD measured at the total hip, femoral neck, total spine, and total body. BMD was measured with dual x‐ray absorptiometry.
Risk of bias:
Sampling: Low risk.
Representativeness: High risk.
Reliability/validity of exposure: High risk.
Reliability/validity of outcome: Low risk.
Blinding of outcome assessment: Low risk.
Risk of selective outcome reporting: Low risk.
Confounding: High risk.
Sources of funding: National Heart, Lung, and Blood Institute; National Institutes of Health; and US Department of Health and Human Services.
Summary of results: When participants were stratified into lower and higher sodium intake groups, based on median salt intake of 7.2 g/d (2892 mg sodium/d), there was no association between sodium intake and risk of total fractures (adjusted hazard ratio [HR], 0.97; 95% CI, 0.92–1.01), lumbar spine fractures (adjusted HR, 0.88; 95% CI, 0.78–1.01), or other fractures (adjusted HR, 0.97; 95% CI, 0.96–1.03); however, there was reduced risk of hip fracture with higher sodium intake (adjusted HR, 0.81; 95% CI, 0.67–0.97). There was no association between sodium intake and changes in BMD at the lumbar spine, total hip, or femoral neck. Higher sodium intake was associated with greater total body BMD, but only at 3‐year follow‐up. There was no association between level of dietary sodium and BMD when participants were stratified based on sodium intake above or below the dietary recommendations (≤5.8 g/d salt, ≤2300 mg/d sodium).
Comment: This study found that the amount of sodium consumed has little or no impact on BMD or fracture risk at most skeletal sites and that intakes within current guideline recommendations are unlikely to influence osteoporosis outcomes in postmenopausal women. There are notable strengths of this study, including the large sample size, prospective design, long‐term follow‐up, and well‐ascertained outcomes in a relevant group of postmenopausal women; however, the data cannot be generalized to men or premenopausal women. There are some concerns related to dietary sodium exposure assessment, which could lead to significant misclassification. Dietary sodium estimates were based on self‐report with an FFQ and then biomarker calibrated using a modified version of a published protocol.40 The mean calibrated sodium levels were higher than the FFQ estimates. However, the authors did not report on the validity of their approach in estimating individual sodium intakes (ie, compared with 24‐hour urinary sodium). In addition, sodium consumption was measured only at baseline; thus, estimates do not reflect any changes in intake during the 11.4‐year follow‐up period. Finally, as discussed by the authors, residual confounding may have occurred, particularly in relation to physical activity, which was excluded from primary multivariable analyses.
3.2. Is there an association between urinary sodium and progression of CKD?
He J, Mills KT, Appel LJ, et al. Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol. 2016;27:1202–1212.
Design: Prospective cohort study.
Setting: Multicenter study of seven medical centers in the United States.
Participants: 3757 participants aged 21 to 74 years (mean age 55.7 years) with mild to moderate CKD (estimated glomerular filtration rate 20–70 mL/min/1.73 m2), of whom approximately half had diabetes mellitus.
Exposure: Urinary sodium excretion, measured by a single 24‐hour urine sodium collection at baseline and at 1‐ and 2‐year follow‐up. Urinary volume and collection time were used to assess completeness of collection. A repeat collection was performed if the first collection was inadequate.
Outcomes: Progression of CKD (defined as halving of estimated glomerular filtration rate from baseline or incident end‐stage renal disease (ie, chronic dialysis or kidney transplant)) and all‐cause mortality.
Risk of bias:
Sampling: High risk.
Representativeness: Low risk.
Reliability/validity of exposure: Low risk.
Reliability/validity of outcome: Low risk.
Blinding of outcome assessment: Low risk.
Risk of selective outcome reporting: Low risk.
Confounding: Low risk.
Source of funding: Funded by National Institute of Diabetes and Digestive and Kidney Diseases research grants.
