Abstract
The purpose of this review was to identify, summarize, and critically appraise studies on dietary salt and health outcomes that were published from August 2016 to March 2017. The search strategy was adapted from a previous systematic review on dietary salt and health. Studies that meet standards for methodological quality criteria and eligible health outcomes are reported in detailed critical appraisals. Overall, 47 studies were identified and are summarized in this review. Two studies assessed all‐cause or disease‐specific mortality outcomes, eight studies assessed morbidity reduction‐related outcomes, three studies assessed outcomes related to symptoms/quality of life/functional status, 25 studies assessed blood pressure (BP) outcomes and other clinically relevant surrogate outcomes, and nine studies assessed physiologic surrogate outcomes. Eight of these studies met the criteria for outcomes and methodological quality and underwent detailed critical appraisals and commentary. Five of these studies found adverse effects of salt intake on health outcomes (BP; death due to kidney disease and initiation of dialysis; total kidney volume and composite of kidney function; composite of cardiovascular disease (CVD) events including, and risk of mortality); one study reported the benefits of salt restriction in chronic BP and two studies reported neutral results (BP and risk of CKD). Overall, these articles confirm the negative effects of excessive sodium intake on health outcomes.
1. INTRODUCTION
Meta‐analyses and systematic reviews examining the relationship between dietary salt and health outcomes have been the basis for consensus1, 2 that excess salt (sodium) consumption is associated with multiple adverse health outcomes including a positive causal relationship with blood pressure (BP).3, 4 This evidence led to the World Health Organization (WHO) dietary salt recommendations, that adults consume <5 g/d of salt (<2000 mg/d of sodium), and children consume lower amounts based on lower energy intakes.2 To prevent and manage noncommunicable diseases associated with excess salt consumption, the WHO set a global target of reducing dietary salt intake by 30% by 2025 and many countries worldwide have implemented salt reduction programs.5, 6
The high profile of dietary salt research has resulted in a rapidly growing literature on the health effects of dietary salt. To keep scientific, clinical, and policy stakeholders up‐to‐date with the growing body of the literature, regularly updated reviews and critical appraisals of studies relating to health outcomes are published in the Journal, alternating with reviews of studies relating to salt reduction implementation programs.7 The objective of this fifth Science of Salt health outcomes review was to summarize published articles on salt and health outcomes from August 2016 to March 2017, and to highlight and critically appraise the highest quality articles that were published since the last review.8
2. METHODOLOGY
A detailed description of the methodological approach used to identify published articles for this review has been previously reported.8 Briefly, articles were identified on a weekly basis through a MEDLINE search strategy, which was adapted from a previous systematic review used to develop the WHO guideline on dietary sodium intake.1, 2 Table S1 reports on the types of studies that are included and excluded in this search. This review includes health outcome studies identified during the weeks of August 1st, 2016 to March 27th, 2017. A list of identified studies, including details of the population, study design, outcomes and results, are reviewed and summarized in Table S2. Among these articles, studies were selected to undergo a detailed critical appraisal based on the outcomes examined and methodological quality, as described previously.8
In summary, articles selected for detailed critical appraisal were first based on a hierarchy of health outcomes, which were outcomes classified based on relevance to patients (Table S2), with (a) mortality, (b) morbidity, (c) symptoms/quality of life/functional status, and (d) the diagnosis, prevention or treatment of hypertension and other clinical surrogate outcomes being considered important. Studies on physiologic surrogate outcomes were excluded from eligibility for detailed critical appraisal.
Methodological quality criteria, adapted from the systematic review used to develop the WHO sodium guidelines,3 were also considered when selecting articles for a detailed critical appraisal and commentary (Table S3). In summary, these studies were randomized controlled trials (RCTs) that allocated at least one group of participants to reduced or high salt intake (control group), were ≥4 weeks in duration, achieved an intake difference of ≥2.3 g salt (920 mg sodium) between intervention and control, and measured sodium intake with 24‐hour urine collection, and had no concomitant interventions (ie, hypertensive drugs, other dietary interventions). Studies with a primary outcome of BP were only eligible for detailed review if they met the minimum methodological criteria for RCTs. Cohort studies included in the detailed appraisals were ≥1 year in duration, included ≥400 participants (continuous outcomes) or events (dichotomous outcomes); measured sodium intake with a 24‐hour urine collection, food record, 24‐hour food recall or semiquantitative food frequency questionnaire (FFQ). Additionally, the exposure variable must be sodium intake or excretion alone. Articles that included only sodium‐to‐potassium ratio or related variables, such as salty food preference, were not eligible for detailed appraisal.
Articles selected for a detailed critical appraisal were assessed for risk of bias by two independent reviewers. Randomized controlled trials were assessed using the Cochrane risk of bias tool.9 Observational, nonrandomized studies were assessed using a modified Cochrane risk of bias tool.10 For meta‐analyses, the AMSTAR (Assessing the Methodological Quality of Systematic Reviews) tool was applied.11
3. RESULTS
The weekly searches identified 6281 citations, of which 95 health outcome studies met the criteria for full review (Figure). A total of 47 dietary salt studies met the inclusion criteria: Six meta‐analyses, six RCTs, 11 prospective cohort studies, two retrospective cohort studies, 20 cross‐sectional studies and two case‐control studies, two post hoc analyses of RCTs. The outcomes examined were diverse: Six studies assessed mortality outcomes,12, 13, 14, 15, 16, 17 four studies assessed morbidity outcomes,18, 19, 20, 21 three studies assessed outcomes related to symptoms/quality of life/functional status,22, 23, 24 studies assessed BP outcomes25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41; eight studies assessed other clinically relevant surrogate outcomes42, 43, 44, 45, 46, 47, 48, 49; and nine studies assessed physiologic outcomes (Table S5).50, 51, 52, 53, 54, 55, 56, 57, 58 A range of outcomes were captured by the studies considered lower quality, including all‐cause mortality,13 gastric cancer,20 end‐stage renal disease requiring dialysis or transplant,15 cardiovascular events,14, 16, 21 hypertension prevalence,18 headaches/migraines,22 quality of life,23 multiple sclerosis,24 BP,26, 27, 28, 29, 30, 32, 33, 34, 35, 37, 38, 39, 40 cognitive function,44 osteoporosis risk48 and prevalence,45 nonalcoholic fatty liver disease,46 type 2 diabetes,42 carotid atherosclerosis,47 bone mineral density,55, 57 changes in left ventricular mass,50 inflammatory markers,51 albuminuria,53 and other urinary markers.52, 54 Most of these studies (n = 40) found adverse effects of dietary salt on health outcomes and benefits of a sodium‐restricted diet on health, except for 7 that were neutral15, 24, 29, 31, 35, 37, 49 and one reported worsening symptoms with a sodium‐restricted diet in the elderly.44
Figure 1.
