Abstract
Hyperuricemia is associated with cardiovascular disease and its prevalence is unknown in black Africans. This study reports hyperuricemia distribution and its association with cardiovascular risk factors in a selected Angolan population. A cross‐sectional study in 585 black Africans was performed. Hyperuricemia was defined as uric acid >7.0 mg/dL in men or >5.7 mg/dL in women. Overall prevalence was 25%. Hyperuricemia was associated with hypertension (odds ratio [OR], 2.20; confidence interval [CI], 95% 1.41–3.47), high waist circumference (OR, 1.67; CI, 95% 1.05–2.65), and metabolic syndrome (OR, 1.66; CI, 95% 1.07–2.57). Compared to those with uric acid levels in the first quartile, individuals in the fourth quartile showed higher body mass index, waist circumference, systolic blood pressure, and plasma levels of creatinine and triglycerides. Hypertension, high waist circumference, and metabolic syndrome were the major cardiovascular risk factors associated with hyperuricemia.
High levels of serum uric acid (SUA) can lead to tissue deposition, which is commonly manifested as gout and kidney stones. According to data from the National Health and Nutrition Examination Survey (NHANES) 2007–2008, the prevalence of hyperuricemia was 21% in American adults, reaching 26% in African Americans. The prevalence of hyperuricemia has been increasing in recent decades, and it varies according to sex, age and, ancestry. Hyperuricemia is associated with cardiovascular risk factors, such as hypertension and metabolic syndrome,1, 2 as well as cardiovascular outcomes, such as coronary artery disease (CAD).3 A recent meta‐analysis concluded that SUA is an independent predictor of cardiovascular mortality.4
African countries have experienced a rapid epidemiological transition, mainly in areas with rapid economic growth and urbanization. In such countries, despite the high incidence of poverty‐related diseases, CAD and stroke have become important public health problems.5 However, the association of SUA levels with cardiovascular risk has not been studied in the black African population. In addition, there is a lack of data on SUA distribution and the prevalence of hyperuricemia in this population. Because cardiovascular disease (CVD) has increased in Africa, it is essential to obtain this information. Therefore, the purpose of this study was to determine the SUA distribution in black Africans and to investigate possible associations between increased SUA levels and traditional cardiovascular risk factors.
Methods
Data were collected in a cross‐sectional study (2009–2010) investigating the prevalence and severity of cardiovascular risk factors in civil servants of a public university in Luanda, Angola. Among 1458 employees of the university, 625 volunteers, aged 20 years or older, visited the Department of Physiology, Faculty of Medicine of Agostinho Neto University (UAN), where they underwent clinical and laboratory examinations. Details of the study design were previously described.6 Of the 625 enrolled volunteers, 585 had black skin color and were included in the present analysis. Data were collected using a modified questionnaire from the World Health Organization Multinational Monitoring of Trends and Determinants in Cardiovascular Disease (WHO‐MONICA) Project and the WHO manuals for stepwise approaches and the surveillance of nontransmitted diseases.7 Formal education was determined by years in school and economic status, according to the participant's income per month by quintiles: first quintile (very low socioeconomic class), second quintile (low socioeconomic class), third (middle socioeconomic class), fourth quintile (upper‐middle socioeconomic class), and fifth quintile (upper socioeconomic class). The study was conducted according to the tenets of the Declaration of Helsinki, and participants signed an informed consent form approved by the ethics committee of UAN.
