Abstract
The aim of this study was to compare the effect of morning and bedtime administration of valsartan/amlodipine combination therapy (80/5 mg) on nocturnal brachial and central blood pressure (BP) measured by ambulatory BP monitoring in patients with hypertension. This was a 16‐week prospective, multicenter, randomized, open‐label, crossover, noninferiority clinical trial. Patients underwent 24‐hour ambulatory BP monitoring at randomization, at switching, and at the end of the study. Twenty‐three patients (mean age, 68.0 years) were studied. The difference in nocturnal brachial systolic BP between the morning and bedtime administrations of combination valsartan/amlodipine was −3.2 mm Hg, and the two‐sided 95% confidence interval ranged from −6.8 to 0.4 mm Hg. The difference in nocturnal central systolic BP was −4.0 mm Hg (95% confidence interval, −7.6 to −0.4 mm Hg). The upper limit of the 95% confidence interval was below the margin of 3.0 mm Hg in both nocturnal brachial and central systolic BP, confirming the noninferiority of morning administration to the bedtime administration of valsartan/amlodipine combination therapy.
Keywords: amlodipine, bedtime administration, clinical trial, crossover, morning administration, nocturnal central blood pressure, noninferiority, valsartan
1. INTRODUCTION
Nocturnal brachial blood pressure (BP) is a better predictor of mortality and cardiovascular morbidity than daytime BP in patients with hypertension.1, 2, 3 Reducing nocturnal brachial BP is associated with cardiovascular protection.3, 4 Further, nocturnal central BP estimated using 24‐hour ambulatory BP monitoring (ABPM) may be associated with cardiovascular disease risk.5 Therefore, nocturnal central BP reduction might be important for the management of hypertension.
The bedtime administration of antihypertensive agents (ie, chronotherapy) has been shown to lower nocturnal brachial BP compared with morning administration in some, but not all,6, 7, 8 studies. However, the effects of chronotherapy on nocturnal central BP have never been tested. A once‐daily antihypertensive agent taken in the morning has been a conventional therapy for the management of hypertension. If physicians describe a long‐acting, once‐daily antihypertensive agent for patients with hypertension, the BP‐lowering effect on nocturnal BP was equal to that by bedtime administration.9 Therefore, we hypothesized that the morning administration of a long‐acting renin‐angiotensin‐aldosterone system (RAAS) inhibitor/calcium channel blocker (CCB) combination would not be inferior to the bedtime administration of the same RAAS inhibitor/CCB combination for the reduction of nocturnal brachial and central BP.
We tested our hypothesis in the CPET (Chronotherapy for Ambulatory Central Pressure) study, a randomized controlled trial designed to compare the effect of morning vs bedtime administration of a valsartan/amlodipine combination on nocturnal brachial and central BP in Japanese patients with hypertension.
2. METHODS
2.1. Study design
The CPET study was a 16‐week prospective, multicenter, randomized, open‐label, crossover, noninferiority clinical trial conducted in Japan. The patients were enrolled between March 2014 and July 2015 at two institutions in Japan: Higashiagatsuma‐machi National Health Insurance Clinic and the Jichi Medical University School of Medicine. The study's purpose was to compare the effects of morning vs bedtime administrations of a valsartan/amlodipine combination on nocturnal brachial and central BP in patients with hypertension.
During a run‐in period, all patients underwent ≥4 weeks of monotherapy with either any type of angiotensin II receptor blocker (ARBs) or CCB with standard or maximum doses (Figure 1). For the randomization, the physicians who enrolled the patients made a telephone call to an independent research center, and the patients were assigned in a blind manner to one of the two treatment arms: morning or bedtime administration of a fixed‐dose valsartan/amlodipine (80/5 mg) combination tablet, over 8 weeks (the first period). In the next 8 weeks (the second period), all patients were switched to the arm other than their original assignment. The total interventional period of this study was 16 weeks. Every 4 weeks throughout the study period, using an interview sheet, physicians recorded adverse events and confirmed that the patients' adherence to the medication was >70%.
Figure 1.