Summary of results: During the 15 807 person‐years of follow‐up, there was a positive association between urinary sodium excretion and the cumulative incidence of CKD progression and mortality, after adjustment for multiple covariates including baseline estimated glomerular filtration rate. Compared with the lowest quartile of sodium excretion (<6.7 g/d salt, <2686 mg/d sodium), the highest quartile of sodium excretion (≥11.2 g/d salt, ≥4476 mg/d sodium) was associated with an increased risk of CKD progression (adjusted HR, 1.54; 95% CI, 1.23–1.92) and mortality (adjusted HR, 1.45; 95% CI, 1.08–1.95). The association between urinary sodium excretion and CKD progression remained similar in all subgroups. Statistical tests for interaction were nonsignificant for sex, race, diabetes mellitus, and the use of renin angiotensin system–blocking agents.
Comment: This prospective study, in a large cohort of individuals with established kidney disease, demonstrated a strong and significant association between high urinary sodium and CKD progression, independent of other important variables known to be associated with renal failure including baseline renal function. Notably, the investigators defined the exposure of interest using three 24‐hour urine collections, spaced over several years. This represents a methodological strength in the longitudinal collection of dietary data and in reducing bias associated with self‐report. Even so, it should be acknowledged that although a 24‐hour urine collection provides a valid estimate of sodium intake, a single 24‐hour urine collection may not be accurate in determining habitual dietary sodium intake in a free‐living population due to day‐to‐day fluctuations in sodium consumption.41 Consequently, the reported association between sodium excretion and CKD progression may have been underestimated or overestimated. While the findings of the study are intriguing, the observational study design does not prove a causal relationship. Clinical trials are needed to formally test the effect of sodium reduction on CKD progression and mortality.
3.3. Does dietary sodium affect the association between hypertension and cognitive decline?
Haring B, Wu C, Coker LH, et al. Hypertension, dietary sodium, and cognitive decline: results from the Women's Health Initiative Memory Study. Am J Hypertens. 2016;29:202–216.
Design: Prospective observational study (post‐RCT follow‐up).
Setting: 39 US clinical centers participating in the Women's Health Initiative Memory Study (WHIMS) RCT.
Participants: N=6426 postmenopausal women aged 65 to 79 years and free of dementia at enrollment; median follow‐up: 9.1 years.
Exposure: Sodium intake, measured by an FFQ, with 24‐hour urine sodium excretion in a subsample to correct dietary self‐report data for potential measurement errors. BP (average of two baseline measurements), measured by certified staff; hypertension, defined as self‐report of current drug therapy for hypertension; BP control (“controlled” defined as clinic BP <140 mm Hg or diastolic BP <90 mm Hg at baseline and “uncontrolled” defined as clinic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg at baseline).
Outcomes: Incidence of mild cognitive impairment or probable dementia. The Modified Mini‐Mental State Examination was performed in all women annually as a screening test. Further neurocognitive and neuropsychiatric examinations were conducted to establish the presence of mild cognitive impairment or probable dementia. The central adjudication committee (two neurologists and one geriatric psychiatrist) independently reviewed cases. A consensus diagnosis was made, and disagreements were resolved with discussion.
Risk of bias:
Sampling: Low risk.
Representativeness: High risk.
Reliability/validity of exposure: High risk.
Reliability/validity of outcome: Low risk.
Blinding of outcome assessment: Unclear risk.
Risk of selective outcome reporting: Low risk.
Confounding: High risk.
Sources of funding: National Heart, Lung, and Blood Institute; National Institutes of Health; and US Department of Health and Human Services.
Summary of results: Postmenopausal women with hypertension had higher risk of mild cognitive impairment or probable dementia compared with women without hypertension (adjusted HR, 1.20; 95% CI, 1.04–1.39), as did women taking antihypertensive treatment with uncontrolled BP compared with women with untreated, controlled BP (adjusted HR, 1.30; 95% CI, 1.05–1.60). There was no significant interaction with sodium intake category (≤3.75 g/d salt, 3.75–7.5 g/d salt, and >7.5 g/d salt, equivalent to ≤1500 mg/d sodium, 1500–2999 mg/d sodium, and >3000 mg/d sodium, respectively).
