Abstract
Although plasma aldosterone concentration (PAC) varies depending on primary aldosteronism (PA) subtypes, patients with different subtypes may have similar blood pressure (BP). The authors hypothesized that hormones other than aldosterone might influence BP in PA patients. A total of 73 PA cases, including 30 cases of aldosterone‐producing adenomas (APAs), 29 cases of bilateral hyperaldosteronism, and 24 control cases of essential hypertension were enrolled retrospectively. The authors examined the levels of aldosterone, cortisol, renin, and adrenocorticotropic hormone (ACTH) measured at 12 am, 6 am, 12 pm, and 6 pm and BP in the early morning (6 am to 7 am), late morning (9 am to 11 am), and early evening (5 pm to 7 pm). Results showed no statistically significant correlation between PAC and BP in the patients with PA; however, early and late morning systolic BP strongly correlated with ACTH at 6 am in patients with APA. These results suggest that hormones other than aldosterone, such as ACTH, may affect BP in patients with APA.
Keywords: ACTH, aldosterone, blood pressure, primary aldosteronism
1. Introduction
Primary aldosteronism (PA) caused by autonomous aldosterone production is one of the most common causes of secondary hypertension. Some reports have found that the incidence of PA is about 10% in hypertensive patients.1, 2, 3 Aldosterone produced by adenomas or hyperplasia has been associated with hypertension, hypokalemia, and periodic paralysis. In addition, it has been reported that the prevalence of metabolic syndrome in patients with PA is more common than in patients with essential hypertension (EH).4, 5, 6 Treatment of aldosterone‐producing adenomas (APA) has been found to induce regression of hypertension and metabolic abnormalities.7 Cardiovascular complications are more prevalent in patients with PA than in patients with EH because of an increased intima‐media thickness of the carotid artery caused by excess aldosterone production.8 Therefore, either adrenalectomy or treatment with aldosterone antagonists is necessary to achieve target blood pressure (BP) and to lower cardiovascular disease risks.
PA is divided into two major subtypes: unilateral hyperaldosteronism (UHA), which is mainly caused by APA, and bilateral hyperaldosteronism (BHA), which is mainly caused by bilateral adrenal hyperplasia. Although baseline plasma aldosterone levels are higher in UHA than in BHA, there are no significant differences in BP between these two entities.9 One report has suggested that cortisol and adrenomedullary hormones might contribute to hypertension in patients with PA.10 However, the effect of adrenal and pituitary hormone levels on BP in patients with PA is not completely understood.
To investigate whether hormones other than aldosterone influence BP level in patients with PA, we examined the association of plasma adrenal and pituitary hormone levels (ie, aldosterone, cortisol, and adrenocorticotropic hormone [ACTH]) at 12 am, 6 am, 12 pm, and 6 pm and BP levels in the early morning, late morning, and early evening in patients with PA and EH and compared the results.
2. Methods
2.1. Setting and patients
The study protocol was approved by the ethics committee of our hospital in accordance with the Declaration of Helsinki. We retrospectively reviewed the cases of 73 patients with PA diagnosed by adrenal venous sampling and 24 patients with EH who were admitted to rule out secondary hypertension between January 2006 and May 2016.
The diagnosis of PA was established according to the Japanese Endocrine Society.11 Hypertensive patients with elevated aldosterone/renin ratios >200, with plasma aldosterone concentration (PAC) expressed in pg/mL and plasma renin activity (PRA) expressed in ng/mL per hour, underwent loading tests using the captopril challenge test, the furosemide upright test, and the saline infusion test (SIT). Medications that influence loading tests were replaced at least 4 weeks before testing where applicable. Results from the captopril challenge test were considered positive if the aldosterone/renin ratio 90 minutes after taking 50 mg captopril was ≥200. Results from the furosemide upright test were considered positive if PRA after 2 hours of upright posture following furosemide infusion was <2.0 ng/mL per hour. Results from SIT were considered positive if the aldosterone level after 4 hours of 2 L of saline infusion was ≥60.0 pg/mL.11 PA was diagnosed if at least one of the three test results were positive.