Clinical examinations were performed between 8 am and 12 pm in temperature‐controlled rooms (22–23°C). Participants were asked to refrain from smoking, physical exercise, and caffeinated beverages for 12 hours before the visit. Venous blood samples were collected under fasting conditions from the forearm by standard techniques and processed immediately using commercially available kits (BioSystems SA, Barcelona, Spain) for determination of serum levels of triglycerides, total cholesterol, high‐density lipoprotein cholesterol (HDL‐C), glucose, creatinine, and uric acid. Biochemical parameters were analyzed using enzymatic methods on a spectrophotometer (BioSystems BTS‐310, Barcelona, Spain). In patients with triglyceride levels <400 mg/dL, low‐density lipoprotein cholesterol (LDL‐C) was calculated according to Friedewald's formula.8
Hyperuricemia was defined as SUA levels >7.0 mg/dL for men and 5.7 mg/dL for women, according to NHANES.9 Diabetes was defined as a fasting glucose level ≥126 mg/dL or the use of antidiabetic drugs.10 Dyslipidemia was defined as the presence of one or more of the following: LDL‐C ≥160 mg/dL, HDL‐C <40 mg/dL for men or <50 mg/dL for women, triglycerides ≥150 mg/dL, or treatment for dyslipidemia.11
Blood pressure (BP) was measured in patients three times in the nondominant arm after 5 minutes of resting in a seated position with the arm at the level of the heart using an automated and validated digital oscillometric sphygmomanometer (Omron 705CP, Tokyo, Japan). The readings were taken at 3‐minute intervals, and the mean value of the two last readings was defined as the clinic BP. Hypertension was defined when the systolic BP (SBP) was ≥140 mm Hg and/or when the diastolic BP (DBP) was ≥90 mm Hg or when the subject was using antihypertensive drugs, including diuretics.12
Waist circumference (WC) was measured at the end of normal expiration, at the midpoint between the lower border of the rib cage and the top of the iliac crest13 and recorded to the nearest 0.1 cm. Body weight was measured using a previously calibrated mechanical scale with participants barefoot and wearing only underclothes. Body mass index (BMI) was calculated by dividing weight by the square of height (kg/m2). Based on the BMI values, individuals were classified as normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9kg/m2), or obese (≥30.0 kg/m2).14
Metabolic syndrome (MetS) was defined according to the Joint Interim Statement (JIS), based on the presence of three or more of the following indexes: WC ≥94 cm or ≥80 cm in men and women, respectively; SBP ≥130 mm Hg and/or DBP ≥85 mm Hg and/or use of BP‐lowering drugs; fasting triglycerides ≥150 mg/dL or treatment for hypertriglyceridemia; HDL‐C <40 mg/dL or <50 mg/dL in men and women, respectively, or treatment for dyslipidemia; and fasting glucose level ≥100 mg/dL or taking antidiabetic medication.15
Statistical Analysis
Continuous variables are shown as the mean±standard deviation. Means were compared using Student t test for independent samples or analysis of variance. Categorical variables were expressed as the number of individuals, percentage, and 95% confidence interval (CI). Logistic regression was adopted to estimate odds ratio (OR). The model was adjusted for age and sex. Data were analyzed using SPSS 20.0 software (IBM, Chicago, IL), and statistical significance was set at P<.05.
Results
The studied sample was balanced with regard to sex, with 301 (51%) of the patients women. The mean age of the sample was 44 years (range 22–72 years). Table 1 shows the demographic, anthropometric, and biochemical characteristics of the participants.
Table 1.