Comment: This large, prospective long‐term study analyzed the association between hypertension and cognitive decline and whether sodium intake modified this association. A subgroup analysis by sodium intake category was performed, rather than having sodium intake as a distinct variable in the multivariable models. The proposed mechanism relating sodium intake with dementia is via small vessel disease–induced elevations in BP. Therefore, by assessing sodium intake as an effect modifier, their analysis was not optimally designed to address the association between sodium intake and mild cognitive impairment/probable dementia. In addition, hypertension was defined as a dichotomous variable, and therefore the severity of hypertension was not taken into account in the models. Several covariates were included in the adjustment, although they were unable to account for other potentially important clinical confounders, such as the presence of liver and renal disease. While the WHIMS RCT used rigorous methods, it is unclear whether the conduct of the post‐trial follow‐up was similar; for example, it is unclear whether the adjudicators were blinded to sodium intake. In addition, after 2008, the cognitive assessments were performed over the telephone rather than face‐to‐face. The study used FFQs to assess sodium intake and therefore is subject to recall bias and underreporting.42 Furthermore, FFQs were available in only a small subset of participants and therefore their analysis may be have been underpowered. Changes in sodium intake over the 9.1‐year follow‐up were not captured, as only baseline FFQs were administered.
3.4. What is the association between salt and hypertension in rural and urban populations in low‐ to middle‐income countries?
Subasinghe AK, Arabshahi S, Busingye D, et al. Association between salt and hypertension in rural and urban populations of low to middle income countries: a systematic review and meta‐analysis of population based studies. Asia Pac J Clin Nutr. 2016;25:402–413.
Design: Meta‐analysis of observational studies.
Methods:
Data sources: PubMed, Web of Science, and Scopus until July 2014.
Study selection and assessment: 19 studies met inclusion criteria (n=134 916 adults from 4 countries, mean age range 39.7 to 55 years). Only studies with multivariable regression adjustment were included. There were 11 studies from India, six from China, one from Nepal, and one from Togo. Studies were pooled using random‐effects models. Heterogeneity was assessed by I 2.
Method of sodium intake measurement: Various methods, including reports of the total amount of salt consumed by a family for 1 year; a direct question about salt added to food; and dietary methods (7‐day food records, FFQ, household questionnaire, 24‐hour recall).
Outcomes: Prevalence of hypertension (systolic BP ≥140 mm Hg and/or diastolic BP ≥90 mm Hg and/or prescription of antihypertensive medication; systolic BP ≥130 mm Hg and/or diastolic BP ≥85 mm Hg)
Subgroup analyses: nonlean body size (body mass index ≥23) vs lean body size (body mass index <23); rural vs urban populations
Risk of bias:
A priori design: No.
Duplicate study selection/data extraction: No.
Comprehensive literature search: Yes.
Status of publication used as an inclusion criterion: No.
List of studies (included and excluded) provided: No.
Characteristics of included studies provided: Yes.
Quality of studies assessed and documented: No.
Quality of included studies used appropriately in formulating conclusions: No.
Methods to combine finding appropriate: Yes.
Publication bias assessed: No.
Conflict of interest stated: Yes. The authors declared no conflict of interest.
Summary of results: The association between salt intake and hypertension was greater in urban populations (pooled odds ratio, 1.42 per 1 g/d greater salt intake; 95% CI, 1.19–1.69) than in rural populations (pooled odds ratio, 1.07; 95% CI, 1.04–1.10). In the rural population studies, there was a stronger association between salt intake and hypertension in patients with body mass index <23 kg/m2 (pooled odds ratio, 1.19; 95% CI, 1.12–1.26) compared with those with body mass index ≥23 kg/m2 (pooled odds ratio, 1.01; 95% CI, 1.00–1.01).
