Abstract
Sleep disordered breathing (SDB) is highly prevalent in patients with high blood pressure (BP). Severity of SDB can be evaluated by the number of apneas and hypopneas per hour (AHI) or by measures of hypoxia. The objective of this study was to assess the association between different measures of SDB and BP. In 134 consecutive patients, polygraphy was performed to determine the AHI. Pulse oximetry was used to determine hypoxemic burden (time below 90% oxygen saturation [T90] and hypoxia load [HL], representing the integrated area above the curve of desaturation). AHI did not correlate with systolic and diastolic BP or pulse pressure. In contrast, HL correlated with pulse pressure during the day (P = .01) and night (P = .0034) before and after adjustment for body mass index. The correlation between systolic BP and HL at night disappeared following adjustment for body mass index. This study generates the hypothesis that nocturnal hypoxemic burden may represent a suitable marker of BP pattern and a potential treatment target in hypertensive patients.
Keywords: blood pressure, hypertension, hypoxia, pulse pressure, sleep apnea
1. INTRODUCTION
Sleep‐disordered breathing (SDB), particularly obstructive sleep apnea (OSA), is highly prevalent in patients with hypertension.1 Approximately half of all patients with OSA have hypertension, and about 30%‐40% of patients with hypertension also have clinically relevant OSA.2 This prevalence of OSA is even higher (up to 90%) in patients with drug‐resistant hypertension.3 Treatment of OSA by continuous positive airway pressure ventilation (CPAP) alleviates intermittent hypoxia.4, 5, 6, 7, 8, 9 The greatest benefits of CPAP therapy for OSA have been seen in patients with resistant hypertension, where CPAP reduced daytime blood pressure (BP) by 6.5 mm Hg, compared with a 3.1 mm Hg increase in untreated patients.10 In a meta‐analysis of 3 randomized‐controlled trials, CPAP‐withdrawal resulted in a clinically relevant increase in BP, which was considerably higher than BP in conventional CPAP trials, and the effects of CPAP‐withdrawal may be underestimated when office BP is used.11 These studies suggest an involvement of SDB in the pathophysiology of increased BP. Besides systolic and diastolic BP, increased pulse pressure typically reflects aortic stiffness, which is attributable to fatigue of elastin and increased pressure wave reflections, and is predictive of stroke12 and cardiovascular mortality.13, 14, 15 The associations between different parameters for severity of SDB and BP parameters are unclear.
Presently, severity of SDB is evaluated by the apnea‐hypopnea index (AHI).16 The AHI is an event‐based measure of severity of SDB that considers the number of hypopneas and apneas per hour during sleep, but does not incorporate the degree and duration of desaturations. Therefore, progression of cardiac remodeling and cardiovascular events triggered by hypoxia is neither detected nor predicted by the AHI. In this cross‐sectional explorative study, we enrolled patients with suspected sleep apnea and performed unattended polygraphy and 24‐hour ambulatory BP monitoring to identify which measure of SDB correlates best with different BP parameters.
2. METHODS
2.1. Patients
A total of 134 consecutive patients’ characteristics (see Table 1) were enrolled in this cross‐sectional observational study. All participants were asymptomatic outpatients admitted for routine check‐up evaluations and underwent a detailed history, physical examination, and overnight polygraphy as part of the work‐up in a cardiology clinic. Exclusion criteria were the presence of drug‐resistant hypertension, acute illnesses, unstable heart diseases, or unstable respiratory disorder. All patients had either self‐reported snoring or self‐reported daytime sleepiness but had no history of sleep apnea and no initiated CPAP treatment.
Table 1.