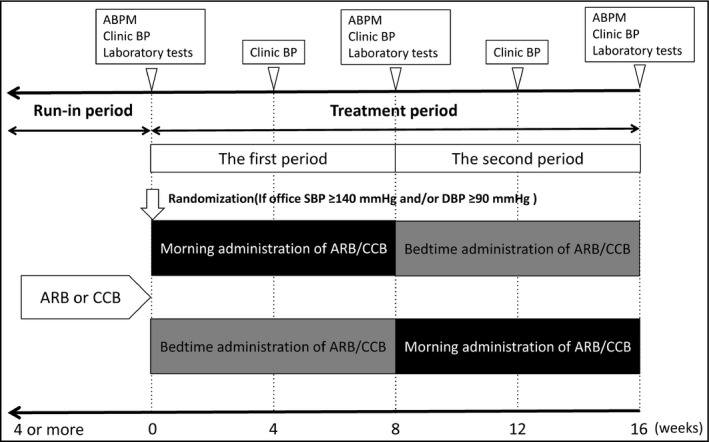
Design of the study. ABPM indicates ambulatory blood pressure monitoring; ARB, angiotensin II receptor blocker; BP, blood pressure; CCB, calcium channel blocker; DBP, diastolic blood pressure; SBP, systolic blood pressure
The study protocol was approved by the ethics committee of the Jichi Medical University School of Medicine (Shimotsuke, Japan), and all of the patients gave written informed consent to participate in the study. The protocol of the CPET study was registered on the University Trials Registry (UMIN‐CTR) website (trial No. UMIN000013519). All data and procedures of this study were regularly monitored by Dilphi Corporation, a contract research organization.
2.2. Patients
The three inclusion criteria were: (1) patients with essential hypertension, defined as clinic brachial systolic BP (SBP) ≥140 mm Hg or diastolic BP (DBP) ≥90 mm Hg; (2) patients receiving ARBs or CCBs before study enrollment; and (3) patients aged 20 to 80 years. The exclusion criteria were: (1) patients who were intolerant to RAAS inhibitors or CCBs; (2) patients who were treated concomitantly with one or more antihypertensive agent other than an ARB or CCB during the run‐in period and a valsartan/amlodipine combination during the treatment period; (3) patients with prevalent heart failure (New York Heart Association functional class III or IV) at baseline; (4) patients who had a serum creatinine level ≥3.0 mg/dL, or were on dialysis at baseline; and 5 patients with prevalent coronary heart disease, cerebrovascular disease, or malignant disease at baseline.
Diabetes mellitus was defined as a fasting glucose level of ≥126 mg/dL and/or a casual glucose level of ≥200 mg/dL or treated diabetes mellitus. Chronic kidney disease was defined as a creatinine‐based estimated glomerular filtration rate <60 mL/min per 1.73 m2 and/or microalbuminuria (a urine albumin to creatinine ratio ≥30 mg/g·Cr).10
2.3. BP measurements
2.3.1. Ambulatory BP
Oscillometric ABPM that records both brachial and central aortic BP was conducted using the Mobil‐O‐Graph NG (IEM). This device was validated to estimate central BP noninvasively, with the use of the ARCSolver algorithm.11 With this algorithm, a generalized transfer function is used and the central aortic pressure is estimated on the basis of the brachial pulse wave.11 The algorithm provides central BP estimations as accurate as those measured invasively,11 and ARCSolver algorithm–derived central BP values are comparable to those obtained invasively.11
Patients underwent 24‐hour ABPM three times: at randomization (week 0), at switch between arms (week 8), and at the end of the study (week 16). Ambulatory BP readings were taken at 30‐minute intervals throughout the 24‐hour day. The data from the ABPM were automatically stored in the memory of the device and transferred electronically to personal computers equipped with IEM software for the analysis of the data.
Nocturnal BP was defined as the average BP value from those taken during the period from when the patient went to bed until he or she got out of bed in the morning. We subclassified the patients according to the percentage of the nocturnal SBP reduction (100 × [1 − sleep SBP/awake SBP]) as follows: “extreme dipper,” when the nocturnal SBP reduction was ≥20%; “dipper,” when the reduction was ≥10% but <20%; “nondipper,” when the reduction was ≥0% but <10%; and “riser,” when the reduction was <0%.12
2.3.2. Clinic BP
Clinic BP was measured at each visit using a validated cuff oscillometric device (HEM‐907, Omron Healthcare) according to the Japanese Society of Hypertension 2014 guidelines.13 BP was measured after the patient rested for at least 2 minutes in a seated position with their legs uncrossed in a quiet environment. Two consecutive BP measurements were taken at a 1‐ to 2‐minute interval, and the average of the measurements was used as the clinic BP value.
2.4. Blood and urine examinations
Fasting blood and spot urine samples were collected from all patients enrolled at randomization (week 0), at switching (week 8), and at the end of the study (week 16). All samples were sent to a single laboratory within 24 hours of collection. Urinary albumin excretion was measured using an immunoturbidity kit (AutoWako Microalbumin, Wako Pure Chemical Industries) and is expressed as urine albumin to creatinine ratio (mg/g·Cr). The intra‐assay and interassay coefficients of the urine albumin to creatinine ratio were <5%.
2.5. Primary outcome
The primary outcome was the extent of nocturnal brachial and central SBP reduction by the intervention (morning vs bedtime administration of the valsartan/amlodipine combination). DBP that is estimated using an oscillometric BP monitor vs a sphygmomanometer is less accurate compared with SBP.14 We therefore selected SBP change as the primary outcome.