Comment: The meta‐analysis included a broad range of participants from both urban and rural populations in low‐ and middle‐income countries. However, most studies were from Asia/South Asia and may not be representative of other regions. The authors utilized a comprehensive search strategy but did not report a priori design or duplicate data extraction. Studies of different methodological type (case‐control vs longitudinal) were combined, although a subgroup analysis based on study design was not performed. In addition, study quality was not assessed. As noted by the authors, salt intake was obtained from different methods, with the majority using self‐report. Therefore, these studies may not provide accurate estimates of salt intake. The authors analyzed salt intake as a categorical variable and as a continuous variable. Because of between‐study differences in cut points used to define high sodium intake, the authors appropriately indicated that the meta‐analysis using sodium intake as a continuous measure provided a more robust interpretation. No studies used 24‐hour urine sodium excretion. Despite these limitations, there was a consistent finding of a positive association between salt intake and hypertension in the included studies.
3.5. What is the effect of modest sodium reduction on BP and urinary albumin excretion in patients with diabetes mellitus or impaired glucose tolerance?
Suckling RJ, He FJ, Markandu ND, et al. Modest salt reduction lowers blood pressure and albumin excretion in impaired glucose tolerance and type 2 diabetes mellitus: a randomized double‐blind trial. Hypertension. 2016;67:1189–1195.
Design: Double‐blind RCT (crossover).
Setting: Blood Pressure Unit at St George's Hospital, London, and General Practice Surgeries in South London, United Kingdom.
Study duration: 12 weeks (two 6‐week periods), preceded by a 4‐week acclimation period.
Participants: 46 participants aged 30 to 80 years (mean 58 years; 52% male), with diet‐controlled diabetes mellitus or impaired glucose tolerance who also had untreated systolic BP 120 to 170 mm Hg or diastolic BP 70 to 100 mm Hg.
Intervention: Reduced‐salt diet group with goal ≈5 g/d salt (2000 mg/d sodium) through dietary advice by trained nurses and provision of salt‐free bread, compared with a group provided salt tablets. Nine tablets of salt per day were consumed daily, each tablet containing 0.58 g of salt (10 mmol or 230 mg sodium).
Achieved sodium intake: 6.8 g/d salt (2720 mg/d sodium) in the placebo group vs 9.7 g/d salt (3880 mg/d sodium) in the salt tablet group. Sodium intake was measured with two consecutive 24‐hour urine collections.
Outcomes: Clinic BP taken in patients in a sitting position, measured by trained research nurses using a validated oscillometric technique; 24‐hour ambulatory BP monitoring; urinary albumin excretion, measured by urine albumin/creatinine ratio; and pulse wave velocity and endothelial function (digital volume pulse analysis using high‐fidelity photoplethysmography).
Risk of bias:
Random sequence generation: Low risk.
Allocation concealment: Low risk.
Blinding of participants and personnel: Low risk.
Blinding of outcome assessors: Low risk.
Incomplete outcome reporting: Low risk for clinic BP; high risk for 24‐hour ambulatory BP monitoring and vascular measures.
Selective reporting: Unclear risk.
Other sources of bias: Low risk.
Source of funding: Hypertension Trust and National Health Service.
Summary of results: Compared with the salt tablet group, the placebo group (reduced salt) had lower BP (systolic BP: –4.2 mm Hg, P<.001; diastolic BP: –1.7 mm Hg, P=.055) and lower urine albumin/creatinine ratio (median 0.64 vs 0.73, P<.05).