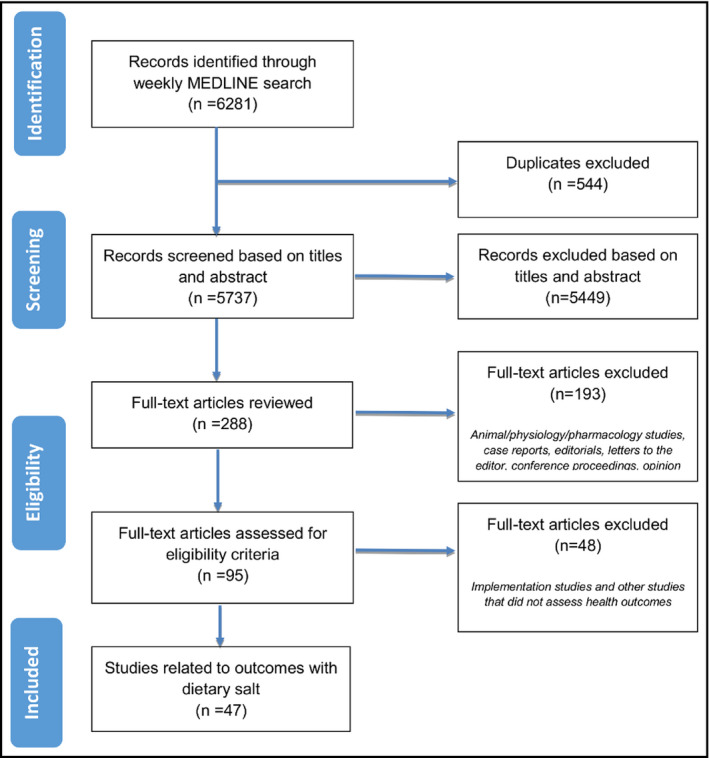
Flow diagram for studies identified from August 2016 to March 2017
Of 47 identified studies, eight met the inclusion criteria for outcomes examined and methodological quality and thus were included in the detailed risk of bias assessments and critical appraisals: Two meta‐analyses,25, 31 one RCT,36 four prospective cohort studies,12, 17, 19, 49 and one post hoc analysis of two RCTs.43 The studies found that: Dietary modifications, including low sodium diets, are associated with a variable reduction in BP25; dietary sodium modification did not affect BP in normotensive individuals31; medium‐high sodium intake in combination with the DASH diet lowered BP and uric acid levels in pre‐stage or stage 1 hypertensive individuals compared to a control diet36; sodium excretion is not associated with risk of CKD49; healthy dietary patterns reduce risk of major renal outcomes (composite of death due to renal causes and dialysis, with death due to a nonrenal cause)17; higher sodium excretion was associated with an increased risk of CVD in individuals with CKD19; higher sodium excretion was associated with increased risk of mortality in pre‐hypertensive adults12; a low sodium diet is associated with improved markers of kidney function in individuals with autosomal dominant polycystic kidney disease (ADPKD).43 Therefore, most of these high‐quality studies (n = 5) found adverse effects of excess sodium on health outcomes; one study reported the benefits of sodium restriction on BP and two studies reported neutral results.
The risk of bias assessments for these eight studies is included in Table S6(a‐i); a summary of study characteristics and results are captured in Table S4; and the written critical appraisals and commentary are below.
3.1. Detailed critical appraisals of selected studies
3.1.1. Is there an association between dietary sodium and all‐cause mortality over 20 years of follow‐up?
Cook, Nancy R., Lawrence J. Appel, and Paul K. Whelton. “Sodium intake and all‐cause mortality over 20 years in the Trials of Hypertension Prevention.” Journal of the American College of Cardiology 68.15 (2016): 1609‐1617.
Design: Prospective cohort study.
Setting: A total of 3126 pre‐hypertensive men and women participating in the Trials of Hypertension Prevention (TOHP) phases I or II. Data from posttrial deaths were ascertained.
Participants: Trials of Hypertension Prevention I (n = 744): 71% male; mean age 43; TOHP II (n = 2382): 66% male; mean age 44. Median follow‐up 24 years. All individuals were pre‐hypertensive and free of other diseases. In the phase I, 327 participants were randomized to a sodium reduction group, and 417 participants were included in a usual care comparison group. In phase II, 1191 participants were included in the sodium reduction group and 1191 in a sodium control group (usual diet).
Exposure: Sodium intake, estimated by three to seven 24‐hour urine collections.
Outcomes: All‐cause mortality.
Risk of bias: Please see ratings in Table 1.
Table 1.
Risk of bias critical assessment—Nonrandomized trials
| Bias domain | Study and ratings | ||||
|---|---|---|---|---|---|
| Kieneker et al50 | Mills et al20 | Smyth et al19 | Cook et al13 | Torres et al44 | |
| Sampling | Low | High | Unclear | N/A | Unclear |
| Representativeness | Low | Low | High | Unclear | High |
| Reliability/validity of outcome | Low | Low | High | Low | Unclear |
| Reliability/validity of exposure | Low | Low | High | Low | Low |
| Blinding of outcome assessment | Low | Low | High | High | Low |
| Risk of selective outcome reporting | Low | Low | Low | Low | Low |
| Confounding | Low | Low | Low | Low | High |
Sources of funding: TOHP Follow‐up Study was supported by a National Institutes of Health/National Heart, Lung, and Blood Institute grant and an American Heart Association award.
Summary of results: Median follow‐up time among survivors was 25.7 years for TOHP I and 22.4 years for TOHP II. For both phases, 251 deaths occurred in both groups. There was a nonsignificant 15% lower risk of death in the sodium reduction group (HR: 0.85; 95% CI: 0.66‐1.09; P = 0.19). Among participants assigned to the sodium control group, there was a direct linear association between average sodium intake and mortality, with an HR of 0.75, 0.95, and 1.00 (reference) and 1.07 (P trend = 0.30) for <5.75, 5.75‐9, 9‐12 and ≥12 g salt/d, respectively; per 2.5 g salt (1000 mg sodium)/d the HR was 1.12 (95% CI: 1.00‐1.26; P = 0.05). The HR per unit increase in sodium/potassium ratio was 1.13 (95% CI: 1.01‐1.27; P = 0.04).
Comment: The methodology used to assess sodium intake is arguably the most robust measurement of usual dietary sodium intake, as estimated by multiple nonconsecutive 24‐hour urine collections.59 While the study shows a significant linear relationship between progressively higher salt intake and increased mortality, the authors state that participant adherence to low sodium diets is unknown and there were no sodium intake measurements during the course of follow‐up after the trial period.
3.1.2. What is the association between urinary sodium excretion and CVD events?
Mills KT, Chen J, Yang W, et al. Sodium Excretion and the Risk of Cardiovascular Disease in Patients with Chronic Kidney Disease. JAMA. 2016; 315(20):2200‐10.
Design: Prospective cohort study.
Setting: Patients with CKD enrolled in the Chronic Renal Insufficiency Cohort (CRIC) Study. CRIC recruited from seven locations in the United States.
Participants: A total of 3757 CKD patients aged 21‐74 years with mild‐to‐moderate CKD defined by age‐specific glomerular filtrate rate (eGFR) criteria between 20 and 70 mL/min/1.73 m2. Approximately half of the patients had diabetes.
Exposure: Urinary sodium excretion estimated from a 24‐hour urine collection at baseline, year 1 and year 2 follow‐up visits. Urinary volume, creatinine excretion, and reported collection time were used to assess completeness. Urinary sodium excretion was calibrated to sex‐specific mean 24‐hour urinary creatinine excretion.
Outcomes: The primary outcome was a composite of CVD events defined as myocardial infarction, stroke, or congestive heart failure. Each of these outcomes was also analyzed individually. CVD outcomes were identified at annual visits and during 6‐month telephone calls in between annual visits. Follow‐up was censored at the first of either death, loss to follow‐up, withdrawal, or March 2013.
Risk of bias: Please see ratings in Table 1.