Patients with a confirmed diagnosis and who consented to surgery underwent adrenal venous sampling with ACTH stimulation. Stimulated adrenal venous cortisol levels of ≥200 μg/dL and/or a selectivity index >5 indicated successful sampling.11 We diagnosed UHA when the ACTH stimulation results showed a lateralization ratio (LR) of ≥4.0 or 2.6<LR<4.0 with CR<1. Among the 73 patients with PA who underwent adrenal venous sampling, bilateral adrenal vein cannulation was successful in 54 patients, 28 and 26 of whom were diagnosed with UHA and BHA, respectively. Although bilateral cannulation of the adrenal veins was unsuccessful in 19 patients, we diagnosed eight patients with UHA based on supporting information, including contralateral suppression in adrenal venous sampling, extremely high aldosterone levels on the side of the tumor, and unilateral uptake of 131I‐adosterol.12 All eight patients underwent surgery. As a result, we confirmed the eight patients as having UHA due to the improvement of postoperative clinical course. Furthermore, 3 of 19 patients with unsuccessful adrenal venous sampling were diagnosed with BHA because of supporting data, including bilateral uptake of 131I‐adosterol, no contralateral suppression, no extremely high aldosterone levels, and no computed tomography–detected adrenal nodules. In eight patients, laterality could not be determined. Overall, we diagnosed UHA and BHA in 36 and 29 patients, respectively. Of the 36 patients with UHA, 32 underwent adrenalectomy and all of them were cured or showed improvement. Of the 32 patients who underwent adrenalectomy, 30 were diagnosed with unilateral APA and two were diagnosed with unilateral adrenal hyperplasia.
A total of 24 patients who were admitted for exclusion of secondary hypertension due to refractory hypertension, juvenile hypertension, or incidentaloma were diagnosed with EH and included as controls. EH was defined as BP ≥140/90 mm Hg for three consecutive measurements. All patients had undergone endocrine testing and imaging studies to exclude secondary hypertension.
2.2. Measurement of clinical and biochemical parameters
Medications that affect plasma aldosterone levels, including angiotensin‐converting enzyme inhibitors, angiotensin II receptor antagonists, β‐blockers, and diuretics, were replaced with calcium channel blockers at least 4 weeks before admission in patients with PA and EH. Only five patients with PA received a concomitant α‐blocker in addition to calcium channel blockers to control BP. In the hospital setting, the patients mostly remained in bed, apart from use of the toilet. Daily salt intake was 6 g. BP was measured by a trained nurse with an automatic device (model ES‐H55; Terumo Corporation, Tokyo, Japan) in the sitting position at three different times (early morning: 6 am to 7 am; late morning: 9 am to 11 am; early evening: 5 pm to 7 pm) per day. The mean of each BP measurement at the same time over 3 days was used.
Biochemical parameters including sodium, potassium, aldosterone, cortisol, PRA, and ACTH were evaluated in the admission setting. The levels of aldosterone, cortisol, and ACTH were measured at 12 am, 6 am, 12 pm, and 6 pm in 67 patients with PA and 24 patients with EH. Aldosterone and cortisol excretion were measured in 24‐hour urine samples of 66 patients with PA and 24 patients with EH.
2.3. Hormone assays
Plasma and urinary aldosterone levels were measured by radioimmunoassay (RIA) using commercial kits (SPAC‐S Aldosterone Kit; Fujirebio, Tokyo, Japan). The coefficient of variation was <20%. Plasma cortisol concentrations were measured by electrochemiluminescence immunoassay (ECLusys; Roche Diagnostics, Tokyo, Japan). The coefficient of variation was <15%. Plasma ACTH concentrations were measured by electrochemiluminescence immunoassay (ECLusys; Roche Diagnostics). The coefficient of variation was <10%. Urinary cortisol concentration was measured by RIA with a commercial kit (Cortisol kit; Immunotech, Marseille, France). The coefficient of variation was <10%. PRA was determined by measuring generation of angiotensin I in vitro using a commercially available RIA kit (PRA; Fujirebio). The lowest detectable PRA was 0.1 ng/mL per hour. The coefficient of variation was <10%. In the case of PRA values <0.1 ng/mL per hour, we calculated the PRA as 0.09 ng/mL per hour.
2.4. Statistical analysis
Results are expressed as median with interquartile range (25%–75%) for continuous variables and as absolute numbers and relative percentages for categorical variables. Differences in measured parameters between groups were evaluated using the Mann‐Whitney U test. Fisher exact test was used to compare differences in sex, smoking rates, and drinking rates. Correlations between continuous variables were examined using Pearson coefficient. All tests for significance and the resulting P values were two‐sided, with a level of significance of 5%. Plasma aldosterone, cortisol, ACTH, urinary aldosterone, and urinary cortisol were calculated as logarithmic transformation because of a skewed distribution. Stepwise multiple linear regression analysis adjusted for age, sex, body mass index, and the variables that significantly correlated with systolic BP in the univariate analysis was performed to identify independent predictors of systolic BP. Statistical analysis was performed using SPSS version 23 (SPSS Inc, Chicago, IL).