Patients′ characteristics
| All (n = 134) | AHI <15/h(n = 84) | AHI ≥15/h (n = 50) | P value | |
|---|---|---|---|---|
| Age (y) | 66.3 ± 11.0 | 65.1 ± 11.6 | 68 ± 9.5 | .168 |
| Sex, male (n [%]) | 104 | 64 (76) | 40 (80) | .672 |
| BMI (kg/m2) | 29.1 ± 6.1 | 27.8 ± 5.8 | 31.8 ± 6.3 | .001 |
| Heart rate (1/min) | 66.0 ± 16.7 | 65.3 ± 16.04 | 67.3 ± 13.8 | .481 |
| Comorbidities | ||||
| Hypertension (n [%]) | 80 | 49 (58) | 31 (62) | .718 |
| DM2 (n [%]) | 27 | 15 (18) | 12 (24) | .504 |
| Medication | ||||
| ACE inhibitor (n [%]) | 97 | 61 (73) | 36 (72) | 1.000 |
| Beta blocker (n [%]) | 87 | 53 (63) | 34 (68) | .581 |
| Diuretics (n [%]) | 63 | 38 (45) | 25 (50) | .720 |
| Dihydropyridin calcium channel blocker (n [%]) | 23 | 15 (18) | 8 (16) | 1.000 |
BMI, body‐mass‐index; CAD, coronary artery disease; DM2, diabetes mellitus type 2.
Bold values indicate significant differences at P < .05.
2.2. Polygraphy
Polygraphy (SomnoScreenTM plus RC Easy, ResMed, Martinsried, Germany) was performed in all subjects using standard polygraphic techniques.16 Respiratory efforts were measured with the use of respiratory inductance plethysmography, and airflow was measured by a nasal pressure cannula. Apnea was defined as a cessation of inspiratory airflow for ≥10 seconds. Hypopneas were defined as a reduction in airflow accompanied by a ≥3% drop in oxygen desaturation from pre‐event baseline.16 The apnea‐hypopnea index (AHI) was defined as the number of apneas and hypopneas per hour recording time. Patients were stratified into those with (AHI ≥15/h) and without (AHI <15/h) moderate to severe SDB. Apneas without evidence of respiratory effort were scored as central, while those with respiratory effort were categorized as obstructive. Patients were classified as predominant OSA if >50% of these AHI events were obstructive. If >50% of these AHI events were central, they were classified as patients with predominant central sleep apnea (CSA). Pulse oximetry was used to determine time with oxygen saturation <90% (T90), mean oxygen saturation (mean SpO2), and minimal oxygen saturation (min SpO2). The hypoxemia load (HL) was defined as the integrated area of the desaturation curve divided by the total recording time obtained by polygraphy. The integrated area of desaturation was approximated by numerical integration using the trapezoidal rule and was divided by total recording time (t total): HL = Σ(½ (t n+1−t n) × (SpO2n + SpO2n+1))/t total. Recording quality was visually determined and artifacts were excluded from the analysis.
2.3. Blood pressure
Twenty‐four‐hour ambulatory BP monitoring was performed at the same time in all patients. Readings were taken every 15 minutes during daytime (7:00 am to 10:00 pm) and every 30 minutes at night (10:00 pm to 7:00 am).
Patients were assessed while adhering to their usual activity and nocturnal sleep routine at home. Only patients with >70% valid BP measurements (either awake or asleep) were included. Mean systolic BP, diastolic BP, and pulse pressure were calculated as overall 24‐hour average as well as day and nighttime average. All BP assessors received explicit instructions for 24‐hour ambulatory BP measurements in compliance with published guidelines.17 Ambulatory recordings were done on a workday. We defined the night period as 1:00 am to 5:59 am to capture sleeping hours. Daytime was defined as 9:00 am to 8:59 pm. A systolic BP drop of less than 10% during nighttime was considered to be a non‐dipping pattern.