Sources of funding: Research grant (R01DK074615) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Summary of results: Mean 24‐hour urinary sodium excretion was 3701 ± 1443 mg/d (salt 9.3 ± 3.6 g/d). During follow‐up (median 6.8 years), there were 804 composite CVD events, 575 congestive heart failure events, 305 myocardial infarction events, and 148 stroke events. From the lowest (sodium <2894 mg/d or 7.2 g/d salt) to highest (sodium ≥4548 mg or 11.4 g/d salt) quartile of sodium excretion, there was a significant increase in cumulative incidence of composite CVD, congestive heart failure, myocardial infarction, and stroke (log‐rank P = 0.001 for all). In the multivariate model, the lowest quartile of sodium excretion compared to the highest quartile was at greater risk of composite CVD (hazard ratio (HR): 1.36, 95% CI: 1.09‐1.70; P = 0.007), heart failure (HR: 1.34, 95% CI: 1.03‐1.74; P = 0.03), and stroke (HR: 1.81 (95% CI: 1.08‐3.02; P = 0.02), but not myocardial infarction (HR: 1.15, 95% CI: 0.79‐ 1.66; P = 0.46). Multivariable‐adjusted restricted cubic spline analysis indicated a significant linear association between calibrated urinary sodium excretion and composite CVD and stroke, but not with MI. These analyses also showed no significance for a nonlinear association between calibrated urinary sodium excretion and composite CVD.
Comment: This large prospective cohort study has several strengths. For example, multiple nonconsecutive 24‐hour urine collections were used to prospectively measure sodium excretion at three time points, the gold standard for sodium intake assessment.7 However, as noted by the authors, whether 24‐hour sodium excretion provides a valid estimate of sodium intake has not been established in individuals with CKD. Another strength of this study is the inclusion of racially and ethnically diverse patients and approximately half the sample had diabetes therefore making the results more generalizable to patients with CKD. Outcome assessment bias was reduced by following the participants up regularly (annually and 6 monthly phone calls) and having two physicians adjudicate all events. Although authors considered many confounding variables in their models, inherently observational designs are prone to confounding. Observational studies also cannot establish causality and the risk of reverse causality is high.
3.1.3. Is there an association between sodium intake and increased risk of death due to renal causes and/or initiation of dialysis?
Smyth A, Griffin M, Yusuf S et al. Diet and Major Renal Outcomes: A Prospective Cohort Study. The NIH‐AARP Diet and Health Study. J Ren Nutr. 2016 Sep;26(5):288‐98.
Design: Prospective cohort study.
Setting: A total of six states and two urban areas in the United States participating in the National Institutes of Health‐American Association of Retired Persons Diet and Health Study.
Participants: N = 544 635 adults (59% men) aged 51‐70 years; mean follow‐up: 14.3 years.
Exposure: Sodium intake measured by a validated 124‐item food frequency questionnaire. Sodium intake was classified into quintiles as follows (mg/d): Q1 < 1700 (4.4 g salt/day); Q2 1700‐2300 (4.3‐5.8 g salt/d); Q3 2300‐2800 (5.8‐7.1 g salt/d); Q4 2800‐3600 (7.1‐9.1 g salt/d), and; Q5 > 3600 (9.1 g salt/d).
Outcomes: Composite of death due to renal causes and the initiation of dialysis. Death was ascertained from the Social Security Administration Death Master File and National Death Index. Death due to renal causes was defined as death where chronic renal disease was identified as a primary or contributing cause of death based on the International Classification of Diseases coding system. Initiation of dialysis was self‐report.
Risk of bias: Please see ratings in Table 1.
Summary of results: The composite of death due to renal causes and initiation of dialysis occurred in 4848 participants. The highest quintile of sodium was associated with an increased risk of the composite endpoint (sub‐hazard ratio (sHR) 1.17; 95% CI: 1.02‐1.33) compared to the lowest sodium quintile.
Comment: There are several strengths of this study, including adjustment for several variables in the analysis, and conducting interaction and sensitivity analyses, which did not result in substantial changes to the outcomes compared to the main analyses. Several limitations must also be considered. Sodium intake was assessed using self‐completed FFQs and therefore is subject to misreporting and recall bias.59 FFQs were also only administered at baseline, so changes in sodium intake over time were not accounted for in the analyses. The outcome of initiation of dialysis was self‐reported and not validated. In addition, the study utilized secondary data from another study with poor baseline response rates, which put the representativeness of the current study in question.
3.1.4. What are the BP lowering effects of specific dietary patterns?
Gay HC, Rao SG, Vaccarino V et al. Effects of Different Dietary Interventions on BP: Systematic Review and Meta‐Analysis of Randomized Controlled Trials. Hypertension, 2016, 67(4), 733‐739.
Design: Meta‐analysis of RCTs of controlled clinical trials.
Methods:
Data sources: PubMed, EMBASE and Web of Science (from January 1, 1990 to March 1, 2015).
Study selection and assessment: RCTs assessing the effect of dietary interventions on net change in systolic and diastolic BP in adults >19 years of age. Twenty‐four trials were selected for inclusion (n = 23 858 adults, mean age range 34‐67 years). Data were pooled using random effects models, and study quality was assessed using the Cochrane risk of bias tool.
Method of sodium intake measurement: Not reported. The trials were grouped according to dietary intervention: (a) Dietary Approaches to Spot Hypertension (DASH); (b) Low sodium diet; (c) Low sodium, high potassium diet; (d) Low sodium, low calorie diet; (e) Low calorie diet; and (f) Mediterranean diet. The low sodium diet was defined as consuming below 2.3 g (2300 mg) of sodium per day.
Outcomes: Changes in systolic and diastolic BP.
Subgroup analyses: Hypertensive status, antihypertensive medication use (yes vs no), age (<50 vs ≥50 years), sex (<50% vs ≥50% male), diabetes status (diabetic vs nondiabetic), study duration (<12 vs 12‐24 vs >24 months), sample size (<100 vs 100‐1000 vs >1000 participants), body mass index at baseline (<30 vs 30‐35 vs >35 kg/m2), physical activity encouraged (yes vs no), and whether BP reduction was considered as the primary outcome.
Risk of bias: Please see ratings in Table 2.
Table 2.
Risk of bias critical assessment—meta‐analyses
| Bias domain | Study and ratings | |
|---|---|---|
| Gay et al26 | Kelly et al32 | |
| A priori design | No | Yes |
| Duplicate study selection/data extraction | Yes, for study selection; unclear for data extraction | Yes |
| Comprehensive literature search | Yes | Yes |
| Status of publication used as an inclusion criterion | No | Yes |
| List of studies (included and excluded) provided | No | Yes |
| Characteristics of included studies provided | Yes | Yes |
| Quality of studies assessed and documented | Yes | Yes |
| Quality of included studies used appropriately in formulating conclusions | Yes | Yes |
| Methods to combine findings appropriate | Yes | Yes |
| Publication bias assessed | Yes | No |
| Conflict of interest stated | Yes, the authors declared no disclosure. | Yes |
Summary of results: The overall pooled estimate of mean change in systolic BP and diastolic BP was −3.07 mm Hg (95% CI: −3.85 to −2.30) and −1.81 mm Hg (−2.24 to −1.38), respectively. All interventions that included sodium restriction led to significant reductions in systolic BP [low sodium diet: −2.06 (−3.50, −0.63); low sodium and high potassium diet: −3.14(−6.27, −0.02); low sodium and low calorie diet: −2.38(−3.79, −0.98)], and diastolic BP [low sodium diet: −1.30 (−2.37, −0.23); low sodium and high potassium diet: −2.01 (−3.40, −0.62); low sodium and low calorie diet: −1.33 (−2.04, −0.62)]. Subgroup analyses did not independently assess dietary sodium.