2.6. Sample size
This crossover‐design study tested the noninferiority of the effect of morning administration compared with bedtime administration of valsartan/amlodipine on nocturnal brachial and central BP reduction. In previous studies, the bedtime administration of valsartan reduced mean 24‐hour SBP levels by a maximum of 3.0 mm Hg compared with the morning administration of the same dosage of valsartan.7, 15, 16 In other studies, the time of a once‐a‐day amlodipine administration did not influence its efficacy for 24‐hour BP control.17, 18 Based on these clinically important differences,19 we fixed the margin of clinical noninferiority at 3.0 mm Hg for the present study.
We thus conducted the statistical analysis to test for noninferiority against a one‐sided alternative hypothesis at the 2.5% significance level and a power of 80%. By assuming a standard deviation for the changes in nocturnal BP of 7.0 mm Hg and a clinically significant difference in the nocturnal brachial SBP reduction between the morning and bedtime administrations from the baseline of 0.43 mm Hg (based on a previous report20), the sample size would be 23 patients. Adjusting by 10% for loss to follow‐up or dropout of patients, a final total of 26 patients were needed at randomization.
2.7. Statistical analysis
We analyzed the data by using a per‐protocol set. Descriptive statistics are presented as means and standard deviations and proportions where appropriate. Data are presented as mean and standard error of the mean or 95% confidence interval (CI). A linear mixed model analysis, proposed by Grizzle21, 22 for a two‐period crossover study, was used to test for a possible carryover or drug‐order effect. The fixed effects in the model were drug‐order (morning/bedtime administration or bedtime/morning administration), period (the first 8‐week period or the second 8‐week period), and treatment (morning administration vs bedtime administration). Since the patients were assigned to one of the drug‐order arms in the first period and all patients were switched to the other drug‐order arm in the second period, they were nested within drug‐order arms. Because the association of the changes in nocturnal brachial and central BP from baseline between each arm in each patient should be evaluated, the random effect in this linear mixed model was set to be the patients. If crossover studies were full‐factorial designs (with factors drug‐order, period, and treatment), it would be possible to evaluate not only the main effects but also the interactions of each or all factors; however, crossover studies are not full‐factorial designs.
For the noninferiority assessments, we used a two‐sided 95% CI of the difference between morning and bedtime administration in the change of nocturnal brachial and central SBP from baseline. If the upper limit of a two‐sided 95% CI for the treatment effect is below the margin of noninferiority, it means that the morning administration is noninferior.23, 24, 25 In the analysis of changes in nocturnal central BP, we adjusted for changes in the nocturnal heart rate as an adjustment factor.26, 27 Statistical analyses were performed using SPSS version 24.0 (SPSS Inc) and SAS version 9.4 (SAS Institute Inc). A P value <.05 was considered significant.
3. RESULTS
3.1. Study population and baseline characteristics
A total of 26 patients consented and were enrolled in this study. Of them, one patient was excluded before randomization because of withdrawing consent. Two patients were excluded during the study; one had the adverse event of renal dysfunction and another was hospitalized for epileptic seizure. Thus, a total of 23 patients completed the treatments for 16 weeks (Figure 2).
Figure 2.
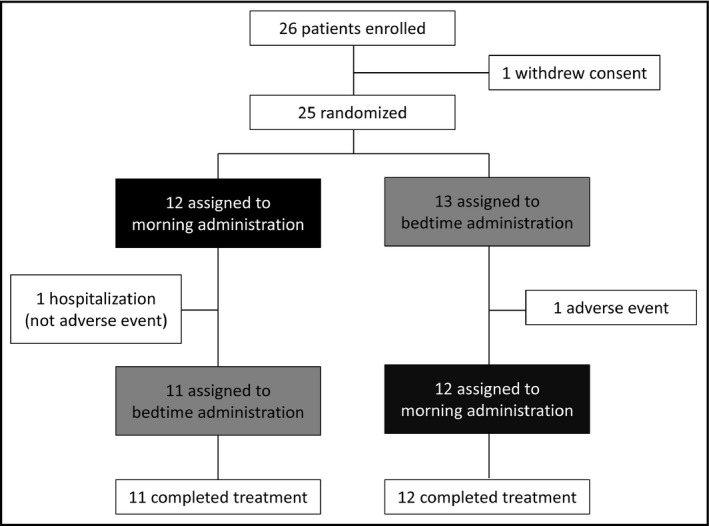
Patients' disposition
Table 1 provides the demographic variables and clinical characteristics of the included patients. Of the 23 patients, 15 (65%) were women; the mean (standard deviation) age was 68.0 (8.7) years and median (interquartile range) age was 68.0 (63.0–77.0) years; and 65% were classified as having chronic kidney disease. There were no significant differences in nocturnal BP levels at the randomization between the ARB and CCB groups during the run‐in period (Table S1). The dipping statuses at the randomization were as follows: extreme dipper, 17%; dipper, 52%; nondipper, 22%; and riser, 9%.