2.4. Statistical analysis
The study was designed as a cross‐sectional explorative study to determine associations between measures of SDB and arterial BP in adults. Adults with a suspect clinical manifestation of sleep apnea were included in the study. HL, T90, AHI, min SpO2, mean SpO2, and predominant OSA or predominant CSA were considered as independent variables. Mean diastolic and systolic BP, dipper or non‐dippers, and mean pulse pressure were considered as dependent variables. Demographic and other baseline characteristics are stated as mean ± standard deviation for normally distributed continuous variables and absolute and relative frequency for categorical variables. Missing data were not imputed. Statistical comparisons between groups were performed using the Pearson χ2 test for categorical variables and the 2 sample unpaired t test or Wilcoxon rank‐sum test for continuous variables where appropriate. For continuous variables and in order to estimate associations between 2 continuous variables a linear regression, analysis was conducted. Uni‐ and multivariable binomial logistic regression models were used in order to estimate the association between classification of sleep apnea and dipping behavior. Due to the explorative and hypothesis generating nature of the study, we did not correct for multiple testing. All P‐values are two‐sided and a significance level of 5% (P < .05) was used. For all statistical calculations, software GraphPad Prism 6 was used.
3. RESULTS
3.1. Patients’ characteristics
Patients’ characteristics are presented in Table 1. Stratified by SDB status, there was no significant difference between patients with an AHI <15/h or with an AHI ≥15/h with respect to age, gender, or cardiovascular risk factors. Patients with SDB had a statistically significant higher body‐mass index compared with those with no SDB. 71% of all patients had predominant OSA, and the remaining patients had predominant CSA.
3.2. Association of event‐based as well as hypoxia‐based measures of SDB and hypoxemic burden in patients
Sleep characteristics are shown in Table 2. Hypoxia‐based measures like mean oxygen saturation, minimal oxygen saturation, and T90 did not differ between patients with or without SDB. The HL tended to be lower in patients without compared to patients with SDB. Particularly patients with an AHI <15/h showed a broad variation in T90 and HL, ranging from very low levels to levels higher than those found in patients with an AHI ≥15/h (Figure).
Table 2.
Event‐based and hypoxia‐based parameters
| AHI < 15/h | AHI ≥ 15/h | P value | |
|---|---|---|---|
| Event‐based measures of SDB | |||
| Apnea‐hypopnea index, AHI (1/h) | 7.5 ± 4.1 | 29.6 ± 13.0 | |
| Hypoxia‐based measures of SDB | |||
| Mean oxygen saturation, mean SpO2 (%) | 92.8 ± 2.4 | 92.0 ± 2.5 | .14 |
| Time below 90% saturation, T90 (s) | 2803 ± 4619 | 4654 ± 5839 | .52 |
| Hypoxia load, HL (%) | 7.0 ± 2.2 | 7.9 ± 2.6 | .06 |
| Minimum oxygen saturation, min SpO2 (%) | 76 ± 13 | 73 ± 10 | .28 |
Figure 1.
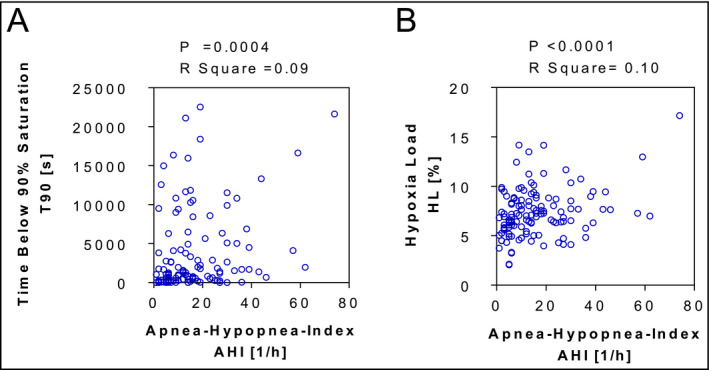
Correlation between the apnea‐hypopnea‐index (AHI) and the time below 90% saturation (T90, A) as well as the hypoxia load (HL, B). AHI, apneas and hypopneas per hour; HL, hypoxia load
3.3. Association of measures of SDB and blood pressure
Different measures of BP did not differ significantly between patients with an AHI <15/h and patients with an AHI ≥15/h (Table 3). The AHI did not correlate significantly with systolic or diastolic BP or pulse pressure either during daytime or during night time. However, in the univariable model, there was a significant correlation between HL and pulse pressure during daytime and night time in all patients (Table 4). Additionally, HL and minimal oxygen saturation were associated with systolic BP during night time but not during daytime. A trend towards an association was also observed for the established hypoxia‐based parameter T90 (Table 4). BMI differed significantly in patients with or without sleep apnea. In a multivariable model adjusted for BMI, pulse pressure during daytime (beta coefficient: 1.0416, P = .0467) and night time (beta coefficient: 1.2536, P = .0324) still correlated with HL. However, the correlation between systolic BP and HL during night time disappeared following adjustment for body mass index (beta coefficient: 1.2483, P = .143).