Comment: This meta‐analysis conducted by Gay and colleagues has a number of strengths. Overall, the search strategy appears to have covered all relevant RCTs or controlled clinical trials to date. The number of trials is relatively large and covered a wide range of dietary interventions. The scientific quality of the studies and publication bias were assessed and incorporated into formulating the study conclusions. Random effects models were used to account for the heterogeneity between studies and several a priori subgroup analyses were conducted to determine the variations in effect size in different subgroups. This study also has some limitations. The authors did not report a priori design and it is unclear if data extraction was completed in duplicate. Also, subgroup analyses were not conducted by diet, therefore whether BP responses to a low sodium diet differed in different subgroups is unclear. The method of sodium intake measurement in the individual studies was not reported; therefore, it was not possible to assess the accuracy of sodium intake estimates.
3.1.5. Are sodium‐induced changes in serum uric acid associated with changes in blood pressure?
Juraschek, S. P., Choi, H. K., Tang, O., et al. Opposing effects of sodium intake on uric acid and blood pressure and their causal implication. Journal of the American Society of Hypertension, 2016; 10(12), 939‐946.
Design: Post hoc analysis of a parallel‐ arm RCT.
Setting: Johns Hopkins University clinical center in Baltimore, Maryland United States.
Study duration: Two 30‐day intervention periods. Prior to the trial initiation subjects consumed a high sodium control diet for 11‐14 days, followed by a 5‐day washout period.
Participants: A total of 103 adults with pre‐stage or stage 1 hypertension (55% women, 75% black) recruited into the DASH trial60 from the aforementioned site only. The mean age was 52, mean serum uric acid baseline was 5.0 mg/dL.
Intervention: In this controlled feeding, study participants were randomized to (a) a control “American diet” group or; (b) a DASH diet group that followed a diet rich in fruits, vegetables, low‐fat dairy foods and was reduced in saturated fat and cholesterol. Within these two groups, participants were fed three different sodium levels, in random order.
Achieved sodium intake: The isocaloric diets contained the following three sodium levels: 1380 mg/d (3.5 g salt/d), 2760 mg/d (7 g salt/d), and 4140 mg/d (10.5 g salt/d).
Outcomes: Fasting serum uric acid was measured via spectrophotometer. The primary outcome of the DASH‐sodium trial was BP, which was measured in triplicate by a protocol that used random‐zero, mercury sphygmomanometers after a 3‐minute rest while the participants were seated.
Risk of bias: Please see ratings in Table 3.
Table 3.
Risk of bias critical assessment—randomized controlled trials
| Bias domain | Study and ratings |
|---|---|
| Juraschek et al37 | |
| Random sequence generation | Low |
| Allocation concealment | Unclear |
| Blinding of participants and personnel | Unclear |
| Blinding of outcome assessors | Unclear |
| Incomplete outcome reporting | Low |
| Selective reporting | Low |
| Other sources of bias | High |
Summary of results: Increasing sodium intake (low to high) decreased uric acid (−0.4 mg/dL, P < 0.001) and increased systolic and diastolic BP (4.3 mm Hg, P < 0.001 and 2.3 mm Hg, P < 0.001 respectively), with similar findings observed in the control and DASH diet interventions. The change in uric acid levels was independent of the change in systolic and diastolic BP, even after controlling for baseline blood pressure, BP response to sodium, or baseline uric acid level.
Comments: This study was a highly controlled feeding trial that included randomization and standardized dietary interventions that required each subject to consume three different levels of sodium, thus they served as their own control. Urine collections were used to estimate dietary sodium at multiple time points; however, it is unclear which urine collections were used as part of this analysis. Additionally, as duly noted by the authors, the findings cannot confirm a causal relationship between uric acid levels and BP. Finally, these findings cannot be generalized to clinical populations that are at high risk of hyperuricemia and hypertension, such as those with CKD and cardiovascular disease as these populations and related disease outcomes were not investigated.
3.1.6. What is the effect of altering dietary sodium on BP in adults with systolic BP of <140 mm Hg?
Kelly J, Khalesi S, Dickinson K, Hines S, Coombes JS, Todd AS. The effect of dietary sodium modification on BP in adults with systolic BP less than 140 mm Hg: a systematic review. JBI Database System Rev Implement Rep 2016; 14 (6):196‐237.
Design: A systematic review of randomized controlled trials and nonrandomized controlled trials.
Methods:
Data sources: MEDLINE, CINAHL, ProQuest, Scopus, Embase, Cochrane Library and Wiley InterScience, clinical trial registrars, ProQuest database, Dissertations and Theses International, Mednar, OpenSIGLE and EAGLE between 1980 and 2014.
Study selection and assessment: Five studies were selected for inclusion (n = 1214; three were in Caucasians, one in African American population and one mixed race population). Only studies where adult participants with systolic BP of <140 mm Hg were included and interventions that evaluated dietary sodium intake for more than 4 weeks of duration were included. Studies that scored poorly on the Joanna Briggs Institute Meta‐Analysis of Statistics Assessment and Review Instruments tool, had a high level of selection bias (for example, studies that exclude people who are truly normotensive) or significant confounding was excluded from the review. Studies were pooled using a random effects model. Heterogeneity was assessed by Chi‐square test and the I 2 value.
Method of sodium intake measurement: Three of five studies included measurements of sodium intake from 24‐hour urine collections. One study estimated sodium intake from regularly collected early morning spot urine samples and the other used a pooled 24‐hour urine collection from three individual 8‐hour collections.
Outcomes: Systolic BP, diastolic BP, pulse wave analysis or flow‐mediated dilation by Doppler ultrasound.
Subgroup analyses: Trials with urinary sodium excretion of >40 mmolday/24 hours vs <40 mmol/24 hours. Other planned subgroup analyses were not possible due to few studies.
Risk of bias: Please see ratings in Table 2.
Summary of results: The estimated change in dietary sodium from usual salt intake was −4000 mg/d (10 g salt/d). The overall reduction in systolic BP (−0.71 mm Hg, 95% CI: −2.62, 1.20, P = 0.47) and diastolic BP (−0.57 mm Hg, 95% CI: −1.26, 0.12, P = 0.10) was not significant. There was no change in pulse wave velocity following dietary sodium reduction for over 4 weeks. Significant heterogeneity was reported for systolic BP (τ2 = 2.19, χ2 = 8.77, df = 4 (P = 0.07; I 2 = 54%).
Comment: The systematic review included five trials of small to moderate size (n = 1214). The authors utilized a comprehensive search strategy for both peer‐reviewed studies and gray literature and study selection and data extraction were done in duplicate. The study quality was assessed and those that scored poorly were excluded. Of the five included studies, two did not measure salt intake using 24‐hour urinary sodium excretion. One study included regular early morning spot urine samples and the other used three individual 8‐hour collections; thus, sodium intake estimates in these studies may be inaccurate. As stated by authors, a limitation of this study was that large, long‐term multicenter trials were excluded as some participants in these studies had systolic BP >140 mm Hg and studies with data for subgroups of normotensive populations could not be retrieved. In addition, significant heterogeneity was noted between studies for systolic BP.