Comment: This double‐blind crossover RCT had several strengths. Sodium intake was well measured, using the mean of two consecutive 24‐hour urine sodium measurements. The study achieved adequate differentiation in sodium intake between the two groups with a 2.9 g/d salt (1160 mg/d sodium) difference between the reduced sodium and control groups. The results of this RCT in patients early in the course of diabetes mellitus (diet‐controlled diabetes mellitus or impaired glucose intolerance with normal to mildly elevated BP) demonstrate consistency with systematic review evidence of the BP‐lowering effect of salt reduction in the general population.3 In this study, the benefit of sodium reduction was observed with a moderate reduction in sodium intake in the low sodium group (difference in sodium intake of 2.9 g/d salt [1160 mg/d sodium] and achieved intake 6.8 g/d salt [2720 mg/d sodium]).43 However, the sample size of the study was small. Although BP data were available for almost all patients, data for vascular measures were not available for 22% of the 46 patients and therefore the study was likely underpowered for these other outcomes.
4. DISCUSSION
This review identified 28 studies relating dietary sodium to health outcomes. Among the studies that met minimum methodological criteria, two studies found that high salt intake has adverse effects on health outcomes and two studies were neutral. For those studies that did not meet minimum methodological criteria, 20 found adverse effects of salt on health, one found beneficial effects of salt on health, and three were neutral.
This is the first Science of Salt review to implement both an outcome hierarchy and methodological quality criteria to prioritize which articles were included for risk of bias assessments and detailed critical appraisal. This approach ensures that the highest‐quality studies, reporting on the most important patient outcomes, are being reviewed, reported, and translated for clinical, research, and policy stakeholders. This is especially relevant given the controversy in the literature regarding the effects of salt on health outcomes, which may be influenced by the publication of lower‐quality research (ie, cross‐sectional design, invalid methods for assessment of sodium exposure, insufficient magnitude of salt reduction in interventions, inadequate duration or number of events).
Up to May 2015, we annually published a summary of studies on salt and health outcomes.44, 45 In June 2015, we replaced the annual reviews with regularly published summaries that include risk of bias assessments and critical appraisals.46, 47, 48 From June 2015 to July 2016, 64 studies were identified that met our inclusion criteria, ie, original human research on salt intake and health outcomes. Of the 58 (91%) articles that were primary research studies (ie, not a meta‐analysis or systematic review of studies), which examined any outcome (category I to VI), only 10 (17%) studies met the minimum methodological criteria. These studies are summarized in Table S3. Among these studies, three were RCTs and seven were prospective cohort designs: six (60%) demonstrated that increased dietary salt has adverse effects on health outcomes, three (30%) were neutral, and one (10%) found that increased salt benefits health outcomes. The latter study was conducted in a heart failure population. The remainder of studies on salt and health outcomes (83%), identified since June 2015, did not meet our methodological quality criteria. These studies found a somewhat similar proportion of articles that reported positive and negative effects associated with dietary salt: 85% found that increased salt had adverse effects on health, 4% found positive effects of increased salt on health, and 11% were neutral. Many of these studies did not meet minimum methodological criteria because they were cross‐sectional in nature or used spot urine collections to assess exposure to sodium. Differentiating low‐ from high‐quality research is critical as the unreliable results of low‐quality research may influence the field in the same way as high‐quality research.
The criteria that the Science of Salt authors have developed and applied are adapted from criteria used by other expert groups that conducted systematic reviews to derive dietary salt recommendations, such as those generated by the WHO.1, 3 An international TRUE Consortium (International Consortium for Quality Research on Dietary Sodium/Salt) of experts on salt and health outcomes is developing recommendations for the conduct of dietary salt research. These recommendations will be based on systematic reviews on topics such as dietary assessment of sodium (ie, food recalls and diaries and FFQs), biomarker assessment of sodium (ie, 24‐hour urine collection and spot urine samples), and other related outcomes including BP.49 These recommendations will provide evidence‐based guidance that can be implemented to improve the quality of research that examines salt and health outcomes.