Table 3.
Measures of blood pressure
| Day | Night | |||||
|---|---|---|---|---|---|---|
| AHI <15/h | AHI ≥15/h | P value | AHI <15/h | AHI ≥15/h | P value | |
| Systolic BP (mm Hg) | 126.1 ± 14.4 | 128.83 ± 15.3 | .433 | 117.28 ± 14.8 | 124.2 ± 16.5 | .059 |
| Diastolic BP (mm Hg) | 76.9 ± 11.8 | 77.10 ± 11.9 | .901 | 69.68 ± 9.6 | 71.9 ± 12.4 | .374 |
| Pulse pressure (mm Hg) | 49.1 ± 9.7 | 51.7 ± 10.7 | .374 | 47.6 ± 10.5 | 52.3 ± 12.1 | .131 |
Table 4.
Univariable model: associations between event‐based and hypoxia‐based parameters and measures of blood pressure
| AHI | HL | T90 | Mean SpO2 | Min SpO2 | |
|---|---|---|---|---|---|
| Day | |||||
| Systolic BP | P = .92 | P = .16 | P = .90 | P = .19 | P = .87 |
| R 2 = 0.0001 | R 2 = 0.02 | R 2 = 0.0001 | R 2 = 0.02 | R 2 = 0.003 | |
| Diastolic BP | P = .13 | P = .72 | P = .18 | P = .56 | P = .071 |
| R 2 = 0.02 | R 2 = 0.001 | R 2 = 0.02 | R 2 = 0.004 | R 2 = 0.03 | |
| Pulse pressure | P = .11 | P = .014 | P = .077 | P = .11 | P = .53 |
| R 2 = 0.03 | R 2 = 0.07 | R 2 = 0.03 | R 2 = 0.031 | R 2 = 0.0004 | |
| Night | |||||
| Systolic BP | P = .46 | P = .043 | P = .06 | P = .051 | P = .02 |
| R 2 = 0.0006 | R 2 = 0.05 | R 2 = 0.04 | R 2 = 0.04 | R 2 = 0.06 | |
| Diastolic BP | P = .38 | P = .98 | P = .98 | P = .9 | P = .63 |
| R 2 = 0.009 | R 2 = 0.001 | R 2 = 0.0001 | R 2 = 0.0003 | R 2 = 0.002 | |
| Pulse pressure | P = .058 | P = .0034 | P = .0075 | P = .16 | P = .32 |
| R 2 = 0.045 | R 2 = 0.1 | R 2 = 0.08 | R 2 = 0.033 | R 2 = 0.01 | |
AHI, apnea‐hypopnea index; HL, hypoxia load; mean SpO2, mean oxygen saturation; min SpO2, minimum oxygen saturation; T90, time below 90% saturation.
Bold values indicate significant differences at P < .05.