3.1.7. Is sodium excretion associated with increased risk of chronic kidney disease?
Kieneker L, Bakker S, Boer R, Navis G, Gansevoort R and Joosten M. Kidney International. Low Potassium Excretion but not High Sodium Excretion is Associated with Increased Risk of Developing Chronic Kidney Disease. 2016;90(4): 888‐96.
Design: Prospective observational cohort study.
Setting: The Prevention of Renal and Vascular End‐Stage Disease (PREVEND) population‐based cohort study, in the Netherlands.
Participants: A total of 5315 (adults aged between 28 and 75 years) participants without chronic kidney disease (CKD) at baseline.
Exposure: Sodium and potassium intake measured by four 24‐hour urine samples (two consecutive samples collected at baseline between 1997 and 1998 and two at the second visit between 2001 and 2003). The average of the paired measurements at each time point was used.
Outcomes: Onset of CKD, defined as de novo development of eGFR <60 mL/min/1.73 m2 or urinary albumin excretion (UAE) >30 mg/24, or both.
Risk of bias: Please see ratings in Table 1.
Sources of funding: The Dutch Kidney Foundation.
Summary of results. At baseline, median urinary sodium was 3105 mg sodium/d (7.7 g salt/d). During a median follow‐up of 10.3 years, 872 individuals developed CKD. There was no significant association between urinary sodium excretion and the risk of CKD after adjustment for age and sex (HR per 2.8 g salt or 1150 mg sodium/day increment, 1.01, 95% CI: 0.94, 1.09), nor after additional adjustment for several other factors (HR per 2.8 g salt or 1150 mg sodium/day increment, 0.97, 95% CI: 0.89, 1.06). Secondary analysis with CKD defined as eGRF < 60 mL/min/1.73 m2 or urinary albumin excretion >30 mg/24‐h alone, revealed similar findings.
Comment: There are several strengths of this study. This was a large longitudinal study that represented the underlying population. Notably, the investigators assessed the exposure of interest using four 24‐hour urine collections collected at two separate time points. 24‐hour urine collection is the gold standard for assessment of sodium intake.61 This method eliminates reporting bias and underestimation, which are known limitations of other subjective dietary intake measures.59 Due to day‐to‐day fluctuations in sodium consumption, a single urine collection may not be indicative of habitual intake; thus, several 24‐hour urine collections are more valuable in estimating true intake, a strength of this study. Some limitations exist; although this study represents the underlying population, it may not be generalizable to other populations as this was a uniform ethnic population including predominantly Caucasian individuals. In addition, the observational study design does not enable causation to be established.
3.1.8. Is there an association between sodium excretion and rates of change in total kidney volume (TKV) and risk of composite endpoint of 50% reduction in eGFR, End‐stage renal disease (ESRD), or death?
Torres V, Abebe K, Schrier R, et al. Dietary salt restriction is beneficial to the management of autosomal dominant polycystic kidney disease. Kidney International. 91(2):493‐500, 2017.
Design: Post hoc analysis of the Halt Progression of Polycystic Kidney Disease study, which consisted of two randomized controlled trials (Study A and B).
Setting: Multicentre study of seven clinical sites in the United States.
Participants: A total of 558 ADPKD patients aged 15‐49 years with an eGFR >60 mL/min/1.73 m2 from Study A, and 486 ADPKD patients aged 18‐64 years with an eGFR of 25‐60 mL/min/1.73 m2 from Study B.
Exposure: Urinary sodium excretion, measured by a single 24‐hour urine collection at baseline, at the end of the 4‐month drug titration period and annually thereafter. At baseline, all patients in both studies were instructed to consume <2300 mg/d sodium (5.75 g/d salt)/day diet.
Outcomes: Percent change in total kidney volume (TKV) measured by magnetic resonance imaging and rate of change in eGFR measured using centralized measurements of serum creatinine level (Study A); composite outcome of 50% reduction in eGFR, ESRD or death, and rate of change in eGFR (Study B).
Sources of funding: Grants from the National Institute of Diabetes and Digestive and Kidney Diseases, National Center for Research Resources General Clinical Research Centers, National Center for Advancing Translational Sciences Clinical and Translational Science Awards; funding from the Zell Family Foundation, and; grant from the PKD Foundation.
Summary of results: In Study A, average and time‐varying sodium excretion were significantly associated with the rate of TKV growth (0.43%/year (P < 0.001) and 0.09%/year (P = 0.005) for each 414 mg/d (1.04 g/d salt) increase in sodium excretion, respectively), but not with the rate of change in eGFR. In Study B, average sodium excretion, but not time‐varying sodium excretion, was significantly associated with an increased risk of the composite outcome of 50% reduction in eGFR, ESRD or death (HR 1.08 95% CI: 1.01, 1.06 for each 414 mg/d (1.04 g/d salt) increase in sodium excretion, P = 0.010) and higher rate of decline in eGFR (−0.09 mL/min/1.73 m2/year for each 414 mg/d (1.04 g/d salt) increase in sodium excretion, P < 0.001).
Comment: A strength of this analysis is that sodium excretion was measured using 24‐hour urine collections at multiple times during the follow‐up period. However, the method used for the 24‐hour collections and any methods used to assess for sample completeness are not reported. Furthermore, the validity of using 24‐hour urine samples to approximate sodium intake in patients with ADPKD has not been established and medical therapies (i.e., loop diuretics) may impact sodium excretion.62 The major limitation of this study is that this prospective analysis was completed on patients enrolled in a randomized double‐blinded placebo controlled trial of rigorous BP control with anti‐hypertensive drug therapy, and participants were given advice to reduce sodium intake at baseline; thus, generalizability is limited. Furthermore, the analyses were only adjusted for age, gender, race, body surface area, and time × BP arm interaction. Due to these limitations, it is likely that confounding may have influenced the results, and since these are post hoc analyses the results should be interpreted as hypothesis generating only.
4. DISCUSSION
This review identified 47 studies relating dietary sodium to health outcomes. Among the studies that met minimum methodological criteria, seven studies demonstrated positive associations between dietary salt and adverse health outcomes, and two studies reported neutral results.
The findings from Science of Salt review are consistent with the previous reviews,8, 63, 64, 65, 66, 67, 68 in that, most high‐quality studies found a relationship between excess sodium intake and adverse health outcomes or that dietary sodium reduction improves health outcomes. Both an outcome hierarchy and methodological quality criteria were used to identify the most relevant and highest quality studies, to prioritize articles to be included for risk of bias assessments and detailed critical appraisal. Like previous reviews, the majority of identified studies did not meet the methodological quality criteria and thus should not be used to inform national or international guidelines. These lower quality studies must be interpreted with caution. In this review, 6 high‐quality studies demonstrated that excess sodium intake has adverse effects on health and that dietary sodium reduction improves health in normotensive, hypertensive, diabetic, and renal disease populations.
Out of the 47 studies identified, 39 did not meet our minimum methodological criteria. The main reasons for this were the cross‐sectional nature or the use of spot urine collections to assess exposure to sodium. Differentiating low‐ from high‐quality research is critical as the low‐quality research should not influence the field to the same extent as high‐quality research. Eight of these studies met criteria for outcomes and methodological quality and underwent detailed critical appraisals and commentary.