Table 1.
Clinical characteristics of the study patients
| Variable | n = 23 |
| Age, y | 68.0 ± 8.7 |
| Women/men, No. (%) | 15 (65.2)/8 (34.8) |
| BMI, kg/m2 | 24.9 ± 4.1 |
| Current smoking, % | 30.4 |
| Habitual drinking, % | 17.4 |
| Previous antihypertensive medication, No. (%) | |
| ARBs | 8 (34.8) |
| Morning administration | 5 (21.7) |
| Evening administration | 3 (13.0) |
| CCBs | 15 (65.2) |
| Morning administration | 15 (65.2) |
| Evening administration | 0 (0) |
| Total cholesterol, mg/dL | 204.8 ± 28.1 |
| High‐density lipoprotein, mg/dL | 64.0 ± 12.7 |
| Triglyceride, mg/dL | 107.6 ± 67.4 |
| Diabetes mellitus, % | 17.4 |
| eGFRcreat, mL/min/1.73 m2 | 68.5 ± 15.7 |
| Urinary albumin excretion | |
| Geometric mean, mg/g·Cr | 77.5 ± 202.8 |
| Mean log‐transformed UACR | 3.04 ± 1.44 |
| Chronic kidney disease, % | 65.2 |
| Blood pressure parameters, mm Hg | |
| Clinic SBP | 154.3 ± 15.4 |
| Clinic DBP | 80.8 ± 8.3 |
| Nocturnal brachial SBP | 122.7 ± 13.0 |
| Nocturnal brachial DBP | 72.4 ± 7.4 |
| Nocturnal central SBP | 113.8 ± 10.7 |
| Nocturnal central DBP | 73.8 ± 7.6 |
| Dipping status, No. (%) | |
| Extreme dipper | 4 (17.4) |
| Dipper | 12 (52.2) |
| Nondipper | 5 (21.7) |
| Riser | 2 (8.7) |
Abbreviations: ARB, angiotensin II receptor blocker; BMI, body mass index; CCB, calcium channel blocker; DBP, diastolic blood pressure; eGFRcreat, creatinine‐based estimated glomerular filtration rate; SBP, systolic blood pressure; UACR, urine albumin to creatinine ratio.
Data are expressed as mean±standard deviation or percentage.
3.2. Changes in nocturnal brachial and central BP
A linear mixed model analysis for nocturnal BP revealed a nonsignificant drug‐order effect (P = .86 for brachial SBP, P = .22 for brachial DBP, P = .93 for central SBP, and P = .12 for central DBP) and a nonsignificant period effect (P = .74 for brachial SBP, P = .82 for brachial DBP, P = .78 for central SBP, and P = .63 for central DBP). After the exclusion of the drug‐order effect and period effect, the basic assumptions of the crossover design were verified.
The nocturnal brachial and central BP levels in the individual patients at each administration period are shown in Figure 3. Table 2 shows the changes in nocturnal brachial and central BP from baseline by the morning and bedtime administrations of valsartan/amlodipine. The morning administration significantly reduced the patients' brachial and central SBP and DBP compared with their baseline values, whereas the bedtime administration significantly reduced central DBP only. The upper limit of the 95% CI for the difference of nocturnal brachial SBP reduction between the morning and bedtime administration was further below the margin of bedtime administration and included zero, which means that morning administration was noninferior compared with the bedtime administration (Figure 4A). The upper limit of the 95% CI for the difference of nocturnal central SBP reduction between the morning and bedtime administration was entirely below zero, which demonstrated that the morning administration was not only noninferior to the bedtime administration, it was superior (Figure 4A).
Figure 3.
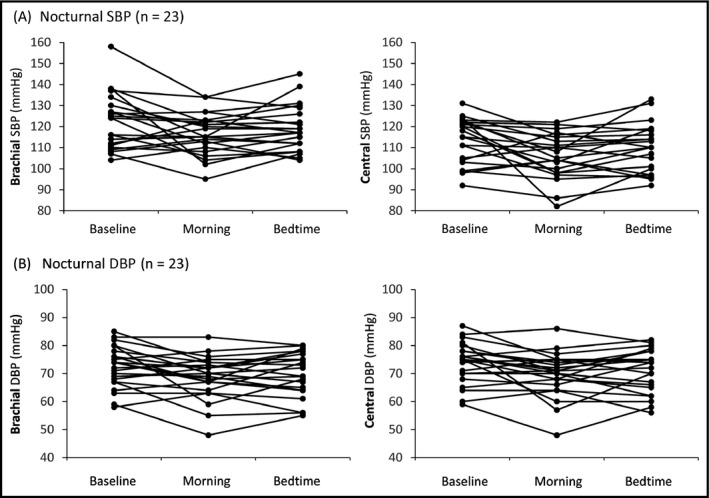
Comparison of nocturnal blood pressure levels in the individual patient at each administration period. DBP indicates diastolic blood pressure; SBP, systolic blood pressure
Table 2.