3.4. Classification of sleep apnea and circadian behavior of blood pressure
The proportion of patients showing a non‐dipping pattern of BP was not different in patients with (AHI ≥15/h) compared to patients without moderate to severe SDB (AHI <15/h) (P = .460). However, results from univariable binomial logistic regression models suggest that the odds of non‐dipping blood pressure by night is 5 times higher in patients with predominant OSA compared with patients with predominant CSA (OR = 5.26, P = .013). After adjusting for covariates such as age, BMI, mean systolic BP at night, number of BP lowering drugs, and sex, the odds of non‐dipping blood pressure by night was almost 5‐times higher in patients with predominant OSA compared with patients with predominant CSA. This just missed significance (OR = 4.67, P = .051). These results should be interpreted with caution due to the smaller number of patients with predominant CSA, resulting in lower estimation accuracy. Univariable linear regression models showed no statistically significant differences between patients with predominant OSA and patients with predominant CSA regarding systolic BP, diastolic BP, or pulse pressure.
4. DISCUSSION
4.1. Association of event‐based as well as hypoxia‐based measures of SDB
Several complications and end‐organ damage are attributed to the amount and severity of intermittent hypoxia in SDB.18 Severity of SDB is currently evaluated by the event‐based measure of SDB “AHI”16 considering the number of hypopneas and apneas per hour during sleep. Apneas are defined as a >90% drop from the peak thoracic excursion of the pre‐event baseline for more than 10 seconds without requisitioning oxygen desaturation. In the case of a hypopnea, the peak thoracic excursion drops by ≥30% for at least 10 seconds accompanied by ≥3% oxygen desaturation from pre‐event baseline or an arousal.16 Therefore, the current scoring system of SDB is mainly focused on the occurrence of respiratory events but does not incorporate the absolute degree and duration of desaturation characterizing and determining the nocturnal hypoxemic burden.16
Initially, we determined the distribution of nocturnal hypoxemic burden and the severity of SDB in patients with suspected SDB. To further characterize hypoxemic burden, we introduced a novel marker describing the hypoxic load (HL) during night. The HL is representing the integrated area above the SO2‐curve and correlates well to T90. However, in contrast to conventional hypoxia markers like T90, HL describes hypoxemic burden independently from any threshold or cause for oxygen desaturation. We showed that markers estimating hypoxemic burden during night, like HL or T90, do not correlate with the event‐based marker AHI either in patients with an AHI >15/h or in patients with an AHI <15/h. Particularly, patients with an AHI <15/h show a broad variation in HL ranging from very low values to values higher than those found in patients with severe SDB.
The observation that severity of SDB determined by the AHI does not represent nocturnal hypoxemic burden has relevant clinical implications. In chronic stable heart failure patients with reduced ejection fraction, the hypoxemic burden per se, determined by eg, T90, and not the number of respiratory events determined by the AHI, but is a predictor of all‐cause mortality.19 Minimal oxygen saturation during sleep, the number of desaturations <90% per hour and T90, were significantly associated with adverse events in heart failure patients with reduced ejection fraction, whereas the AHI was not.20, 21 Nocturnal hypoxemia in OSA has been shown to be an important predictor of poor prognosis for patients after myocardial infarction.22 In comparison to the AHI, T90 and HL describe the hypoxemic burden during night and may be particularly important to predict progression of cardiac remodeling and cardiovascular events triggered by hypoxia.
4.2. Nocturnal hypoxic burden and blood pressure
Of note, AHI did not correlate significantly with systolic BP, diastolic BP, or pulse pressure. However, we observed a robust association between nocturnal hypoxemic burden determined by HL and pulse pressure during day‐ and night‐time. Pulse pressure is a risk factor for cardiovascular complications,23, 24 like myocardial infarction,24, 25 and is related to increased cardiovascular mortality and morbidity.26 Additionally, pulse pressure partly reflects reduced vascular compliance and increased arterial stiffness,27 which results in increased pulsatile load and impaired left ventricular relaxation.28, 29 Interestingly, a relationship between arterial stiffness and OSA was shown in middle‐aged hypertensive subjects.30 The correlation between pulse pressure and HL shown in this study further supports the hypothesis that nocturnal hypoxia may be involved in the pathophysiology of reduced vascular compliance and subsequent increase in pulse pressure.31 A numerical trend towards a correlation was observed for T90, though not always significant. Interestingly, the correlation between systolic BP and HL at night disappeared following adjustment for body mass index, suggesting that not just hypoxemic burden but also other concomitant conditions, like obesity and metabolic syndrome, contribute to the complex regulation BP.