5. CONCLUSIONS
This review identified and summarized 28 studies on dietary salt and health outcomes and critically reviewed 5 studies that were of the highest methodological quality and examined outcomes that are most important to patients. Three of these high‐quality studies found adverse effects of salt on health outcomes (CKD and BP) and two were neutral (fracture risk and BMD, and cognitive function).
CONFLICT OF INTEREST
NC is a member of World Action on Salt and Health (a dietary salt reduction organization) and is a paid consultant for the Novartis Foundation, which involves travel expenses and personal fees for site visits, and a one‐time contract (2016) to develop a survey. JA is supported by an Emerging Research Leaders Award from the Heart and Stroke Foundation of Canada. AAL is supported by the Hypertension Canada New Investigator Award. MMYW is a research consultant with Arbor Research Collaborative for Health. KT is supported by a National Health and Medical Research Council of Australia postgraduate scholarship. JW is Director of the WHO Collaborating Centre on Population Salt Reduction and receives funding for work on salt reduction from WHO, VicHealth, the Australian National Health and Medical Research Council, and the National Heart Foundation.
Supporting information
ACKNOWLEDGMENTS
We thank Dr Bradley Johnston, University of Toronto, for his input and expertise in developing the hierarchy of outcomes. The process to provide regular updates on the science of sodium is supported by the World Hypertension League, WHO Collaborating Centre on Population Salt Reduction (George Institute for Global Health), Pan American Health Organization/WHO Technical Advisory Group on Cardiovascular Disease Prevention through Dietary Sodium, and World Action on Salt and Health.
Arcand J, Wong MM, Santos JA, et al. More evidence that salt increases blood pressure and risk of kidney disease from the Science of Salt: A regularly updated systematic review of salt and health outcomes (April–July 2016). J Clin Hypertens. 2017;19:813‐823. 10.1111/jch.13049
REFERENCES
- 1. WHO . Guideline: Sodium Intake for Adults and Children. Geneva: World Health Organization (WHO); 2012. [PubMed] [Google Scholar]
- 2. Institute of Medicine . Dietary reference intakes for water, potassiums sodium, chloride, and sulfate. The National Academies Press: Washington DC; 2005. [Google Scholar]
- 3. Aburto NJ, Ziolkovska A, Hooper L, et al. Effect of lower sodium intake on health: systematic review and meta‐analyses. BMJ. 2013;346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta‐analysis of prospective studies. BMJ. 2009;339:b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization . Report of the formal meeting of member states to conclude the work on the comprehensive global monitoring framework, including indicators, and a set of voluntary global targets for the prevention and control of noncommunicable diseases. Report, 1‐6. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 6. Arcand J, Webster J, Johnson C, et al. Announcing “Up to Date in the Science of Sodium.” J Clin Hypertens (Greenwich). 2016;18:85‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence–imprecision. J Clin Epidemiol. 2011;64:1283‐1293. [DOI] [PubMed] [Google Scholar]
- 8. Higgins JP, Deeks JJ, Altman DG, eds. Chapter 16: special topics in statistics. In: Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration; 2011. http://www.cochrane-handbook.org. Accessed February 8, 2017. [Google Scholar]
- 9. McLaren L, Sumar N, Barberio AM, et al. Population‐level interventions in government jurisdictions for dietary sodium reduction. Cochrane Database Syst Rev. 2016;9:CD010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Okayama A, Okuda N, Miura K, et al. Dietary sodium‐to‐potassium ratio as a risk factor for stroke, cardiovascular disease and all‐cause mortality in Japan: the NIPPON DATA80 cohort study. BMJ Open. 2016;6:e011632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He J, Mills KT, Appel LJ, et al. Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol. 2016;27:1202‐1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carbone L, Johnson KC, Huang Y, et al. Sodium intake and osteoporosis. Findings from the women's health initiative. J Clin Endocrinol Metab. 2016;101:1414‐1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haring B, Wu C, Coker LH, et al. Hypertension, dietary sodium, and cognitive decline: results from the women's health initiative memory study. Am J Hypertens. 2016;29:202‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pogoda JM, Gross NB, Arakaki X, et al. Severe headache or migraine history is inversely correlated with dietary sodium intake: NHANES 1999–2004: a response. Headache. 2016;56:1216‐1218. [DOI] [PubMed] [Google Scholar]