3. Results
3.1. Characteristics of patients with PA and EH
Of the 73 patients with PA, 36 were diagnosed with UHA and 29 with BHA. The laterality of eight patients with PA could not be decided because of adrenal venous sampling failure. Of the 36 patients with UAH, 30 were confirmed to have APA after adrenalectomy. The 24 control patients were confirmed to have EH. Baseline characteristics of the PA, APA, BHA, and EH groups are shown in Table 1. The patients with EH were significantly older than the patients with PA (P=.02). There were no statistically significant differences in sex, body mass index, or BP among the four groups. Serum potassium levels were significantly lower in patients with APA and BHA than those with EH (P=.0001 and P=.009, respectively). In addition, serum potassium levels were significantly lower in patients with APA than those with BHA (P=.0001). PAC at 12 am, 6 am, 12 pm, and 6 pm and 24‐hour urinary aldosterone levels were significantly higher in patients with APA and BHA than those with EH. In addition, PAC at 12 am, 6 am, 12 pm, and 6 pm were significantly higher in those with APA than those with BHA (P=.0001, P=.0001, P=.0001, and P=.0001, respectively). In addition, PAC at 6 am and 12 pm were significantly higher in patients with BHA than those with EH (P=.048 and P=.028, respectively). The only statistically significant difference in plasma cortisol concentration (PFC) among the four groups was in the 6 pm measurement, which was significantly higher in patients with APA than in those with BHA (P=.016). There were no statistically significant differences in ACTH among the four groups. PRA at 12 am, 6 am, 12 pm, and 6 pm were significantly lower in patients with APA than those with EH (P=.012, P=.004, P=.012, and P=.037, respectively). In addition, PRA at 12 am and 6 pm were significantly lower in patients with BHA than those with EH (P=.028 and P=.027, respectively). There were no statistically significant differences in PRA between the groups with APA and BHA.
Table 1.
Baseline Characteristics of Patients With PA, APA, BHA, and EH
| PA | APA | BHA | EH | P Value | |
|---|---|---|---|---|---|
| (n=73) | (n=30) | (n=29) | (n=24) | (PA vs EH) | |
| Age, y | 47.0 (40.0–58.0) | 45.5 (40.0–52.5) | 46.0 (37.0–59.0) | 59.5 (42.0–69.8) | .02 |
| Male sex, % | 48 (35/73) | 53 (16/30) | 38 (11/29) | 50 (12/24) | .52 |
| Smokers, % | 37 (27/73) | 47 (14/30) | 24 (7/29) | 42 (10/24) | .81 |
| Alcohol drinkers, % | 32 (23/73) | 40 (12/30) | 17 (5/29) | 54 (14/24) | .06 |
| Body mass index, kg/m2 | 24.4 (22.4–28.2) | 23.9 (20.5–26.5) | 24.7 (23.0–31.3) | 24.5 (23.0–27.0) | .88 |
| Serum Na, mmol/L | 142.0 (140.0–144.0) | 143.0 (141.0–144.0) | 141.0 (140.0–142.5) | 141.0 (140.0–142.8) | .10 |
| Serum K, mmol/L | 3.7 (3.1–4.0) | 3.1 (2.9–3.7) | 3.8 (3.7–4.1) | 4.2 (3.8–4.3) | .0001 |
| eGFR, mL/min per 1.73 m2 | 79.4 (70.3–94.7) | 81.6 (71.0–94.4) | 83.7 (71.7–98.3) | 80.5 (66.9–92.9) | .64 |
| Early morning SBP, mm Hg | 133.7 (128.2–143.0) | 135.6 (127.3–145.4) | 133.3 (128.2–143.3) | 144.0 (128.2–149.8) | .10 |
| Early morning DBP, mm Hg | 83.7 (79.0–90.5) | 87.0 (79.8–93.0) | 82.3 (78.3–87.5) | 81.5 (72.8–91.2) | .37 |
| Late morning SBP, mm Hg | 135.3 (127.8–141.8) | 135.1 (128.9–144.0) | 137.7 (126.0–141.2) | 139.7 (127.0–149.9) | .31 |
| Late morning DBP, mm Hg | 84.3 (79.0–91.2) | 85.3 (81.4–91.8) | 83.0 (79.0–91.3) | 81.3 (70.5–92.3) | .22 |
| Early evening SBP, mm Hg | 139.0 (131.3–146.8) | 139.5 (128.4–150.9) | 138.7 (132.0–146.3) | 145.3 (134.0–153.7) | .17 |
| Early evening DBP, mm Hg | 86.0 (81.7–91.8) | 87.8 (81.1–93.3) | 86.3 (81.2–90.7) | 87.0 (76.0–97.8) | .71 |
| PAC at 12 am, pg/mL | 155.0 (96.7–247.3) | 232.5 (144.5–403.8) | 104.1 (70.0–138.5) | 75.8 (63.3–109.3) | .0001 |
| PAC at 6 am, pg/mL | 276.5 (156.5–540.3) | 546.5 (411.3–770.0) | 159.5 (110.5–217.8) | 119.5 (92.3–143.8) | .0001 |