Demographic, Anthropometric, and Biochemical Characteristics of the Study Participants by Sex
| Characteristics | Total | Men | Women | P Value |
|---|---|---|---|---|
| (N=585) | (n=284) | (n=301) | ||
| Age, y | 44±11 | 45±11 | 44±10 | .051 |
| Education level, y in school | .001 | |||
| ≤4 | 110 (18.8) | 45 (15.85) | 65 (21.6) | |
| 5–12 | 100 (17.1) | 65 (22.9) | 35 (11.6) | |
| ≥13 | 375 (64.1) | 174 (61.3) | 201 (66.8) | |
| Socioeconomic class | .008 | |||
| Very low | 11 (1.9) | 3 (1.1) | 8 (2.7) | |
| Low | 87 (14.9) | 49 (17.3) | 38 (12.6) | |
| Middle | 153 (26.2) | 64 (22.5) | 89 (11.6) | |
| Upper‐middle | 132 (22.6) | 55 (19.4) | 77 (66.8) | |
| Upper | 202 (34.5) | 113 (39.8) | 89 (29.6) | |
| BMI, kg/m2 | 25.6 (5.4) | 24.0±4.2 | 27.2±5.9 | <.001 |
| WC, cm | 81.8±13.3 | 79.7±12.6 | 83.3±13.7 | <.001 |
| Glucose, mg/dL | 94±8 | 95±20 | 93±22 | .268 |
| Creatinine, mg/dL | 1.1±0.2 | 1.2±0.2 | 1.0±0.2 | .059 |
| Total cholesterol, mg/dL | 192±39 | 190±42 | 194±36 | .197 |
| Triglycerides, mg/dL | 100±40 | 102±41 | 99±38 | .318 |
| HDL cholesterol, mg/dL | 46±11 | 44±10 | 48±11 | <.001 |
Abbreviations: BMI, body mass index; HDL, high‐density lipoprotein; WC, waist circumference. Continuous values are expressed as the mean±standard deviation and were analyzed using Student t test for independent samples. Proportions, expressed as number of individuals (percentage), were analyzed using the chi‐square test.
SUA distribution in men and women is shown in the 1. The mean SUA levels were 6.1±1.7 mg/dL in men and 4.8±1.4 mg/dL in women. The overall prevalence of hyperuricemia was 25% (95% CI, 21.3–28.3) and was higher (P<.05) in postmenopausal women (31%; 95% CI, 26.8–34.2). The prevalence and mean SUA levels categorized by sex and age are presented in Table 2.
Figure 1.
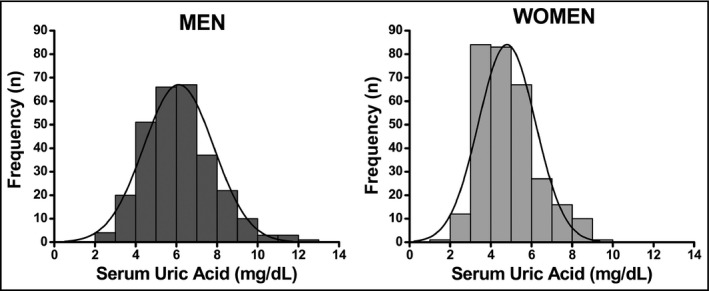
Serum uric acid distribution by sex.
Table 2.
Prevalence of Hyperuricemia and Mean Serum Urate Levels
| Prevalence of Hyperuricemia, | Serum Urate Level, Mean (95% CI), | |
|---|---|---|
| % (95% CI) | mg/dL | |
| Overall | 25 (21.3–28.3) | 5.5 (5.3–5.6) |
| Men | 26 (22.8–30.0) | 6.1 (5.9–6.3) |
| All women | 23 (19.9–26.7) | 4.8 (4.7–5.0) |
| Postmenopausal women | 31 (26.8–34.2) | 5.1 (4.8–5.3) |
| Age category, y | ||
| 20–29 | 19 (15.6–22.0) | 5.0 (4.6–5.4) |
| 30–39 | 22 (18.8–25.6) | 5.2 (5.0–5.5) |
| 40–49 | 25 (21.5–28.5) | 5.5 (5.2–5.7) |
| 50–59 | 28 (23.9–31.1) | 5.7 (5.4–6.0) |
| 60–69 | 30 (25.9–33.3) | 5.8 (5.2–6.5) |
Abbreviation: CI, confidence interval.
Hyperuricemia was defined as serum urate levels >7.0 mg/dL in men and >5.7 mg/dL in women according to National Health and Nutrition Examination Survey criteria.