- 16. Nakano M, Eguchi K, Sato T, et al. Effect of intensive salt‐restriction education on clinic, home, and ambulatory blood pressure levels in treated hypertensive patients during a 3‐month education period. J Clin Hypertens (Greenwich). 2016;18:385‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naseem S, Ghazanfar H, Assad S, Ghazanfar A. Role of sodium‐restricted dietary approaches to control blood pressure in Pakistani hypertensive population. J Pak Med Assoc. 2016;66:837‐842. [PubMed] [Google Scholar]
- 18. Ravi S, Bermudez OI, Harivanzan V, et al. Sodium intake, blood pressure, and dietary sources of sodium in an adult south Indian population. Ann Glob Health. 2016;82:234‐242. [DOI] [PubMed] [Google Scholar]
- 19. Subasinghe AK, Arabshahi S, Busingye D, et al. Association between salt and hypertension in rural and urban populations of low to middle income countries: a systematic review and meta‐analysis of population based studies. Asia Pac J Clin Nutr. 2016;25:402‐413. [DOI] [PubMed] [Google Scholar]
- 20. Suckling RJ, He FJ, Markandu ND, MacGregor GA. Modest salt reduction lowers blood pressure and albumin excretion in impaired glucose tolerance and type 2 diabetes mellitus: a randomized double‐blind trial. Hypertension. 2016;67:1189‐1195. [DOI] [PubMed] [Google Scholar]
- 21. Feng W, Cai Q, Yuan W, et al. Low response of renin‐angiotensin system to sodium intake intervention in chinese hypertensive patients. Medicine (Baltimore). 2016;95:e2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dolansky MA, Schaefer JT, Hawkins MA, et al. The association between cognitive function and objective adherence to dietary sodium guidelines in patients with heart failure. Patient Prefer Adherence. 2016;10:233‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. El Darouti MA, Zeid OA, Abdel Halim DM, et al. Salty and spicy food; are they involved in the pathogenesis of acne vulgaris? A case controlled study. J Cosmet Dermatol. 2016;15:145‐149. [DOI] [PubMed] [Google Scholar]
- 24. Grimes CA, Riddell LJ, Campbell KJ, He FJ, Nowson CA. 24‐h urinary sodium excretion is associated with obesity in a cross‐sectional sample of Australian schoolchildren. Br J Nutr. 2016;115:1071‐1079. [DOI] [PubMed] [Google Scholar]
- 25. Hwang SY, Kim J. An examination of the association of cognitive functioning, adherence to sodium restriction and Na/K ratios in Korean heart failure patients. J Clin Nurs. 2016;25:1766‐1776. [DOI] [PubMed] [Google Scholar]
- 26. McDonald J, Graves J, Waldman A, et al. A case‐control study of dietary salt intake in pediatric‐onset multiple sclerosis. Mult Scler Relat Disord. 2016;6:87‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park Y, Kwon SJ, Ha YC. Association between urinary sodium excretion and bone health in male and female adults. Ann Nutr Metab. 2016;68:189‐196. [DOI] [PubMed] [Google Scholar]
- 28. Umesawa M, Iso H, Fujino Y, et al. Salty food preference and intake and risk of gastric cancer: the JACC study. J Epidemiol. 2016;26:92‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cavka A, Jukic I, Ali M, et al. Short‐term high salt intake reduces brachial artery and microvascular function in the absence of changes in blood pressure. J Hypertens. 2016;34:676‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chun YH, Han K, Kim do H, et al. Association of urinary sodium excretion with insulin resistance in Korean adolescents: results from the Korea National Health and Nutrition Examination Survey 2009‐2010. Medicine (Baltimore). 