| PAC at 12 pm, pg/mL | 210.0 (162.0–348.5) | 314.0 (209.0–374.0) | 184.5 (140.3–226.8) | 118.0 (101.0–192.0) | .0001 |
| PAC at 6 pm, pg/mL | 180.0 (121.8–303.0) | 291.5 (144.3–373.0) | 127.5 (92.6–185.8) | 91.9 (74.1–143.5) | .0001 |
| PFC at 12 am, pg/mL | 3.1 (2.2–6.5) | 3.1 (2.4–7.4) | 3.0 (1.9–6.3) | 3.5 (2.6–6.4) | .55 |
| PFC at 6 am, pg/mL | 16.6 (13.3–19.7) | 16.5 (13.2–20.1) | 15.8 (13.5–18.9) | 15.4 (10.4–19.1) | .38 |
| PFC at 12 pm, pg/mL | 8.8 (5.7–10.9) | 9.2 (5.8–11.6) | 7.2 (4.9–10.0) | 9.1 (7.2–10.6) | .37 |
| PFC at 6 pm, pg/mL | 5.6 (3.4–8.0) | 6.3 (4.7–9.0) | 4.0 (3.1–7.2) | 4.7 (3.8–6.8) | .69 |
| ACTH at 12 am, pg/mL | 7.7 (4.5–12.8) | 5.3 (3.3–11.2) | 10.0 (5.8–13.4) | 10.4 (4.3–14.0) | .92 |
| ACTH at 6 am, pg/mL | 31.9 (18.2–48.6) | 22.5 (16.5–44.0) | 30.0 (16.3–45.9) | 32.1 (24.5–47.4) | .99 |
| ACTH at 12 pm, pg/mL | 14.9 (10.8–22.8) | 12.6 (10.1–20.0) | 14.0 (10.9–21.3) | 16.5 (11.6–20.1) | .89 |
| ACTH at 6 pm, pg/mL | 9.8 (7.2–16.1) | 8.8 (6.3–19.6) | 9.6 (5.9–15.0) | 8.2 (6.8–13.8) | .27 |
| PRA at 12 am, ng/mL per h | 0.2 (0.09–0.4) | 0.2 (0.09–0.4) | 0.3 (0.1–0.4) | 0.4 (0.2–0.8) | .001 |
| PRA at 6 am, ng/mL per h | 0.2 (0.09–0.4) | 0.2 (0.09–0.4) | 0.3 (0.2–0.4) | 0.4 (0.2–0.6) | .003 |
| PRA at 12 pm, ng/mL per h | 0.3 (0.09–0.6) | 0.3 (0.2–0.5) | 0.4 (0.2–0.7) | 0.5 (0.3–1.9) | .005 |
| PRA at 6 pm, ng/mL per h | 0.3 (0.2–0.4) | 0.3 (0.1–0.4) | 0.4 (0.2–0.6) | 0.5 (0.2–1.7) | .003 |
| Urine‐aldosterone, μg/d | 14.0 (9.1–26.3) | 28.0 (18.0–37.0) | 9.0 (7.4–11.0) | 7.5 (5.2–10.0) | .0001 |
| Urine‐cortisol, μg/d | 34.8 (22.2–56.4) | 32.1 (21.1–55.1) | 38.9 (20.7–68.3) | 24.3 (18.4–50.5) | .10 |
Data are expressed as median (25th–75th percentile). Abbreviations: ACTH, adrenocorticotropic hormone; APA, aldosterone‐producing adenoma; BHA, bilateral hyperaldosteronism; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; EH, essential hypertension; PA, primary hyperaldosteronism; PAC, plasma aldosterone concentration; PFC, plasma cortisol concentration; PRA, plasma renin activity; SBP, systolic blood pressure.
3.2. Association of biochemical parameters with BP levels
In the univariate correlation analysis of patients with PA, systolic and diastolic BP at three different times did not correlate with log (24‐hour urinary aldosterone excretion) or log PAC at 12 am, 6 am, 12 pm, and 6 pm. There was also no significant correlation between systolic or diastolic BP and log PFC or log PRA at 12 am, 6 am, 12 pm, and 6 pm. However, systolic BP at three different times correlated positively with log ACTH at 12 pm in PA patients (r=.28, P=.027; r=.41, P=.001; and r=.36, P=.004, respectively). In addition, stepwise multiple linear regression analysis adjusted for age, sex, body mass index, and the variables that significantly correlated with systolic BP in the univariate analysis indicated that log ACTH at 12 pm was the only independent predictor for systolic BP at the three different times.
Next, we separately analyzed the groups with APA and BHA. In patients with APA, there was no significant correlation between BP and log PAC, log PFC, or log PRA at 12 am, 6 am, 12 pm, and 6 pm. However, systolic BP in the early and late morning correlated positively with log ACTH at 6 am and 12 pm in patients with APA (Table 2, Figure). Multiple linear regression analysis was performed with age, sex, body mass index, and the variables that significantly correlated with systolic BP in the univariate analysis. After stepwise selection, log ACTH at 6 am had an independent positive correlation with systolic BP (β=.42, P=.031; β=.62, P=.001; Table 3). In patients with BHA, there was no statistically significant correlation between BP and log PAC, log PFC, log ACTH, or log PRA at 12 am, 6 am, 12 pm, or 6 pm.