Changes in nocturnal BP parameters
| Variable | Morning administration (n = 23) | Bedtime administration (n = 23) | Comparison between groups | ||
|---|---|---|---|---|---|
| Change, mean (95% CI) | P value | Change, mean (95% CI) | P value | P value | |
| Nocturnal brachial SBP, mm Hg | −6.1 (−11.3 to −0.9) | .023 | −2.9 (−8.0 to 2.3) | .261 | .076 |
| Nocturnal brachial DBP, mm Hg | −3.8 (−6.5 to −1.1) | .008 | −2.5 (−5.3 to 0.2) | .064 | .254 |
| Nocturnal central SBPa mm Hg | −7.1 (−11.9 to −2.3) | .006 | −3.1 (−7.9 to 1.7) | .196 | .033 |
| Nocturnal central DBPa mm Hg | −4.3 (−7.2 to −1.4) | .006 | −3.1 (−6.0 to −0.2) | .037 | .342 |
Abbreviations: DBP, diastolic blood pressure; SBP, systolic blood pressure.
Data are expressed as mean (95% confidence interval [CI]).
P value refers to the changes in each blood pressure (BP) parameter from baseline and the comparison of morning and bedtime administration of valsartan/amlodipine combination by a linear mixed model analysis.
Adjusted for the change of nocturnal heart rate.
Figure 4.
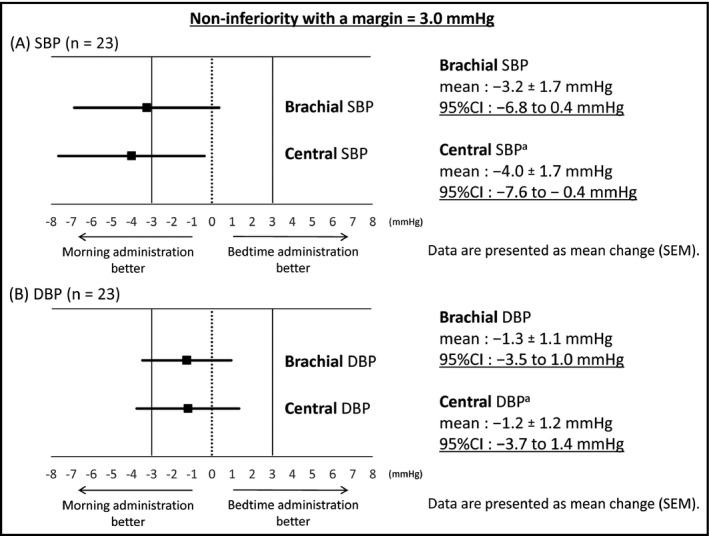
(A) The 95% confidence interval (CI) of the difference between the morning and bedtime administrations in the changes of nocturnal brachial and central systolic blood pressure (SBP) from baseline. aAdjusted for the change of nocturnal heart rate. (B) The 95% CI of the difference between the morning and bedtime administrations in the changes of nocturnal brachial and central diastolic blood pressure (DBP) from baseline. aAdjusted for the change of nocturnal heart rate. SEM indicates standard error of the mean
Regarding DBP, the upper limit of the 95% CI for the difference of nocturnal brachial and central DBP reduction between the morning and bedtime administrations was below the margin of the bedtime administration and included zero, which means that morning administration was noninferior compared with the bedtime administration (Figure 4B).
3.3. Adverse events
Both the morning and bedtime administrations of valsartan/amlodipine therapy were well tolerated except for in one patient who had renal dysfunction (creatinine‐based estimated glomerular filtration rate: 40.4 from 57.0 mL/min per 1.73 m2 at baseline) with increasing uric acid at week 8 (Table S2). We immediately instructed this patient to stop taking the medication.
3.4. Adherence
The overall compliance during the study period was good in both administration groups. There was no patient with a compliance rate ≤70% in either administration group.
4. DISCUSSION
This is the first study to highlight the effect of chronotherapy using a valsartan/amlodipine combination on nocturnal brachial and central BP in Japanese patients with hypertension. We observed that the morning administration of a valsartan/amlodipine combination was not inferior to the bedtime administration in the reduction of nocturnal brachial and central BP. There was no increase in adverse events by chronotherapy of the valsartan/amlodipine combination.