The characteristics of the patients divided by SUA quartiles are shown in Table 3. SUA increased with age only in women. In both sexes, SUA increased with fat accumulation (as evaluated by BMI or WC, with a stronger association with WC), creatinine, and BP. In relation to plasma lipids, a positive association between SUA and triglycerides was observed only in men. Table 4 depicts clinical outcomes in the sample. As expected from the data shown in Table 3, individuals with hyperuricemia showed higher rates of hypertension (adjusted OR, 2.20; 95% CI, 1.41–3.47), high WC (adjusted OR, 1.67; 95% CI, 1.05–2.65), and MetS (adjusted OR, 1.66; 95% CI, 1.07–2.57).
Table 3.
Demographic, Anthropometric, and Biochemical Characteristics According to Quartiles of Serum Uric Acid by Sex
| Characteristics | Men | P Value | Women | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n=284 | n=301 | |||||||||
| 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | |||
| (<4.9) | (4.9–6.0) | (6.0–7.1) | (>7.1) | (<3.8) | (3.8–4.6) | (4.6–5.7) | (>5.7) | |||
| Age, y | 44±12 | 45±11 | 46±11 | 46±11 | .501 | 40±9 | 44±10 | 45a±9 | 46a±10 | <.001 |
| BMI, kg/m2 | 23.4±3.7 | 23.7±4.3 | 23.7±4.5 | 25.3a±4.2 | .025 | 25.8±5.0 | 26.6±6.2 | 28.3a±5.6 | 28.0±6.5 | .031 |
| WC, cm | 77.2±12.4 | 78.0±12.3 | 79.7±12.2 | 83.8a±12.6 | .009 | 80.8±12.1 | 81.8±14.2 | 86.5a±13.4 | 86.3±14.3 | .013 |
| Glucose, mg/dL | 94±18 | 94±27 | 96±19 | 96±13 | .744 | 91±18 | 93±18 | 96±28 | 92±23 | .621 |
| Creatinine, mg/dL | 1.1±0.2 | 1.1±0.2 | 1.2±0.2 | 1.2a±0.2 | <.001 | 1.0±0.2 | 1.0±0.2 | 1.0±0.2 | 1.1a±0.2 | .005 |
| TC, mg/dL | 183±39 | 189±41 | 196±42 | 192±42 | .286 | 183±37 | 195±37 | 199a±35 | 200a±35 | .017 |
| TG, mg/dL | 96±40 | 93±34 | 105±36 | 114a±51 | .008 | 93±36 | 96±36 | 103±44 | 103±37 | .282 |
| LDL‐C, mg/dL | 120±40 | 127±41 | 131±43 | 123±46 | .384 | 116±40 | 129±38 | 132±38 | 130±37 | .059 |
| HDL‐C, mg/dL | 44±11 | 43±10 | 44±10 | 45±10 | .654 | 48±11 | 47±10 | 47±11 | 49±13 | .460 |
| SBP, mm Hg | 131±22 | 135±22 | 137±24 | 143a±24 | .031 | 125±23 | 131±25 | 139a±28 | 139a±29 | .002 |
| DBP, mm Hg | 80±14 | 83±14 | 82±13 | 86±15 | .055 | 78±15 | 82±12 | 85a±13 | 85a±14 | .002 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; WC, waist circumference.
Continuous values are expressed as mean±standard deviation and were analyzed by one‐way analysis of variance. Bold values indicate significance.
P value <.05 (post‐hoc Tukey's test compared with the first quartile).
Table 4.