Twenty‐four‐hour urine collection is the gold standard for assessment of sodium intake62 as it is estimated to account for 95% of intake.69 This objective method eliminates reporting bias and thus minimizes underestimation of sodium intake, which is known limitations of other subjective dietary reporting measures.70 In this review, three studies investigated sodium intake in a chronic disease population (in patients with CKD) using single or multiple 24‐hour urinary sodium excretion as a measure of intake. In chronic disease settings, such as in CKD, there are many factors that can influence retention or excretion of sodium.62 For example, in CKD, renal excretion of sodium is impaired due to a decline in functioning nephrons. Conversely, diuretic use, common medical therapy for this population, increases sodium excretion.71 Due to abnormalities in renal sodium handling in CKD, interpretation of sodium intake using 24‐hour urinary collections may not be precise. Currently, it is unknown whether 24‐hour urinary sodium excretion provides a valid estimate of sodium intake for individuals with CKD.19 Interestingly, in a heart failure population, not reported in this review, poor agreement was reported between food records and 24‐hour urinary sodium excretion in patients on loop diuretics, while strong agreement was established for those not on diuretics.62 The results from this study highlight the need for including other methods of dietary intake, such as food records, when disturbances in urinary excretion may be present. In addition, some dietary survey methods such as the FFQ may not sufficiently characterize sodium intake72 because of limited precision in estimating nutrient intake and discriminating high from low sodium foods.73, 74, 75, 76 To our knowledge, one clinical screening FFQ tool has been validated in this population.77 In an Australian CKD population, the Scored Sodium Questionnaire tool was moderately associated with assessments of 24‐hour urinary sodium and dietary data. Overall, there is a need to establish the validity of sodium assessment methods in disease states associated with disturbed sodium homeostasis.
The criteria the Science of Salt authors have developed and applied are adapted from criteria used by other expert groups that have conducted systematic reviews to derive dietary salt recommendations, such as those generated by the World Health Organization.2, 3 An international TRUE Consortium (in Ternational consoRtium for qUality resEarch on dietary sodium/salt) of experts on salt and health outcomes is developing recommendations for the conduct of dietary salt research. These recommendations will be based on systematic reviews on topics such as dietary assessment of sodium (ie, food recalls and diaries, FFQ), biomarker assessment of sodium (ie, 24‐hour urine collection, spot urine samples) and other related outcomes such as measurement of BP.78 These recommendations will provide evidence‐based guidance that can be implemented to improve the quality of research examining salt and health outcomes. In the meantime, our regular systematic reviews provide a practical way of keeping up‐to‐date on the science of salt.
5. CONCLUSIONS
This review identified and summarized 47 studies on dietary salt and health outcomes; and critically reviewed eight studies that were of the highest methodological quality that examine outcomes most important to patients. Five of these high‐quality studies found adverse effects of salt on health outcomes (BP; death due to kidney disease and initiation of dialysis; total kidney volume and composite of kidney function; composite of CVD events including, and risk of mortality); one study reported the benefits of sodium restriction in lowering BP and two studies reported neutral results (BP and risk of CKD). Overall, and especially in subgroup populations such as CKD, there is a need for high‐quality studies and meta‐analyses to further define the relationship of sodium intake to outcomes.
CONFLICT OF INTEREST
NRCC was a paid consultant to the Novartis Foundation (2016‐2017) to support their program to improve hypertension control in low‐ to middle‐income countries which include travel support for site visits and a contract to develop a survey. NRCC has provided paid consultative advice on accurate blood pressure assessment to Midway Corporation (2017) and is an unpaid member of World Action on Salt and Health (WASH). JW is Director of the World Health Organization Collaborating Centre on Salt Reduction and receives funding for work on salt reduction from the World Health Organization, the National Health and Medical Research Council, The Victorian Health Promotion Foundation. DM, KSP, KT, JA, and AAL have no conflict of interest to declare. MMYW is a research consultant with Renal Research Institute and Arbor Research Collaborative for Health.
Supporting information
ACKNOWLEDGMENTS
The process to provide regular updates on the science of sodium is supported by the World Hypertension League, World Health Organization Collaborating Centre on Population Salt Reduction (George Institute for Global Health), Pan American Health Organization/WHO Technical Advisory Group on Cardiovascular Disease Prevention through Dietary Sodium, and World Action on Salt and Health.
Malta D, Petersen KS, Johnson C, et al. High sodium intake increases blood pressure and risk of kidney disease from the Science of Salt: A regularly updated systematic review of salt and health outcomes (August 2016 to March 2017). J Clin Hypertens. 2018;20:1654‐1665. 10.1111/jch.13408
REFERENCES
- 1. Institute of Medicine, Food and Nutrition Board . Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate. Washington, DC: National Academy Press, 2005. [Google Scholar]
- 2. WHO . Guideline: Sodium intake for adults and children. Geneva: WHO; 2012.http://apps.who.int/iris/bitstream/handle/10665/77985/9789241504836_eng.pdf;jsessionxml:id=16AC1337961907B8B3FBA7254C4AD7AC?sequence=1. Accessed February 02, 2018. [PubMed] [Google Scholar]
- 3. Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: Systematic review and meta‐analyses. BMJ. 2013;346:f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strazzullo P, D'Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: Meta‐analysis of prospective studies. BMJ. 2009;339:b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Orginization . Report of the Formal Meeting of Member States to conclude the work on the comprehensive global monitoring framework, including indicators, and a set of voluntary global targets for the prevention and control of noncommunicable diseases. Geneva, Switzerland: World Health Orginization;2012. [Google Scholar]
- 6. Trieu K, Neal B, Hawkes C, et al. Salt reduction initiatives around the world—A systematic review of progress towards the global target. PLoS ONE. 2015;10(7):e0130247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arcand J, Webster J, Johnson C, et al. Announcing “Up to Date in the Science of Sodium”. J Clin Hypertens. 2016;18(2):85‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arcand J, Wong MMY, Santos JA, et al. More evidence that salt increases blood pressure and risk of kidney disease from the Science of Salt: A regularly updated systematic review of salt and health outcomes (April‐July 2016). J Clin Hypertens. 2017;19(8):813‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins JPT DJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPTGS, ed. Cochrane Handbook for Systematic Reviews of Interventions Vol Version 5.1.0 The Cochrane Collaboration; 2011. [Google Scholar]