4.3. Circadian rhythm in blood pressure and measures of SDB
Sleep disordered breathing is known to impact the circadian variation of BP. The non‐dipping pattern of BP, characterized by a <10% decrease in nocturnal systolic and diastolic BP, has been shown to correlate to SDB severity.32 Its prevalence is high among non‐treated OSA patients,33, 34 and CPAP therapy can restore normal circadian rhythm.35, 36 Repetitive hypoxia and hypercapnia may result in chemoreceptor activation, increased sympathetic activity, and vasoconstriction during day and night.37 The proportion of patients showing a non‐dipping pattern of BP was not different in patients with (AHI ≥15/h) compared to patients without moderate to severe SDB (AHI <15/h). However, the risk to show a dipping‐pattern of BP was 5‐times higher in patients with predominant OSA compared to patients with predominant CSA, pointing towards a more relevant role of obstructive apneas on circadian rhythm of BP compared to central apneas. However, neither HL nor T90, both markers for hypoxemic burden during night, correlated with the occurrence of non‐dipping pattern of BP.
4.4. Strengths and limitations of the study
We selected an explorative approach to allow the analysis of the suspected association between BP parameters and features of SDB. This study generates the hypothesis that nocturnal hypoxemic burden may contribute to the complex regulation of BP and may represent a suitable treatment target in hypertension. Whether this association can be confirmed in a prospectively characterized cohort needs to be investigated in a future study. Severity of self‐reported daytime sleepiness was not accessed by a questionnaire and tobacco and alcohol use was not captured in this study. Patients who did not have >70% BP measurements were excluded. This might have resulted in a selection bias as this was not an intention to treat design. The mechanisms which may explain the association between nocturnal hypoxemic burden and pulse pressure should be investigated in future clinical and preclinical studies. The mean age of the subjects included in this study was relatively high and the association between nocturnal hypoxemic burden and BP might be different in a younger cohort.
4.5. Summary
The data of this cross‐sectional study showed that nocturnal hypoxemic burden, but not the AHI as an event‐based measure of SDB severity, was associated with high pulse pressure. This association remained significant even after adjustment for body mass index. The correlation between systolic BP and HL at night, however, disappeared following adjustment for body mass index. The presence of predominant OSA, but not hypoxemic burden, increased the odds for non‐dipping blood pressure profile. Whether nocturnal hypoxemia, which can be quantified by low‐cost nocturnal oximetry, can be used to further characterize sleep apnea severity or represents a suitable marker of BP pattern and a potential treatment target in hypertensive patients warrants further research.
CONFLICT OF INTEREST
None.