Table 2.
Pearson Correlation Analysis Between Blood Pressure and Hormones in Patients With APA
| No. | Early Morning | Late Morning | Early Evening | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBP | DBP | SBP | DBP | SBP | DBP | ||||||||
| r | P Value | r | P Value | r | P Value | r | P Value | r | P Value | r | P Value | ||
| Age | 30 | −.22 | .25 | −.24 | .20 | −.054 | .78 | −.12 | .54 | −.42 | .022 | −.36 | .051 |
| Sex | 30 | .085 | .66 | .28 | .13 | .25 | .18 | .45 | .014 | .072 | .70 | .14 | .45 |
| Body mass index | 30 | .12 | .54 | .31 | .092 | .14 | .46 | .36 | .053 | .13 | .50 | .24 | .20 |
| eGFR | 30 | .055 | .77 | .24 | .21 | .026 | .89 | .24 | .21 | .12 | .53 | .15 | .43 |
| Log PAC at 12 am | 28 | −.052 | .79 | .003 | .99 | −.12 | .53 | .15 | .44 | −.12 | .54 | .080 | .68 |
| Log PAC at 6 am | 28 | .20 | .30 | .12 | .54 | .11 | .57 | .17 | .39 | .20 | .31 | .29 | .14 |
| Log PAC at 12 pm | 28 | .017 | .93 | −.015 | .94 | −.13 | .53 | .16 | .43 | .22 | .28 | .13 | .52 |
| Log PAC at 6 pm | 28 | −.035 | .86 | −.16 | .41 | −.031 | .88 | .091 | .65 | .0001 | .99 | −.033 | .87 |
| Log PFC at 12 am | 28 | −.28 | .15 | −.13 | .51 | −.088 | .66 | −.008 | .97 | −.27 | .16 | −.19 | .33 |
| Log PFC at 6 am | 28 | .13 | .50 | .36 | .058 | .14 | .48 | .18 | .36 | .15 | .45 | .31 | .11 |
| Log PFC at 12 pm | 28 | .042 | .84 | .34 | .09 | .054 | .79 | .27 | .19 | .049 | .81 | .18 | .39 |
| Log PFC at 6 pm | 28 | −.14 | .47 | .034 | .086 | −.019 | .92 | .085 | .67 | −.30 | .12 | −.30 | .12 |
| Log ACTH at 12 am | 28 | −.18 | .35 | −.11 | .58 | .062 | .75 | .085 | .67 | −.12 | .29 | −.21 | .30 |
| log ACTH at 6 am | 28 | .42 | .027 | .28 | .15 | .62 | .0001 | .33 | .089 | .35 | .068 | .27 | .16 |
| Log ACTH at 12 pm | 28 | .38 | .050 | .23 | .26 | .42 | .034 | .40 | .043 | .35 | .079 | .21 | .30 |
| Log ACTH at 6 pm | 28 | −.022 | .91 | −.15 | .45 | .22 | .27 | .12 | .55 | −.14 | .48 | −.28 | .15 |
| Log PRA at 12 am | 28 | −.078 | .69 | .061 | .76 | −.18 | .36 | −.10 | .60 | −.19 | .35 | −.20 | .30 |
| Log PRA at 6 am | 28 | .10 | .63 | .12 | .55 | .007 | .97 | .076 | .70 | −.054 | .79 | −.15 | .44 |
| Log PRA at 12 pm | 28 | −.064 | .76 | .044 | .83 | −.15 | .46 | −.071 | .73 | −.18 | .38 | −.12 | .55 |
| Log PRA at 6 pm | 28 | −.085 | .67 | .23 | .24 | −.18 | .37 | .01 | .96 | −.11 | .57 | −.039 | .85 |
| Log U‐Aldosterone | 28 | .14 | .48 | .12 | .57 | .15 | .46 | .28 | .15 | .22 | .27 | .28 | .16 |
| Log U‐Cortisol | 28 | −.14 | .48 | −.15 | .45 | −.18 | .38 | .13 | .53 | −.14 | .49 | −.041 | .84 |
Abbreviations: APA, aldosterone‐producing adenoma; ACTH, adrenocorticotropic hormone; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; PAC, plasma aldosterone concentration; PFC, plasma cortisol concentration; PRA, plasma renin activity; SBP, systolic blood pressure.
Figure 1.