Clinical Outcomes of Study Participants by Hyperuricemia Status
| Characteristics | Normal Uric Acid | Hyperuricemia | Crude OR (95% CI) | Adjusteda OR (95% CI) |
|---|---|---|---|---|
| (n=440, 75%) | (n=145, 25%) | |||
| Hypertension | 181 (41.1) | 86 (59.3) | 2.09 (1.42–3.05) | 2.20 (1.41–3.47) |
| High WC | 149 (33.9) | 59 (40.7) | 1.34 (0.91–1.97) | 1.67 (1.05–2.65) |
| Diabetes | 23 (5.2) | 9 (6.2) | 1.20 (0.54–2.65) | 1.17 (0.49–2.55) |
| Dyslipidemia | 268 (60.9) | 91 (62.8) | 1.08 (0.73–1.59) | 1.07 (0.72–1.60) |
| Overweight | 120 (27.3) | 50 (34.5) | 1.40 (0.94–2.09) | 1.42 (0.95–2.14) |
| Obesity | 82 (18.6) | 33 (22.7) | 1.29 (0.81–2.03) | 1.32 (0.81–2.13) |
| Smoking | 24 (5.4) | 11 (7.6) | 1.42 (0.68–2.98) | 1.25 (0.56–2.60) |
| Metabolic syndrome | 108 (24.5) | 49 (33.8) | 1.57 (1.04–2.36) | 1.66 (1.07–2.57) |
Abbreviations: CI, confidence interval; OR, odds ratio; WC, waist circumference. Values are expressed as number (percentage). Chi‐square test. Hyperuricemia was defined as serum urate levels >7.0 mg/dL in men and >5.7 mg/dL in women, according to National Health and Nutrition Examination Survey criteria.
The model was adjusted for age and sex.
Of the 585 individuals, 157 (27%) had MetS and the prevalence of subcomponents were: 46% elevated BP, 36% high WC, 24% fasting glucose >100 mg/dL or taking antidiabetic medication, 12% elevated triglycerides, and 50% low HDL cholesterol.
We also estimated creatinine clearance using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation for individuals of African descent (Levey and colleagues, 2009). The values were lower in patients with hyperuricemia (106.1±12.0 mL/min/1.73 m2) than in those with normal SUA levels (110.0±12.1 mL/min/1.73 m2–P=.002), but all values had a range of 85.2 to 148.6 mL/min/1.73 m2.
Of the 585 individuals evaluated, 267 (45.6%) were hypertensive (SBP ≥140 or DBP ≥90 mm Hg, or use of antihypertensive drugs). Of these, 143 were using antihypertensive drugs. Excluding the individuals that used antihypertensive drugs, 124 were hypertensive individuals who didn't begin the use of antihypertensive drugs. This last group showed higher mean SUA levels (5.8±1.7 mg/dL) than normotensive patients (5.2±1.6 mg/dL; P<.001). Among the 442 individuals without antihypertensive drugs, the frequency of hypertension was also higher among patients with hyperuricemia: 41 of 100 (41%) had hyperuricemia, while 83 of 342 (24%) had normal uric acid (OR, 2.17; 95% CI, 1.36–3.47; P=.001).
Among hypertensive individuals, 86 (32%) had hyperuricemia, while only 59 (19%) normotensive individuals had hyperuricemia (OR, 2.09; CI, 95% 1.42–3.06; P<.001). Similarly, among the 157 individuals with the MetS, hyperuricemia was more common (31%) than among those without MetS (19%; OR, 1.57; 95% CI, 1.05–2.36; P=.029).
Discussion
To our knowledge, this is the first report of SUA distribution in a black sub‐Saharan African population. Previous studies have reported SUA distribution in South African communities.16, 17, 18, 19 However, the prevalence of hyperuricemia and the associations of uric acid with traditional cardiovascular risk factors were not described in these studies.
In our study, the prevalence of hyperuricemia was 25%, a similar value to that found in African Americans (26%) and higher than that found in white Americans in NHANES (22%).9 In a study performed in Brazil, no relationship between ethnicity and the prevalence of hyperuricemia was found,20 which could be explained by the high degree of racial mixing in the Brazilian population.
Hyperuricemia is related to the consumption of purine‐rich foods, such as meat and fish,21 and beer intake.22 Angola, similar to other African countries, is now facing a rapid epidemiological and nutritional transition following rapid economic growth. Currently, the prevalence of noncommunicable chronic diseases, such as obesity and hypertension, is increasing,23, 24 which is confirmed by our data.