Khoshkish S, Hohl M, Linz B, et al. The association between different features of sleep‐disordered breathing and blood pressure: A cross‐sectional study. J Clin Hypertens. 2018;20:575–581. 10.1111/jch.13202
REFERENCES
- 1. Linz D, Woehrle H, Bitter T, et al. The importance of sleep‐disordered breathing in cardiovascular disease. Clin Res Cardiol. 2015;104:705‐718. [DOI] [PubMed] [Google Scholar]
- 2. Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008;118:1080‐1111. [DOI] [PubMed] [Google Scholar]
- 3. Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug‐resistant hypertension. J Hypertens. 2001;19:2271‐2277. [DOI] [PubMed] [Google Scholar]
- 4. Sullivan CE, Issa FG, Berthon‐Jones M, et al. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862‐865. [DOI] [PubMed] [Google Scholar]
- 5. Parati G, Lombardi C, Hedner J, et al. Position paper on the management of patients with obstructive sleep apnea and hypertension: joint recommendations by the European Society of Hypertension, by the European Respiratory Society and by the members of European COST (COoperation in Scientific and Technological research) ACTION B26 on obstructive sleep apnea. J Hypertens. 2012;30:633‐646. [DOI] [PubMed] [Google Scholar]
- 6. Bratton DJ, Stradling JR, Barbé F, et al. Effect of CPAP on blood pressure in patients with minimally symptomatic obstructive sleep apnoea: a meta‐analysis using individual patient data from four randomised controlled trials. Thorax. 2014;69:1128‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fava C, Dorigoni S, Dalle Vedove F, et al. Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta‐analysis. Chest. 2014;145:762‐771. [DOI] [PubMed] [Google Scholar]
- 8. Schein AS, Kerkhoff AC, Coronel CC, et al. Continuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea; a systematic review and meta‐analysis with 1000 patients. J Hypertens. 2014;32:1762‐1773. [DOI] [PubMed] [Google Scholar]
- 9. Narkiewicz K, Kato M, Phillips BG, et al. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332‐2335. [DOI] [PubMed] [Google Scholar]
- 10. Iftikhar IH, Valentine CW, Bittencourt LR, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta‐analysis. J Hypertens. 2014;32:2341‐2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schwarz EI, Schlatzer C, Rossi VA, et al. The effect of CPAP withdrawal on blood pressure in OSA: data from three randomised‐controlled trials. Eur Resp J. 2014;44(suppl 58):P1740. [DOI] [PubMed] [Google Scholar]
- 12. SHEP Cooperative Research Group . Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255‐3264. [PubMed] [Google Scholar]
- 13. Staessen JA, Gasowski J, Wang JG, et al. Risks of untreated and treated isolated systolic hypertension in the elderly: meta‐analysis of outcome trials. Lancet. 2000;355:865‐872. [DOI] [PubMed] [Google Scholar]
- 14. Mackenzie IS, McEniery CM, Dhakam Z, et al. Comparison of the effects of antihypertensive agents on central blood pressure and arterial stiffness in isolated systolic hypertension. Hypertension. 2009;54:409‐413. [DOI] [PubMed] [Google Scholar]
- 15. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents developed in collaboration with the American Academy of Neurology, American Geriatrics Society, American Society for Preventive Cardiology, American Society of Hypertension, American Society of Nephrology, Association of Black Cardiologists, and European Society of Hypertension. J Am Coll Cardiol. 2011;57:2037‐2114. [DOI] [PubMed] [Google Scholar]
- 16. Iber C. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. 2007: American Academy of Sleep Medicine.