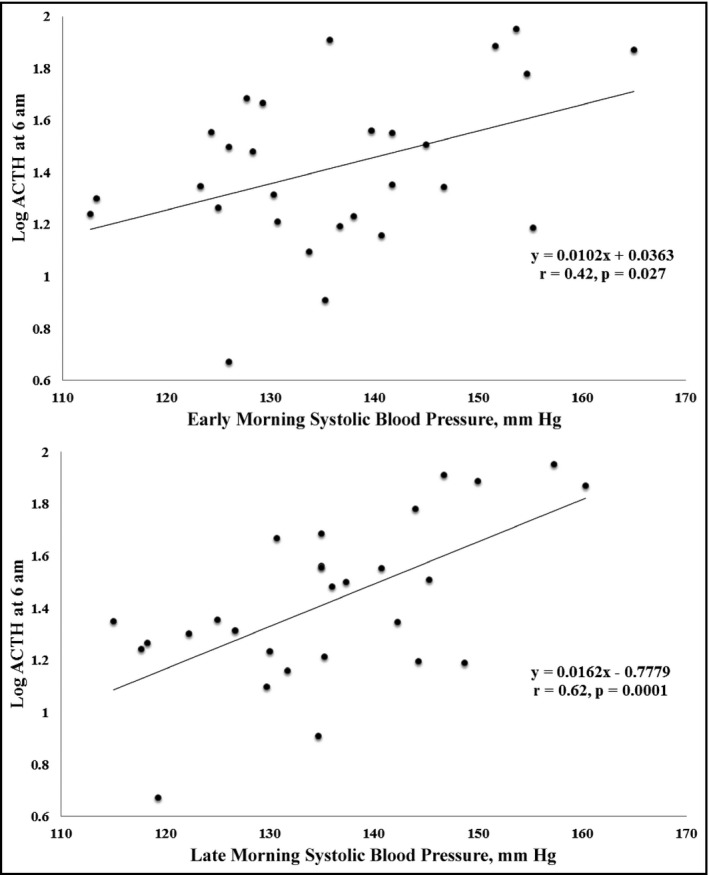
Relationship between log adrenocorticotropic hormone (ACTH) at 6 am and morning systolic blood pressure in patients with aldosterone‐producing adenoma (APA). The horizontal axis indicates morning systolic blood pressure (mm Hg) and the vertical axis shows log ACTH at 6 am in patients with APA. Early morning systolic blood pressure was significantly correlated with log ACTH at 6 am (r=.42, P=.027), and late morning blood pressure was significantly correlated with log ACTH at 6 am (r=.62, P=.0001)
Table 3.
Multiple Linear Regression Analysis for SBP in Patients With APA
| Variables | β | t | P Value | Adjusted R 2 |
|---|---|---|---|---|
| Early morning SBP | ||||
| Log ACTH at 6 am | .424 | 2.292 | .031 | .145 |
| Late morning SBP | ||||
| Log ACTH at 6 am | .615 | 3.818 | .001 | .352 |
Adjusted for age, sex, body mass index, and log adrenocorticotropic hormone (ACTH) at 12 pm. Abbreviations: APA, aldosterone‐producing adenoma; SBP, systolic blood pressure.
In contrast, in patients with EH, systolic and diastolic BP at three different times were all positively correlated with log PAC at 12 pm (systolic BP: r=.52, P=.11; r=.44, P=.036; r=.44, P=.034; and diastolic BP: r=.70, P=.0001; r=.63, P=.001; r=.78, P=.0001). In addition, systolic BP in early morning and late evening had a statistically significant positive correlation with log PAC at 6 am (r=.47, P=.022 and r=.56, P=.005). Furthermore, diastolic BP at every time point was positively correlated with log (24‐hour urinary aldosterone excretion: r=.56, P=.004; r=.62, P=.001; and r=.53, P=.008). No correlation was found between systolic or diastolic BP and log PFC or log ACTH in the EH group. Systolic BP and diastolic BP at every time point except early evening systolic BP had a statistically significant positive correlation with log PRA at 12 am, 6 am, 12 pm, or 6 pm in the EH group.
4. Discussion
Although baseline plasma aldosterone level differs among different PA subtypes, a number of studies have demonstrated that patients with PA who have different subtypes have similar BP levels.10, 13, 14 We hypothesized that hormones other than aldosterone might influence BP level in patients with PA.
We examined the association of adrenocortical hormones and ACTH measured at four different times and BP measurements at three different times in patients with PA, including those with APA and BHA, and patients with EH.
We made two important clinical findings. First, early morning and late morning systolic BP strongly correlated with ACTH at 6 am, which was the peak measurement in a day in patients with APA. Second, there were no statistically significant correlations between PAC and BP measurements in patients with PA.