An earlier study indicated that morning and evening administrations of valsartan/amlodipine combination therapy had equivalent effects on nocturnal brachial BP reduction.20 In ACROBAT (ARB and CCB Longest Combination Treatment on Ambulatory and Home BP in Hypertension With Atrial Fibrillation Multicenter Study on Time of Dosing), there were no significant differences in nocturnal brachial BP reduction between morning and bedtime administrations of an ARB/CCB combination.28 Conversely, there is also a report that the bedtime administration relative to the morning administration of an ARB/CCB combination more effectively reduced nocturnal brachial BP in patients with nondipping status.29 These studies vary in several respects, including the populations, baseline comorbidities, and treatment regimens, which might have contributed to the inconsistent results. In the present study, we used a valsartan/amlodipine combination that was composed of long‐acting antihypertensive drugs and reported to provide a strong and long‐term reduction of BP throughout 24‐hour periods.30, 31, 32 This might be a reason why the morning administration of the valsartan/amlodipine combination showed noninferiority to the bedtime administration in the reduction of nocturnal brachial BP in the present study.
Our present findings revealed that the morning administration of the valsartan/amlodipine combination had a noninferior effect for reducing nocturnal central SBP compared with bedtime administration. We previously reported that a RAAS inhibitor/CCB combination resulted in greater reductions in central SBP compared with a RAAS inhibitor/diuretic combination (despite the lack of a significant difference in brachial SBP reduction) in the J‐CORE (Japan‐Combined Treatment With Olmesartan and a Calcium Channel Blocker Versus Olmesartan and Diuretics Randomized Efficacy) study.33 Several reports also indicated that a RAAS inhibitor/CCB combination is more effective in lowering central BP than other antihypertensive combinations. In the CAFE (Conduit Artery Function Evaluation) study, a substudy of ASCOT (Anglo‐Scandinavian Cardiac Outcomes Trial), an atenolol±thiazide‐based treatment was much less effective than an amlodipine±perindopril‐based treatment at lowering central BP despite an insignificant difference in brachial BP.34 The EXPLOR (Effect of the Fixed Dose Combination Amlodipine/Valsartan on Central Aortic Blood Pressure in Uncontrolled Essential Hypertension With Amlodipine 5 mg) study showed that an amlodipine/valsartan combination significantly decreased central SBP more than an amlodipine/atenolol combination, despite similar changes in brachial SBP.35 Our CPET results support the prior findings by demonstrating that the morning administration of the valsartan/amlodipine combination showed noninferiority in the change of nocturnal central BP compared with the bedtime administration.
We observed that the morning administration of ARB/CCB therapy had a stronger effect on nocturnal central SBP reduction compared with the bedtime administration. However, caution is required in interpreting our results because this was a noninferiority (not a superiority) study. Regarding the issue of whether morning administration of ARB/CCB therapy is more likely than bedtime administration to have a stronger effect on nocturnal central SBP reduction, randomized controlled clinical trials with the objective of determining the optimal treatment timing are required before any definitive conclusion can be made.
5. STUDY LIMITATIONS
There are some limitations of this study. First, each treatment period was short (8 weeks), and the long‐term effects are not yet known. Second, the margin of clinical noninferiority for nocturnal central SBP was set based on a previous study of the effect on nocturnal brachial SBP because no studies examined the effect for nocturnal central SBP. However, the upper limit of the 95% CI for the difference in nocturnal central SBP reduction between morning and bedtime administrations was entirely below zero, which indicates noninferiority. Third, the mean age of the patients in this study was 68.0 years, and individuals at that age are likely to have stiffer conduit arteries, which could exacerbate any different drug effects on central BP. Whether similar results would have been seen in much younger patients is unknown and merits further investigation. Fourth, it was not clear whether this novel device, the Mobil‐O‐Graph NG, provided a realistic estimation of accurate ambulatory central BP values. However, the ARCSolver algorithm provided accurate values for central BP when calibrated with invasive pressure and central BP as obtained with the ARCSolver and brachial cuff–based waveforms, and the data obtained with this algorithm showed good agreement with the reference method (SphygmoCor, AtCor Medical Inc) in suitable conditions.11 These results also confirm the accuracy of the central BP estimation in ambulatory conditions. Fifth, we did not set a washout period in the study design. However, we excluded the presence of a drug‐order effect and a period effect. Sixth, the treatment timing and the class of antihypertensive medication during the run‐in period might have affected the nocturnal BP in the first period. For example, a greater BP‐lowering effect was observed when the time of ARB administration was changed from the morning to bedtime,16, 36 whereas corresponding studies of CCBs showed mixed results.17, 18 Seventh, we used a fixed‐dose valsartan/amlodipine combination tablet (80/5 mg per tablet), which is the standard dose for Japanese patients. Further investigations are required to determine whether a dose‐dependent effect is observed in chronotherapy. Finally, several patients with chronic kidney disease and/or diabetes mellitus were included in this study. Abnormal circadian rhythms, ie, no dipping and nocturnal hypertension, have been reported to be highly prevalent in patients with chronic kidney disease8 and/or diabetes mellitus.37 A subgroup analysis excluding these factors should be performed, but we could not do so because of the small patient sample (n = 23).