- 10. McLaren L, Sumar N, Barberio AM, et al. Population‐level interventions in government jurisdictions for dietary sodium reduction. Cochrane Database Syst Rev. 2016;9:CD010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: A measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cook NR, Appel LJ, Whelton PK. Sodium intake and all‐cause mortality over 20 years in the trials of hypertension prevention. J Am Coll Cardiol. 2016;68(15):1609‐1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saulnier PJ, Gand E, Ragot S, et al. Urinary sodium concentration is an independent predictor of all‐cause and cardiovascular mortality in a type 2 diabetes cohort population. J Diabetes Res. 2017;2017:5327352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lamelas PM, Mente A, Diaz R, et al. Association of urinary sodium excretion with blood pressure and cardiovascular clinical events in 17,033 latin Americans. Am J Hypertens. 2016;29(7):796‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mazarova A, Molnar AO, Akbari A, et al. The association of urinary sodium excretion and the need for renal replacement therapy in advanced chronic kidney disease: A cohort study. BMC Nephrol. 2016;17(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mente A, O'Donnell M, Rangarajan S, et al. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: A pooled analysis of data from four studies. Lancet. 2016;388(10043):465‐475. [DOI] [PubMed] [Google Scholar]
- 17. Smyth A, Griffin M, Yusuf S, et al. Diet and Major Renal Outcomes: A Prospective Cohort Study . The NIH‐AARP diet and health study. J Renal Nutr. 2016;26(5):288‐298. [DOI] [PubMed] [Google Scholar]
- 18. Glatz N, Chappuis A, Conen D, et al. Associations of sodium, potassium and protein intake with blood pressure and hypertension in Switzerland. Swiss Med Wkly. 2017;147:w14411. [DOI] [PubMed] [Google Scholar]
- 19. Mills KT, Chen J, Yang W, et al. Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney disease. JAMA. 2016;315(20):2200‐2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peleteiro B, Barros S, Castro C, Ferro A, Morais S, Lunet N. Worldwide burden of gastric cancer in 2010 attributable to high sodium intake in 1990 and predicted attributable burden for 2030 based on exposures in 2010. Br J Nutr. 2016;116(4):728‐733. [DOI] [PubMed] [Google Scholar]
- 21. Polonia J, Monteiro J, Almeida J, Silva JA, Bertoquini S. High salt intake is associated with a higher risk of cardiovascular events: A 7.2‐year evaluation of a cohort of hypertensive patients. Blood Pressure Monitoring. 2016;21(5):301‐306. [DOI] [PubMed] [Google Scholar]
- 22. Chen L, Zhang Z, Chen W, Whelton PK, Appel LJ. Lower sodium intake and risk of headaches: Results from the trial of nonpharmacologic interventions in the elderly. Am J Public Health. 2016;106(7):1270‐1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mashura HY, Hanych TM, Rishko AA. Quality of life in patients with nonalcoholic fatty liver disease in combination with essential hypertension considering taste sensitivity to sodium chloride. Wiad Lek. 2016;69(2 Pt 2):204‐207. [PubMed] [Google Scholar]
- 24. Nourbakhsh B, Graves J, Casper TC, et al. Dietary salt intake and time to relapse in paediatric multiple sclerosis. J Neurol Neurosurg Psychiatry. 2016;87(12):1350‐1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gay HC, Rao SG, Vaccarino V, Ali MK. Effects of different dietary interventions on blood pressure: Systematic review and meta‐analysis of randomized controlled trials. Hypertension. 2016;67(4):733‐739. [DOI] [PubMed] [Google Scholar]
- 26. Scheelbeek PF, Khan AE, Mojumder S, Elliott P, Vineis P. Drinking water sodium and elevated blood pressure of healthy pregnant women in salinity‐affected coastal areas. Hypertension. 2016;68(2):464‐470. [DOI] [PubMed] [Google Scholar]
- 27. Hashimoto T, Takase H, Okado T, et al. Significance of adjusting salt intake by body weight in the evaluation of dietary salt and blood pressure. J Am Soc Hypertens. 2016;10(8):647‐655 e643. [DOI] [PubMed] [Google Scholar]
- 28. Castiglioni P, Parati G, Lazzeroni D, et al. Hemodynamic and autonomic response to different salt intakes in normotensive individuals. J Am Heart Assoc. 2016;5(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Inoue M, Tsuchihashi T, Hasuo Y, et al. Salt intake, home blood pressure, and perinatal outcome in pregnant women. Circulation J. 2016;80(10):2165‐2172. [DOI] [PubMed] [Google Scholar]
- 30. Park J, Kwock CK, Yang YJ. The effect of the sodium to potassium ratio on hypertension prevalence: A propensity score matching approach. Nutrients. 2016;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kelly J, Khalesi S, Dickinson K, Hines S, Coombes JS, Todd AS. The effect of dietary sodium modification on blood pressure in adults with systolic blood pressure less than 140 mmHg: A systematic review. JBI Database Syst Rev Implement Rep. 2016;14(6):196‐237. [DOI] [PubMed] [Google Scholar]
- 32. Lee S, Kim SH, Shin C. Interaction according to urinary sodium excretion level on the association between ATP2B1 rs17249754 and incident hypertension: The Korean genome epidemiology study. Clin Exp Hypertens. 2016;38(4):352‐358. [DOI] [PubMed] [Google Scholar]
- 33. Aparicio A, Rodriguez‐Rodriguez E, Cuadrado‐Soto E, Navia B, Lopez‐Sobaler AM, Ortega RM. Estimation of salt intake assessed by urinary excretion of sodium over 24 h in Spanish subjects aged 7‐11 years. Eur J Nutr. 2017;56(1):171‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barnado A, Oeser A, Zhang Y, et al. Association of estimated sodium and potassium intake with blood pressure in patients with systemic lupus erythematosus. Lupus. 2016;25(13):1463‐1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brian MS, Dalpiaz A, Matthews EL, Lennon‐Edwards S, Edwards DG, Farquhar WB. Dietary sodium and nocturnal blood pressure dipping in normotensive men and women. J Hum Hypertens. 2017;31(2):145‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Juraschek SP, Choi HK, Tang O, Appel LJ, Miller ER 3rd. Opposing effects of sodium intake on uric acid and blood pressure and their causal implication. J Am Soc Hypertension. 2016;10(12):939‐946 e932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li N, Yan LL, Niu W, et al. The effects of a community‐based sodium reduction program in rural china—A cluster‐randomized trial. PLoS ONE. 2016;11(12):e0166620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mizehoun‐Adissoda C, Houinato D, Houehanou C, et al. Dietary sodium and potassium intakes: Data from urban and rural areas. Nutrition. 2017;33:35‐41. [DOI] [PubMed] [Google Scholar]
- 39. Ndanuko RN, Tapsell LC, Charlton KE, Neale EP, O'Donnell KM, Batterham MJ. Relationship between sodium and potassium intake and blood pressure in a sample of overweight adults. Nutrition. 2017;33:285‐290. [DOI] [PubMed] [Google Scholar]
- 40. Ozkayar N, Dede F, Ates I, Akyel F, Yildirim T, Altun B. The relationship between dietary salt intake and ambulatory blood pressure variability in non‐diabetic hypertensive patients. Nefrologia. 2016;36(6):694‐700. [DOI] [PubMed] [Google Scholar]
- 41. Yilmaz ZV, Akkas E, Turkmen GG, Kara O, Yucel A, Uygur D. Dietary sodium and potassium intake were associated with hypertension, kidney damage and adverse perinatal outcome in pregnant women with preeclampsia. Hypertens Pregnancy. 2017;36(1):77‐83. [DOI] [PubMed] [Google Scholar]
- 42. Radzeviciene L, Ostrauskas R. Adding salt to meals as a risk factor of type 2 diabetes mellitus: A case‐control study. Nutrients. 2017;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Torres VE, Abebe KZ, Schrier RW, et al. Dietary salt restriction is beneficial to the management of autosomal dominant polycystic kidney disease. Kidney Int. 2017;91(2):493‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rush TM, Kritz‐Silverstein D, Laughlin GA, Fung TT, Barrett‐Connor E, McEvoy LK. Association between dietary sodium intake and cognitive function in older adults. J Nutr Health Aging. 2017;21(3):276‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu ZH, Tang ZH, Zhang KQ, Shi L. Salty food preference is associated with osteoporosis among Chinese men. Asia Pacific J Clin Nutr. 2016;25(4):871‐878. [DOI] [PubMed] [Google Scholar]