The first important clinical finding is that early morning and late morning systolic BP strongly correlated with ACTH at 6 am, which was the peak measurement in a day in patients with APA. The synthesis of aldosterone, which affects BP level, is regulated by ACTH stimulation,15, 16 and aldosterone secretion stimulated by ACTH via the ACTH receptor (melanocortin 2 receptor [MC2R]) occurs through Ca2+ pathways as an acute secretion process.17, 18 Importantly, some reports have demonstrated that MC2R is overexpressed in APA.19, 20 Indeed, APA has higher circadian variability and baseline aldosterone levels than BHA.9 In our present study, however, BP level did not correlate with plasma aldosterone levels; instead, morning systolic BP significantly correlated with ACTH at 6 am in patients with APA. This result indicates that ACTH, and not aldosterone, may influence BP levels in APA. Stimulation of the ACTH receptor on APAs might have some effect on BP without the influence of aldosterone secretion. In addition, Hatakeyama and colleagues21 demonstrated that human aortic endothelial cells possess the ACTH receptor and that ACTH downregulates the expression of 11‐hydroxysteroid dehydrogenase type 2, which is essential for the control of vascular tone.21 These findings suggest that ACTH may play a significant role in the control of vascular tone through an interaction with the vascular receptor, and that this interaction might explain the correlation of plasma ACTH and BP in the condition of hyperaldosteronemia.
Second, our data also showed that there was no correlation between BP and PAC in patients with PA. Aldosterone is a potent steroid hormone that promotes sodium retention and leads to the elevation of arterial pressure.22, 23 Indeed, our data show that PAC positively correlates with BP in patients with EH. However, we could not find the same correlation in patients with PA, albeit our sample size was small. This result might be due to aldosterone escape.24, 25 When plasma aldosterone levels are elevated under inappropriate conditions, such as in PA, sodium chloride reabsorption in the renal tubule is decreased despite the continued presence of aldosterone via activation of the mechanism of aldosterone escape. Aldosterone escape by PA might itself explain the absence of correlation between BP and PAC in our study.10
4.1. Limitations
Our study was limited by its small sample size and retrospective design. Accordingly, we cannot conclusively state that there is no association between BP and cortisol and aldosterone because the correlation is obvious. Confirmation of the relationship between BP levels and hormone levels awaits a large‐scale trial. In addition, most of our patients with PA received calcium channel blockers, which might have diminished circadian variation in BP levels. There may be a direct effect of calcium antagonists on APAs, because the overproduction of aldosterone is related to mutations of calcium channels genes in some cases.26 Furthermore, we did not measure adrenomedullary hormones, including epinephrine or norepinephrine. The absence of 24‐hour ambulatory BP recordings may be another limitation of this study.
5. Conclusions
We demonstrated that in patients with APA, morning systolic BP strongly correlated with peak ACTH level measured at 6 am. In addition, we found no statistically significant correlation between PAC and BP in patients with PA. These results suggest that, although it is presumed that aldosterone contributes to high BP in patients with APA, other factors besides aldosterone might be stimulated by ACTH and also contribute to hypertension. Further basic studies are needed to establish the pathophysiology of hypertension in patients with PA.
Disclosure Statement
The authors have nothing to disclose.
Kobayashi, H. , Haketa, A. , Takahiro, U. , Otsuka, H. , Tanaka, S. , Hatanaka, Y. , Ikeda, Y. , Abe, M. , Fukuda, N. and Soma, M. (2017), Plasma adrenocorticotropic hormone but not aldosterone is correlated with blood pressure in patients with aldosterone‐producing adenomas. Journal of Clinical Hypertension, 19:280‐286. doi: 10.1111/jch.12956
References
- 1. Rossi GP, Bernini G, Caliumi C, et al. A prospective study of the prevalence of primary aldosteronism in 1125 hypertensive patients. J Am Coll Cardiol 2006;48:2293–2300. [DOI] [PubMed] [Google Scholar]
- 2. Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66:607–618. [DOI] [PubMed] [Google Scholar]
- 3. Mantero F, Gion M, Armanini D, Opocher G. Aldosterone regulation in primary aldosteronism: differences between adenoma and bilateral hyperplasia. Clin Sci Mol Med Suppl. 1976;3:329–332. [DOI] [PubMed] [Google Scholar]
- 4. Krug AW, Ehrhart‐Bornstein M. Aldosterone and metabolic syndrome: is increased aldosterone in metabolic syndrome patients an additional risk factor? Hypertension. 2008;51:1252–1258. [DOI] [PubMed] [Google Scholar]