6. CONCLUSIONS
The morning administration of a valsartan/amlodipine combination showed noninferiority to the bedtime administration in the change of nocturnal brachial and central SBP in Japanese patients with hypertension. When selecting a valsartan/amlodipine combination for the treatment of hypertension, physicians should take into account this noninferiority result for controlling nocturnal BP levels. Future large‐scale studies are needed to reveal the clinically meaningful effects of chronotherapy for nocturnal central BP reduction.
CONFLICTS OF INTEREST
The authors have no potential conflicts of interest to declare.
Supporting information
ACKNOWLEDGMENTS
We gratefully acknowledge Ms Kimiyo Saito and Ms Miki Sato for their coordination and data management of this study, and Ms Ayako Okura for her editorial assistance.
Fujiwara T, Hoshide S, Yano Y, Kanegae H, Kario K. Comparison of morning vs bedtime administration of the combination of valsartan/amlodipine on nocturnal brachial and central blood pressure in patients with hypertension. J Clin Hypertens. 2017;19:1319–1326. 10.1111/jch.13128
Funding information
Financial support for this study was provided by Novartis Pharma (K.K.).
Clinical Trial ID: UMIN000013519
REFERENCES
- 1. Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219‐1229. [DOI] [PubMed] [Google Scholar]
- 2. Salles GF, Reboldi G, Fagard RH, et al. Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: the Ambulatory Blood Pressure Collaboration in Patients With Hypertension (ABC‐H) meta‐analysis. Hypertension. 2016;67:693‐700. [DOI] [PubMed] [Google Scholar]
- 3. Hermida RC, Ayala DE, Mojon A, et al. Decreasing sleep‐time blood pressure determined by ambulatory monitoring reduces cardiovascular risk. J Am Coll Cardiol. 2011;58:1165‐1173. [DOI] [PubMed] [Google Scholar]
- 4. Yano Y, Hoshide S, Shimizu M, et al. Association of home and ambulatory blood pressure changes with changes in cardiovascular biomarkers during antihypertensive treatment. Am J Hypertens. 2012;25:306‐312. [DOI] [PubMed] [Google Scholar]
- 5. Williams B, Lacy PS, Baschiera F, et al. Novel description of the 24‐hour circadian rhythms of brachial versus central aortic blood pressure and the impact of blood pressure treatment in a randomized controlled clinical trial: the Ambulatory Central Aortic Pressure (AmCAP) study. Hypertension. 2013;61:1168‐1176. [DOI] [PubMed] [Google Scholar]
- 6. Omboni S, Parati G, Palatini P, et al. Reproducibility and clinical value of nocturnal hypotension: prospective evidence from the SAMPLE study. J Hypertens. 1998;16:733‐738. [DOI] [PubMed] [Google Scholar]
- 7. Hermida RC, Calvo C, Ayala DE, et al. Administration time‐dependent effects of valsartan on ambulatory blood pressure in hypertensive subjects. Hypertension. 2003;42:283‐290. [DOI] [PubMed] [Google Scholar]
- 8. Pogue V, Rahman M, Lipkowitz M, et al. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension. 2009;53:20‐27. [DOI] [PubMed] [Google Scholar]
- 9. Zappe DH, Crikelair N, Kandra A, et al. Time of administration important? Morning versus evening dosing of valsartan. J Hypertens. 2015;33:385‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Japanese Society of Nephrology . Evidence‐based clinical practice guideline for CKD 2013. Clin Exp Nephrol. 2014;18:346‐423. [Google Scholar]
- 11. Weber T, Wassertheurer S, Rammer M, et al. Validation of a brachial cuff‐based method for estimating central systolic blood pressure. Hypertension. 2011;58:825‐832. [DOI] [PubMed] [Google Scholar]
- 12. Kario K, Pickering TG, Umeda Y, et al. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401‐1406. [DOI] [PubMed] [Google Scholar]
- 13. Shimamoto K, Ando K, Fujita T, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res. 2014;37:253‐390. [DOI] [PubMed] [Google Scholar]
- 14. Pannarale G, Bebb G, Clark S, et al. Bias and variability in blood pressure measurement with ambulatory recorders. Hypertension. 1993;22:591‐598. [DOI] [PubMed] [Google Scholar]
- 15. Hermida RC, Calvo C, Ayala DE, et al. Administration time‐dependent effects of valsartan on ambulatory blood pressure in elderly hypertensive subjects. Chronobiol Int. 2005;22:755‐776. [DOI] [PubMed] [Google Scholar]
- 16. Hermida RC, Calvo C, Ayala DE, et al. Treatment of non‐dipper hypertension with bedtime administration of valsartan. J Hypertens. 2005;23:1913‐1922. [DOI] [PubMed] [Google Scholar]