- 17. O'Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731‐1768. [DOI] [PubMed] [Google Scholar]
- 18. Linz D, Linz B, Hohl M, et al. Atrial arrhythmogenesis in obstructive sleep apnea: therapeutic implications. Sleep Med Rev. 2016;26:87‐94. [DOI] [PubMed] [Google Scholar]
- 19. Oldenburg O, Wellmann B, Buchholz A, et al. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. Eur Heart J. 2016;37:1695‐1703. [DOI] [PubMed] [Google Scholar]
- 20. Gellen B, Canouï‐Poitrine F, Boyer L, et al. Apnea–hypopnea and desaturations in heart failure with reduced ejection fraction: are we aiming at the right target? Int J Cardiol. 2016;203:1022‐1028. [DOI] [PubMed] [Google Scholar]
- 21. Asano K, Takata Y, Usui Y, et al. New index for analysis of polysomnography’, integrated area of desaturation’, is associated with high cardiovascular risk in patients with mild to moderate obstructive sleep apnea. Respiration. 2009;78:278‐284. [DOI] [PubMed] [Google Scholar]
- 22. Xie J, Sert Kuniyoshi FH, Covassin N, et al. Nocturnal hypoxemia due to obstructive sleep apnea is an independent predictor of poor prognosis after myocardial infarction. J Am Heart Assoc. 2016;5:e003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benetos A, Rudnichi A, Safar M, Guize L. Pulse pressure and cardiovascular mortality in normotensive and hypertensive subjects. Hypertension. 1998;32:560‐564. [DOI] [PubMed] [Google Scholar]
- 24. Franklin SS, Khan SA, Wong ND, et al. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham heart study Circulation. 1999;100:354‐360. [DOI] [PubMed] [Google Scholar]
- 25. Madhavan S, Ooi WL, Cohen H, et al. Relation of pulse pressure and blood pressure reduction to the incidence of myocardial infarction. Hypertension. 1994;23:395‐401. [DOI] [PubMed] [Google Scholar]
- 26. Verdecchia P, Schillaci G, Borgioni C, et al. Ambulatory pulse pressure a potent predictor of total cardiovascular risk in hypertension. Hypertension. 1998;32:983‐988. [DOI] [PubMed] [Google Scholar]
- 27. Lee WH, Hsu PC, Chu CY, et al. Associations of pulse pressure index with left ventricular filling pressure and diastolic dysfunction in patients with chronic kidney disease. Am J Hypertens. 2014;27:454‐459. [DOI] [PubMed] [Google Scholar]
- 28. Jelic S, Bartels MN, Mateika JH, et al. Arterial stiffness increases during obstructive sleep apneas. Sleep. 2002;25:850‐855. [PubMed] [Google Scholar]
- 29. Drager LF, Bortolotto LA, Figueiredo AC, et al. Obstructive sleep apnea, hypertension, and their interaction on arterial stiffness and heart remodeling. Chest. 2007;131:1379‐1386. [DOI] [PubMed] [Google Scholar]
- 30. Tsioufis C, Thomopoulos K, Dimitriadis K, et al. The incremental effect of obstructive sleep apnoea syndrome on arterial stiffness in newly diagnosed essential hypertensive subjects. J Hypertens. 2007;25:141‐146. [DOI] [PubMed] [Google Scholar]
- 31. Chung S, Yoon IY, Lee CH, et al. The association of nocturnal hypoxemia with arterial stiffness and endothelial dysfunction in male patients with obstructive sleep apnea syndrome. Respiration. 2010;79:363‐369. [DOI] [PubMed] [Google Scholar]
- 32. Pankow W, Nabe B, Lies A, et al. Influence of sleep apnea on 24‐hour blood pressure. Chest. 1997;112:1253‐1258. [DOI] [PubMed] [Google Scholar]
- 33. Loredo JS, Ancoli‐Israel S, Dimsdale JE. Sleep quality and blood pressure dipping in obstructive sleep apnea. Am J Hypertens. 2001;14:887‐892. [DOI] [PubMed] [Google Scholar]
- 34. Portaluppi F, Provini F, Cortelli P, et al. Undiagnosed sleep‐disordered breathing among male nondippers with essential hypertension. J Hypertens. 1997;15:1227‐1233. [DOI] [PubMed] [Google Scholar]
- 35. Akashiba T, Minemura H, Yamamoto H, et al. Nasal continuous positive airway pressure changes blood pressure” non‐dippers” to” dippers” in patients with obstructive sleep apnea. Sleep. 1999;22:849‐853. [DOI] [PubMed] [Google Scholar]
- 36. Engleman HM, Gough K, Martin SE, et al. Ambulatory blood pressure on and off continuous positive airway pressure therapy for the sleep apnea/hypopnea syndrome: effects in” non‐dippers”. Sleep. 1996;19:378‐81. [DOI] [PubMed] [Google Scholar]
- 37. Somers VK, Dyken ME, Clary MP, et al. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897. [DOI] [PMC free article] [PubMed] [Google Scholar]