- 5. Matrozova J, Steichen O, Amar L, Zacharieva S, Jeunemaitre X, Plouin PF. Fasting plasma glucose and serum lipids in patients with primary aldosteronism: a controlled cross‐sectional study. Hypertension. 2009;53:605–610. [DOI] [PubMed] [Google Scholar]
- 6. Somlóová Z, Widimský J Jr, Rosa J, et al. The prevalence of metabolic syndrome and its components in two main types of primary aldosteronism. J Hum Hypertens. 2010;24:625–630. [DOI] [PubMed] [Google Scholar]
- 7. Fallo F, Federspil G, Veglio F, Mulatero P. The metabolic syndrome in primary aldosteronism. Curr Diab Rep. 2008;8:42–47. [DOI] [PubMed] [Google Scholar]
- 8. Holaj R, Zelinka T, Wichterle D, Petrák O, Strauch B, Widimský J Jr. Increased intima‐media thickness of the common carotid artery in primary aldosteronism in comparison with essential hypertension. J Hypertens. 2007;25:1451–1457. [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi H, Haketa A, Ueno T, et al. Subtype prediction in primary aldosteronism: measurement of circadian variation of adrenocortical hormones and 24‐h urinary aldosterone. Clin Endocrinol (Oxf). 2016;84:814–821. [DOI] [PubMed] [Google Scholar]
- 10. Ye F, Tang ZY, Wu JC, et al. Hormones other than aldosterone may contribute to hypertension in 3 different subtypes of primary aldosteronism. J Clin Hypertens (Greenwich). 2013;15:264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nishikawa T, Omura M, Satoh F, et al. Guidelines for the diagnosis and treatment of primary aldosteronism—the Japan Endocrine Society 2009. Endocr J. 2011;58:711–721. [DOI] [PubMed] [Google Scholar]
- 12. Yen RF, Wu VC, Liu KL, et al. 131I‐6beta‐iodomethyl‐19‐norcholesterol SPECT/CT for primary aldosteronism patients with inconclusive adrenal venous sampling and CT results. J Nucl Med 2009;50:1631–1637. [DOI] [PubMed] [Google Scholar]
- 13. Wu VC, Chueh SC, Chang HW, et al. Bilateral aldosterone‐producing adenomas: differentiation from bilateral adrenal hyperplasia. QJM. 2008;101:13–22. [DOI] [PubMed] [Google Scholar]
- 14. Giacchetti G, Ronconi V, Turchi F, et al. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: an observational study. J Hypertens. 2007;25:177–186. [DOI] [PubMed] [Google Scholar]
- 15. Willenberg HS, Schinner S, Ansurudeen I. New mechanisms to control aldosterone synthesis. Horm Metab Res. 2008;40:435–441. [DOI] [PubMed] [Google Scholar]
- 16. Williams GH. Aldosterone biosynthesis, regulation, and classical mechanism of action. Heart Fail Rev. 2005;10:7–13. [DOI] [PubMed] [Google Scholar]
- 17. Gallo‐Payet N, Grazzini E, Côté M, et al. Role of Ca2 in the action of adrenocorticotropin in cultured human adrenal glomerulosa cells. J Clin Invest. 1996;98:460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bornstein SR, Chrousos GP. Clinical review 104: Adrenocorticotropin (ACTH)‐ and non‐ACTH‐mediated regulation of the adrenal cortex: neural and immune inputs. J Clin Endocrinol Metab. 1999;84:1729–1736. [DOI] [PubMed] [Google Scholar]
- 19. Arnaldi G, Mancini V, Costantini C, et al. ACTH receptor mRNA in human adrenocortical tumors: overexpression in aldosteronomas. Endocr Res. 1998;24:845–849. [DOI] [PubMed] [Google Scholar]
- 20. Schubert B, Fassnacht M, Beuschlein F, Zenkert S, Allolio B, Reincke M. Angiotensin II type 1 receptor and ACTH receptor expression in human adrenocortical neoplasms. Clin Endocrinol (Oxf). 2001;54:627–632. [DOI] [PubMed] [Google Scholar]
- 21. Hatakeyama H, Inaba S, Taniguchi N, Miyamori I. Functional adrenocorticotropic hormone receptor in cultured human vascular endothelial cells: possible role in control of blood pressure. Hypertension. 2000;36:862–865. [DOI] [PubMed] [Google Scholar]
- 22. Lösel R, Feuring M, Wehling M. Non‐genomic aldosterone action: from the cell membrane to human physiology. J Steroid Biochem Mol Biol. 2002;83:167–171. [DOI] [PubMed] [Google Scholar]
- 23. Christ M, Wehling M. Rapid actions of aldosterone: lymphocytes, vascular smooth muscle and endothelial cells. Steroids. 1999;64:35–41. [DOI] [PubMed] [Google Scholar]
- 24. Turban S, Wang XY, Knepper MA. Regulation of NHE3, NKCC2, and NCC abundance in kidney during aldosterone escape phenomenon: role of NO. Am J Physiol Renal Physiol. 2003;285:F843–F851. [DOI] [PubMed] [Google Scholar]
- 25. Knox FG, Burnett JC Jr, Kohan DE, Spielman WS, Strand JC. Escape from the sodium‐retaining effects of mineralocorticoids. Kidney Int. 1980;17:263–276. [DOI] [PubMed] [Google Scholar]
- 26. Scholl UI, Goh G, Stölting G, de Oliveira RC, Choi M, Overton JD. Somatic and germline CACNA1D calcium channel mutations in aldosterone‐producing adenomas and primary aldosteronism. Nat Genet. 2013. Sep;45:1050–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]