Beyond dietetic factors, hyperuricemia can also be related to the genetic predisposition for higher urate reabsorption in the kidneys. Previous studies have shown that the ABCG2 protein, a uric acid transporter, shows differences in its expression and function by ethnicity.25 The higher SUA levels in men may be partially explained by the increased renal ABCG2 expression in men compared with women.26 However, the association of a mutation in ABCG2 is significantly stronger in postmenopausal women.27 This change is associated with hormonal modifications that may explain the higher SUA values in postmenopausal women compared with those with normal estrogen production, as shown in our study.
Our study confirmed the positive association between increased SUA levels and the traditional cardiovascular risk factors because patients with hyperuricemia also showed a higher prevalence of hypertension and MetS. However, because of the cross‐sectional design of our study, there is no evidence of a causal relationship among these variables.
The African population is a high‐risk group for development of hypertension.24, 28 Our study showed a direct association between SUA levels and SBP. As some antihypertensive drugs have hyperuricemic effects, such as thiazide diuretics,29 or hypouricemic effects, such as losartan,30 BP was also measured in patients not receiving antihypertensive drugs. Nevertheless, hypertensive patients showed mean SUA levels that were greater than normotensive participants.
Accordingly, Palmer and colleagues18 showed a significant increase in BP from the lower to higher uric acid tertiles in African women. Other studies, such as the Bogalusa Heart Study,31 showed that elevated levels of SUA in childhood were associated with a faster BP increase in childhood, and higher BP levels persisted into adulthood in both white and black individuals. The Coronary Artery Risk Development in Young Adults Study (CARDIA)32 showed that uric acid was an independent predictor of incident hypertension in black American men. The participants of the Atherosclerosis Risk in Communities (ARIC) study33 also showed that higher SUA levels were associated with hypertension, especially in black American men. Furthermore, a meta‐analysis concluded that the hyperuricemia was associated with a higher risk of incident hypertension, independent of confounding variables.34 These studies, therefore, support the predictive value of high uric acid in hypertension development. Thus, the high SUA levels in the black population may be related to the higher BP and hypertension found in this group.
SUA and glomerular filtration rate (GFR) are inversely correlated.35 Several studies have reported a link between hyperuricemia and chronic kidney disease (CKD). In this scenario, hyperuricemia has been identified as an independent risk factor for progression of renal disease. Our data show that creatinine values were proportionally higher according to the progression of SUA quartiles. The estimated creatinine clearance by the CKD‐EPI equation was lower among those with hyperuricemia, but all values ranged from 85.2 to 148.6 mL/min/1.73 m2. However, despite the reduction in uric acid excretion with the decrease in the GFR, this occurs only when CKD is in the advanced stages, particularly for creatinine clearance of <15 mL/min.36, 37 Thus, hyperuricemia in our findings could not be justified by the alteration of renal function and is likely correlated with others factors, such as BP and MetS.
Our findings show a higher prevalence of MetS among individuals with hyperuricemia. This was similar to a Brazilian study, where individuals with insulin resistance (higher quartiles of Homeostasis Model Assessment insulin resistance) also showed high levels of uric acid,38 a finding confirmed in a meta‐analysis.39 Regarding the temporal relationship between these variables, a study with an 11‐year follow‐up showed that SUA predicted both current as well as future incidence of MetS.40
In our study, the prevalence of MetS was high (27%). However, when we utilized an internal cutoff for high WC,41 the MetS prevalence was even higher (29.4%). An explanation for this could be the low frequency of high WC in black men because the definition of MetS by international consensus (using the European data) does not include specific values for individuals from sub‐Saharan Africa.15
Study Limitations
There were some limitations in our study. Data were collected from a select Angolan population, in a single professional category, which is not representative of the whole Angolan population. However, the sample shows the distribution of income and education level found in the general population. Moreover, the cross‐sectional design does not allow for the establishment of causal relationships, only associations.
Conclusions
The prevalence of hyperuricemia in black African men and women was shown to be high. Hypertension, high WC, and MetS were the major cardiovascular risk factors associated with hyperuricemia in this population.