- 46. Choi Y, Lee JE, Chang Y, et al. Dietary sodium and potassium intake in relation to non‐alcoholic fatty liver disease. Br J Nutr. 2016;116(8):1447‐1456. [DOI] [PubMed] [Google Scholar]
- 47. Dai XW, Wang C, Xu Y, Guan K, Su YX, Chen YM. Urinary sodium and potassium excretion and carotid atherosclerosis in chinese men and women. Nutrients. 2016;8(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vafa M, Soltani S, Zayeri F, Niroomand M, Najarzadeh A. The relationship between sodium intake and some bone minerals and osteoporosis risk assessment instrument in postmenopausal women. Med J Islamic Rep Iran. 2016;30:377. [PMC free article] [PubMed] [Google Scholar]
- 49. Kieneker LM, Bakker SJ, de Boer RA, Navis GJ, Gansevoort RT, Joosten MM. Low potassium excretion but not high sodium excretion is associated with increased risk of developing chronic kidney disease. Kidney Int. 2016;90(4):888‐896. [DOI] [PubMed] [Google Scholar]
- 50. Catena C, Colussi G, Novello M, et al. Dietary salt intake is a determinant of cardiac changes after treatment of primary aldosteronism: A prospective study. Hypertension. 2016;68(1):204‐212. [DOI] [PubMed] [Google Scholar]
- 51. Gruppen EG, Connelly MA, Vart P, Otvos JD, Bakker SJ, Dullaart RP. GlycA, a novel proinflammatory glycoprotein biomarker, and high‐sensitivity C‐reactive protein are inversely associated with sodium intake after controlling for adiposity: The Prevention of Renal and Vascular End‐Stage Disease study. Am J Clin Nutr. 2016;104(2):415‐422. [DOI] [PubMed] [Google Scholar]
- 52. Humalda JK, Keyzer CA, Binnenmars SH, et al. Concordance of dietary sodium intake and concomitant phosphate load: Implications for sodium interventions. NMCD. 2016;26(8):689‐696. [DOI] [PubMed] [Google Scholar]
- 53. Yan L, Guo X, Wang H, et al. Population‐based association between urinary excretion of sodium, potassium and its ratio with albuminuria in Chinese. Asia Pacific J Clin Nutr. 2016;25(4):785‐797. [DOI] [PubMed] [Google Scholar]
- 54. Huang F, Yu P, Yuan Y, et al. The relationship between sodium excretion and blood pressure, urine albumin, central retinal arteriolar equivalent. BMC Cardiovasc Disord. 2016;16(1):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cao WT, He J, Chen GD, Wang C, Qiu R, Chen YM. The association between urinary sodium to potassium ratio and bone density in middle‐aged Chinese adults. Osteoporos Int. 2017;28(3):1077‐1086. [DOI] [PubMed] [Google Scholar]
- 56. Moosavian SP, Haghighatdoost F, Surkan PJ, Azadbakht L. Salt and obesity: A systematic review and meta‐analysis of observational studies. Int J Food Sci Nutr. 2017;68(3):265‐277. [DOI] [PubMed] [Google Scholar]
- 57. Kwon SJ, Ha YC, Park Y. High dietary sodium intake is associated with low bone mass in postmenopausal women: Korea National Health and Nutrition Examination Survey, 2008‐2011. Osteoporos Int. 2017;28(4):1445‐1452. [DOI] [PubMed] [Google Scholar]
- 58. Saran R, Padilla RL, Gillespie BW, et al. A randomized crossover trial of dietary sodium restriction in stage 3‐4 CKD. CJASN. 2017;12(3):399‐407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McLean R, Williams S, Mann J. Monitoring population sodium intake using spot urine samples: Validation in a New Zealand population. J Hum Hypertens. 2014;28(11):657‐662. [DOI] [PubMed] [Google Scholar]
- 60. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. New England J Med. 1997;336(16):1117‐1124. [DOI] [PubMed] [Google Scholar]
- 61. Bentley B. A review of methods to measure dietary sodium intake. J Cardiovasc Nurs. 2006;21(1):63‐67. [DOI] [PubMed] [Google Scholar]
- 62. Arcand J, Floras JS, Azevedo E, Mak S, Newton GE, Allard JP. Evaluation of 2 methods for sodium intake assessment in cardiac patients with and without heart failure: The confounding effect of loop diuretics. Am J Clin Nutr. 2011;93(3):535‐541. [DOI] [PubMed] [Google Scholar]
- 63. Wong MM, Arcand J, Leung AA, et al. The science of salt: A regularly updated systematic review of salt and health outcomes (August to November 2015). J Clin Hypertension. 2016;18(10):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wong MM, Arcand J, Leung AA, Thout SR, Campbell NR, Webster J. The science of salt: A regularly updated systematic review of salt and health outcomes (December 2015‐March 2016). J Clin Hypertension. 2017;19(3):322‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Arcand J, Wong MM, Trieu K, et al. The science of salt: A regularly updated systematic review of salt and health outcomes (June and July 2015). J Clin Hypertension. 2016;18(5):371‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Trieu K, McLean R, Johnson C, et al. The science of salt: A regularly updated systematic review of the implementation of salt reduction interventions (June‐October 2015). J Clin Hypertension. 2016;18(6):487‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Santos JA, Trieu K, Raj TS, et al. The Science of Salt: A regularly updated systematic review of the implementation of salt reduction interventions (March‐August 2016). J Clin Hypertens. 2017;19(4):439‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Johnson C, Raj TS, Trieu K, et al. The Science of Salt: A systematic review of quality clinical salt outcome studies June 2014 to May 2015. J Clin Hypertens. 2016;18(9):832‐839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schachter J, Harper PH, Radin ME, Caggiula AW, McDonald RH, Diven WF. Comparison of sodium and potassium intake with excretion. Hypertension. 1980;2(5):695‐699. [DOI] [PubMed] [Google Scholar]
- 70. Kipnis V, Midthune D, Freedman L, et al. Bias in dietary‐report instruments and its implications for nutritional epidemiology. Public Health Nutr. 2002;5(6A):915‐923. [DOI] [PubMed] [Google Scholar]
- 71. Reyes AJ, Leary WP. Renal excretory responses to single and repeated administration of diuretics in healthy subjects: Clinical connotations. Cardiovasc Drugs Ther. 1993;7(Suppl 1):29‐44. [DOI] [PubMed] [Google Scholar]
- 72. McLean RM, Farmer VL, Nettleton A, et al. Assessment of dietary sodium intake using a food frequency questionnaire and 24‐hour urinary sodium excretion: A systematic literature review. J Clin Hypertens. 2017;19(12):1214‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : The Eating at America's Table Study. Am J Epidemiol. 2001;154(12):1089‐1099. [DOI] [PubMed] [Google Scholar]
- 74. Ishihara J, Inoue M, Kobayashi M, et al. Impact of the revision of a nutrient database on the validity of a self‐administered food frequency questionnaire (FFQ). J Epidemiol. 2006;16(3):107‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Block G, Woods M, Potosky A, Clifford C. Validation of a self‐administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43(12):1327‐1335. [DOI] [PubMed] [Google Scholar]
- 76. Date C, Fukui M, Yamamoto A, et al. Reproducibility and validity of a self‐administered food frequency questionnaire used in the JACC study. J Epidemiol. 2005;15(Suppl 1):S9‐S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Mason B, Ross L, Gill E, Healy H, Juffs P, Kark A. Development and validation of a dietary screening tool for high sodium consumption in Australian renal patients. J Ren Nutr. 2014;24(2):123‐134; e121‐e123. [DOI] [PubMed] [Google Scholar]
- 78. Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: The National Institutes of Health‐American Association of Retired Persons Diet and Health Study. Am J Epidemiol. 2001;154(12):1119‐1125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