2016;95:e3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Graudal NA, Hubeck‐Graudal T, Jurgens G. Reduced dietary sodium intake increases heart rate. A meta‐analysis of 63 randomized controlled trials including 72 study populations. Front Physiol. 2016;7:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heye AK, Thrippleton MJ, Chappell FM, et al. Blood pressure and sodium: association with MRI markers in cerebral small vessel disease. J Cereb Blood Flow Metab. 2016;36:264‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hou L, Zhang M, Han W, et al. Influence of salt intake on association of blood uric acid with hypertension and related cardiovascular risk. PLoS ONE. 2016;11:e0150451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Imaizumi Y, Eguchi K, Murakami T, et al. High salt intake is independently associated with hypertensive target organ damage. J Clin Hypertens (Greenwich). 2016;18:315‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matthews EL, Brian MS, Ramick MG, et al. High dietary sodium reduces brachial artery flow‐mediated dilation in humans with salt‐sensitive and salt‐resistant blood pressure. J Appl Physiol 1985. 2015;118:1510‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rhee MY, Kim JH, Na SH, et al. Elevation of heart‐femoral pulse wave velocity by short‐term low sodium diet followed by high sodium diet in hypertensive patients with sodium sensitivity. Nutr Res Pract. 2016;10:288‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Won JC, Hong JW, Noh JH, Kim DJ. Association between estimated 24‐h urinary sodium excretion and metabolic syndrome in Korean adults: the 2009 to 2011 Korea national health and nutrition examination survey. Medicine (Baltimore). 2016;95:e3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Y, Li F, Liu FQ, et al. Elevation of fasting ghrelin in healthy human subjects consuming a high‐salt diet: a novel mechanism of obesity? Nutrients. 2016;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mozaffarian D, Fahimi S, Singh GM, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371:624‐634. [DOI] [PubMed] [Google Scholar]
- 40. Huang Y, Van Horn L, Tinker LF, et al. Measurement error corrected sodium and potassium intake estimation using 24‐hour urinary excretion. Hypertension. 2014;63:238‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Caggiula AW, Wing RR, Nowalk MP, et al. The measurement of sodium and potassium intake. Am J Clin Nutr. 1985;42:391‐398. [DOI] [PubMed] [Google Scholar]
- 42. McLean RM. Measuring population sodium intake: a review of methods. Nutrients. 2014;6:4651‐4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Campbell NR, Correa‐Rotter R, Cappuccio FP, et al. Proposed nomenclature for salt intake and for reductions in dietary salt. J Clin Hypertens (Greenwich). 2015;17:247‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Johnson C, Raj TS, Trudeau L, et al. The science of salt: a systematic review of clinical salt studies 2013 to 2014. J Clin Hypertens (Greenwich). 2015;17:401‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johnson C, Raj TS, Trieu K, et al. The science of salt: a systematic review of quality clinical salt outcome studies June 2014 to May 2015. J Clin Hypertens (Greenwich). 2016;18:832‐839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wong MM, Arcand J, Leung AA, Thout SR, Campbell NR, Webster J. The science of salt: a regularly updated systematic review of salt and health outcomes (December 2015 to March 2016). J Clin Hypertens (Greenwich). 2017;19:322‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arcand J, Wong MM, Trieu K, et al. The science of salt: a regularly updated systematic review of salt and health outcomes (June and July 2015). J Clin Hypertens (Greenwich). 2016;18:371‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wong MM, Arcand J, Leung AA, et al. The science of salt: a regularly updated systematic review of salt and health outcomes (August to November 2015). J Clin Hypertens (Greenwich). 2016;18:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. TRUE Consortium . Recommended standards for assessing blood pressure in human research where blood pressure or hypertension is a major focus. J Clin Hypertens (Greenwich). 2017;19:108‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