Acknowledgment
This work was supported by grants from the Fundação para Ciência e Desenvolvimento de Angola and CAPES (Brazil).
Conflict of Interest
None.
J Clin Hypertens (Greenwich). 2017;19:45–50. DOI: 10.1111/jch.12863. © 2016 Wiley Periodicals, Inc.
References
- 1. Coutinho Tde A, Turner ST, Peyser PA, et al. Associations of serum uric acid with markers of inflammation, metabolic syndrome, and subclinical coronary atherosclerosis. Am J Hypertens. 2007;20:83–89. [DOI] [PubMed] [Google Scholar]
- 2. Kanbay M, Jensen T, Solak Y, et al. Uric acid in metabolic syndrome: from an innocent bystander to a central player. Eur J Intern Med. 2016; 29: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grossman C, Shemesh J, Koren‐Morag N, et al. Serum uric acid is associated with coronary artery calcification. J Clin Hypertens (Greenwich). 2014;16:424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhao G, Huang L, Song M, et al. Baseline serum uric acid level as a predictor of cardiovascular disease related mortality and all‐cause mortality: a meta‐analysis of prospective studies. Atherosclerosis. 2013;231:61–68. [DOI] [PubMed] [Google Scholar]
- 5. Vorster HH. The emergence of cardiovascular disease during urbanisation of Africans. Public Health Nutr. 2002;5:239–243. [DOI] [PubMed] [Google Scholar]
- 6. Capingana DP, Magalhaes P, Silva AB, et al. Prevalence of cardiovascular risk factors and socioeconomic level among public‐sector workers in Angola. BMC Public Health. 2013;13:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . Chronic diseases and health promotion: Stepwise approach to surveillance (STEPS). http://www.who.int/chp/en/. Accessed June 7, 2016.
- 8. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 9. Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63:3136–3141. [DOI] [PubMed] [Google Scholar]
- 10. American Diabetes Association . Standards of medical care in diabetes–2007. Diabetes Care. 2007;30(suppl 1):S4–S41. [DOI] [PubMed] [Google Scholar]
- 11. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive summary of The Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 12. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 13. Lean ME, Han TS, Morrison CE. Waist circumference as a measure for indicating need for weight management. BMJ. 1995;311:158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization . Obesity: Preventing and managing the global epidemic: Report of a WHO Consultation on Obesity. Geneva. 1997. [PubMed]
- 15. Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 16. Beighton P, Daynes G, Soskolne CL. Serum uric acid concentrations in a Xhosa community in the Transkei of Southern Africa. Ann Rheum Dis. 1976;35:77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beighton P, Solomon L, Soskolne CL, et al. Serum uric acid concentrations in an urbanized South African Negro population. Ann Rheum Dis. 1974;33:442–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palmer IM, Schutte AE, Huisman HW, et al. A comparison of uric acid levels in Black African vs Caucasian women from South Africa: the POWIRS study. Ethn Dis. 2007;17:676–681. [PubMed] [Google Scholar]
- 19. Palmer IM, Schutte AE, Huisman HW. Uric acid and the cardiovascular profile of African and Caucasian men. J Hum Hypertens. 2010;24:639–645. [DOI] [PubMed] [Google Scholar]
- 20. Rodrigues SL, Baldo MP, Capingana P, et al. Gender distribution of serum uric acid and cardiovascular risk factors: population based study. Arq Bras Cardiol. 2012;98:13–21. [DOI] [PubMed] [Google Scholar]
- 21. Choi HK, Liu S, Curhan G. Intake of purine‐rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthr Rheum. 2005;52:283–289. [DOI] [PubMed] [Google Scholar]
- 22. Gaffo AL, Roseman JM, Jacobs DR, et al. Serum urate and its relationship with alcoholic beverage intake in men and women: findings from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort. Ann Rheum Dis. 2010;69:1965–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bourne LT, Lambert EV, Steyn K. Where does the black population of South Africa stand on the nutrition transition? Public Health Nutr. 2002;5:157–162. [DOI] [PubMed] [Google Scholar]