- 17. Mengden T, Binswanger B, Spühler T, et al. The use of self‐measured blood pressure determinations in assessing dynamics of drug compliance in a study with amlodipine once a day, morning versus evening. J Hypertens. 1993;11:1403‐1411. [DOI] [PubMed] [Google Scholar]
- 18. Nold G, Strobel G, Lemmer B. Morning versus evening amlodipine treatment: effect on circadian blood pressure profile in essential hypertensive patients. Blood Press Monit. 1998;3:17‐25. [PubMed] [Google Scholar]
- 19. Wiens BL. Choosing an equivalence limit for noninferiority or equivalence studies. Control Clin Trials. 2002;23:2‐14. [DOI] [PubMed] [Google Scholar]
- 20. Asmar R, Gosse P, Quere S, et al. Efficacy of morning and evening dosing of amlodipine/valsartan combination in hypertensive patients uncontrolled by 5 mg of amlodipine. Blood Press Monit. 2011;16:80‐86. [DOI] [PubMed] [Google Scholar]
- 21. Grizzle JE. The two‐period change‐over design and its use in clinical trials. Biometrics. 1965;21:467‐480. [PubMed] [Google Scholar]
- 22. Castellana JV, Patel HI. Analysis of two‐period crossover design in a multicenter clinical trial. Biometrics. 1985;41:969‐977. [PubMed] [Google Scholar]
- 23. Piaggio G, Elbourne DR, Altman DG, et al. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA. 2006;295:1152‐1160. [DOI] [PubMed] [Google Scholar]
- 24. Piaggio G, Elbourne DR, Pocock SJ, et al. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308:2594‐2604. [DOI] [PubMed] [Google Scholar]
- 25. Juneja A, Aggarwal AR, Adhikari T, et al. Testing of hypothesis in equivalence and non inferiority trials—a concept. J Clin Diagn Res. 2016;10:LG01‐LG03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1‐13. [DOI] [PubMed] [Google Scholar]
- 27. McEniery CM, Cockcroft JR, Roman MJ, et al. Central blood pressure: current evidence and clinical importance. Eur Heart J. 2014;35:1719‐1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kario K, Hoshide S, Uchiyama K, et al. Dose timing of an angiotensin II receptor blocker/calcium channel blocker combination in hypertensive patients with paroxysmal atrial fibrillation. J Clin Hypertens (Greenwich). 2016;18:1036‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoshino A, Nakamura T, Matsubara H. The bedtime administration ameliorates blood pressure variability and reduces urinary albumin excretion in amlodipine‐olmesartan combination therapy. Clin Exp Hypertens. 2010;32:416‐422. [DOI] [PubMed] [Google Scholar]
- 30. Weycker D, Keskinaslan A, Levy DG, et al. Effectiveness of add‐on therapy with amlodipine in hypertensive patients receiving valsartan. Blood Press Suppl. 2008;2:5‐12. [DOI] [PubMed] [Google Scholar]
- 31. Allemann Y, Fraile B, Lambert M, et al. Efficacy of the combination of amlodipine and valsartan in patients with hypertension uncontrolled with previous monotherapy: the Exforge in Failure after Single Therapy (EX‐FAST) study. J Clin Hypertens (Greenwich). 2008;10:185‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hermida RC, Ayala DE, Fontao MJ, et al. Chronotherapy with valsartan/amlodipine fixed combination: improved blood pressure control of essential hypertension with bedtime dosing. Chronobiol Int. 2010;27:1287‐1303. [DOI] [PubMed] [Google Scholar]
- 33. Matsui Y, Eguchi K, O'Rourke MF, et al. Differential effects between a calcium channel blocker and a diuretic when used in combination with angiotensin II receptor blocker on central aortic pressure in hypertensive patients. Hypertension. 2009;54:716‐723. [DOI] [PubMed] [Google Scholar]
- 34. Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure‐lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213‐1225. [DOI] [PubMed] [Google Scholar]
- 35. Boutouyrie P, Achouba A, Trunet P, et al. Amlodipine‐valsartan combination decreases central systolic blood pressure more effectively than the amlodipine‐atenolol combination: the EXPLOR study. Hypertension. 2010;55:1314‐1322. [DOI] [PubMed] [Google Scholar]
- 36. Hermida RC, Ayala D, Calvo C. Optimal timing for antihypertensive dosing: focus on valsartan. Ther Clin Risk Manag. 2007;3:119‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cuspidi C, Meani S, Lonati L, et al. Short‐term reproducibility of a non‐dipping pattern in type 2 diabetic hypertensive patients. J Hypertens. 2006;24:647‐653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