- 24. Pires JE, Sebastiao YV, Langa AJ, et al. Hypertension in Northern Angola: prevalence, associated factors, awareness, treatment and control. BMC Public Health. 2013;13:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sakiyama M, Matsuo H, Takada Y, et al. Ethnic differences in ATP‐binding cassette transporter, sub‐family G, member 2 (ABCG2/BCRP): genotype combinations and estimated functions. Drug Metab Pharmacokinetics. 2014;29:490–492. [DOI] [PubMed] [Google Scholar]
- 26. Dehghan A, Kottgen A, Yang Q, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome‐wide association study. Lancet. 2008;372:1953–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang L, Spencer KL, Voruganti VS, et al. Association of functional polymorphism rs2231142 (Q141K) in the ABCG2 gene with serum uric acid and gout in 4 US populations: the PAGE Study. Am J Epidemiol. 2013;177:923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guwatudde D, Nankya‐Mutyoba J, Kalyesubula R, et al. The burden of hypertension in sub‐Saharan Africa: a four‐country cross sectional study. BMC Public Health. 2015;15:1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vandell AG, McDonough CW, Gong Y, et al. Hydrochlorothiazide‐induced hyperuricaemia in the pharmacogenomic evaluation of antihypertensive responses study. J Intern Med. 2014;276:486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wolff ML, Cruz JL, Vanderman AJ, et al. The effect of angiotensin II receptor blockers on hyperuricemia. Ther Adv Chronic Dis. 2015;6:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alper AB Jr, Chen W, Yau L, et al. Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension. 2005;45:34–38. [DOI] [PubMed] [Google Scholar]
- 32. Dyer AR, Liu K, Walsh M, et al. Ten‐year incidence of elevated blood pressure and its predictors: the CARDIA study. Coronary Artery Risk Development in (Young) Adults. J Hum Hypertens. 1999;13:13–21. [DOI] [PubMed] [Google Scholar]
- 33. Mellen PB, Bleyer AJ, Erlinger TP, et al. Serum uric acid predicts incident hypertension in a biethnic cohort: the atherosclerosis risk in communities study. Hypertension. 2006;48:1037–1042. [DOI] [PubMed] [Google Scholar]
- 34. Wang J, Qin T, Chen J, et al. Hyperuricemia and risk of incident hypertension: a systematic review and meta‐analysis of observational studies. PLoS One. 2014;9:e114259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson RJ, Nakagawa T, Jalal D, et al. Uric acid and chronic kidney disease: which is chasing which? Nephrol Dial Transplantation. 2013;28:2221–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Steele TH, Rieselbach RE. The contribution of residual nephrons within the chronically diseased kidney to urate homeostasis in man. Am J Med. 1967;43:876–886. [DOI] [PubMed] [Google Scholar]
- 37. Rastegar A, Thier SO. The physiologic approach to hyperuricemia. New Engl J Med. 1972;286:470–476. [DOI] [PubMed] [Google Scholar]
- 38. Genelhu VA, Celoria BM, Duarte SF, et al. Not all obese subjects of multiethnic origin are at similar risk for developing hypertension and type 2 diabetes. Eur J Intern Med. 2009;20:289–295. [DOI] [PubMed] [Google Scholar]
- 39. Yuan H, Yu C, Li X, et al. Serum uric acid levels and risk of metabolic syndrome: a dose‐response meta‐analysis of prospective studies. J Clin Endocrin Metab. 2015;100:4198–4207. [DOI] [PubMed] [Google Scholar]
- 40. Osgood K, Krakoff J, Thearle M. Serum uric acid predicts both current and future components of the metabolic syndrome. Metab Syndrome Rel Dis. 2013;11:157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Magalhaes P, Capingana DP, Mill JG. Prevalence of the metabolic syndrome and determination of optimal cut‐off values of waist circumference in university employees from Angola. Cardiovasc J Africa. 2014;25:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]


