Abstract
The aim of this prospective study was to evaluate total sleep duration as a potential risk factor for the development of hypertension after a mean of 2.6 years of follow‐up. The study participants comprised 1715 Korean adults aged 40 to 70 years. The participants were without hypertension at baseline (2005–2008) and during follow‐up (2008–2011) to determine the incident cases of hypertension. Based on a self‐reported questionnaire, the individuals were stratified according to total sleep duration (<6 hours, 6–7.9 hours, 8–9.9 hours, ≥10 hours). Hypertension was defined according to the Eighth Joint National Committee (JNC 8) guidelines. After an average of 2.6 years of follow‐up, 164 (9.56%) participants developed hypertension. In multivariate adjusted models, the odds ratio for new‐onset hypertension was 1.71 (95% confidence interval, 1.01–2.89) in participants with a short sleep duration (<6 hours) compared with those who reported 6 to 7.9 hours of sleep. Long sleep duration (more than 8 hours) did not have any significant difference on incident hypertension. Among middle‐aged and elderly Korean adults, short sleepers were independently associated with a higher risk of developing hypertension.
Keywords: hypertension, Korean adults, prospective study, sleep duration
1. Introduction
Hypertension is a major public health concern worldwide because of its high prevalence and status as an important risk factor for renal and cardiovascular diseases.1, 2 High blood pressure (BP) aggravates the risk of diabetes and related complications of microvascular and macrovascular diseases.3, 4 Despite the implementation of various intervention and preventative measures, the prevalence of hypertension has increased to 35% in East Asia.5 The Korean National and Nutritional Examination Survey revealed that the prevalence of hypertension was 28.5% in Korea.6 Hypertensive patients typically must modify their lifestyle and dietary habits to reduce the effects of the condition. Given the high prevalence of hypertension and its potential consequences, there is substantial interest in understanding its lifestyle risk factors in population‐based longitudinal studies.
Sleep plays a vital role in maintaining the overall growth of the human body and mind. Epidemiological studies have suggested that chronic sleep deprivation results in various negative health outcomes, including obesity, type 2 diabetes, metabolic syndrome, and coronary heart disease.7, 8, 9, 10, 11 Sleep restriction impedes many essential homeostatic mechanisms and is accompanied by detrimental effects on the hormonal and metabolic systems.12 A sleep deficiency study observed a significant elevation in BP in healthy participants and in patients with preexisting hypertension after sleep was curtailed to 3.6 to 4.5 hours per night.13, 14
The association between short sleep duration and hypertension has recently been reported by many researchers.15, 16, 17, 18, 19 However, most of these studies have been conducted in Western populations. Hence, their results are not generalizable to other ethnicities, including Koreans. Moreover, the association between sleep duration and hypertension was based on a cross‐sectional design; very few prospective studies have been performed on the cause and effect relationship between these factors.18, 19, 20 Clinicians and policymakers should focus on these risk factors when developing preventive approaches to reduce the burden associated with hypertension. In contrast, other studies have shown that short sleep duration was not associated with hypertension in a longitudinal setting, which produced inconsistent findings.21, 22 Therefore, we need to study this relationship using a population‐based approach to identify sleep duration as a major predictor of hypertension and its associated morbidity.
In this study, we investigated the prospective association between short sleep duration and the risk of incident hypertension in a Korean cohort study. We hypothesized that short sleep duration (<6 hours) would be a predictor of progression to hypertension.
2. Methods
2.1. Study participants
Our study data were collected from a prospective cohort within the Korean Genome and Epidemiology Study on Atherosclerosis Risk in Rural Areas in the Korean General Population (KoGES‐ARIRANG), a continuing community‐based cohort. The basic aim of this longitudinal cohort was to estimate the risk factors for chronic metabolic diseases, such as diabetes, dyslipidemia, obesity, metabolic syndrome, and cardiovascular disease.23, 24 Participants in this survey comprised 5178 men and women aged 40 to 70 years. The study individuals lived in rural areas of Wonju and Pyeongchang in South Korea. This region is less likely to experience demographic shifts; therefore, the study participants should be available for long‐term follow‐up.
Figure 1 shows the flow chart description of the study population. The baseline study included 5178 adults from November 2005 to January 2008. The first follow‐up survey invited participants to take part in the study from April 2008 to January 2011, and 3862 (74.6%) participants responded. We excluded 2108 participants who had hypertension at baseline, 26 participants with a history or current diagnosis of cardiovascular disease, and 13 participants with missing data. Finally, 1715 participants without hypertension at baseline were recruited for the present analysis. Before the study began, written informed consent was obtained from the study participants to declare their willingness to take part in the survey. The protocol for the study was approved by the institutional review board of Wonju Christian Hospital (approval number: CR105024‐026).
Figure 1.
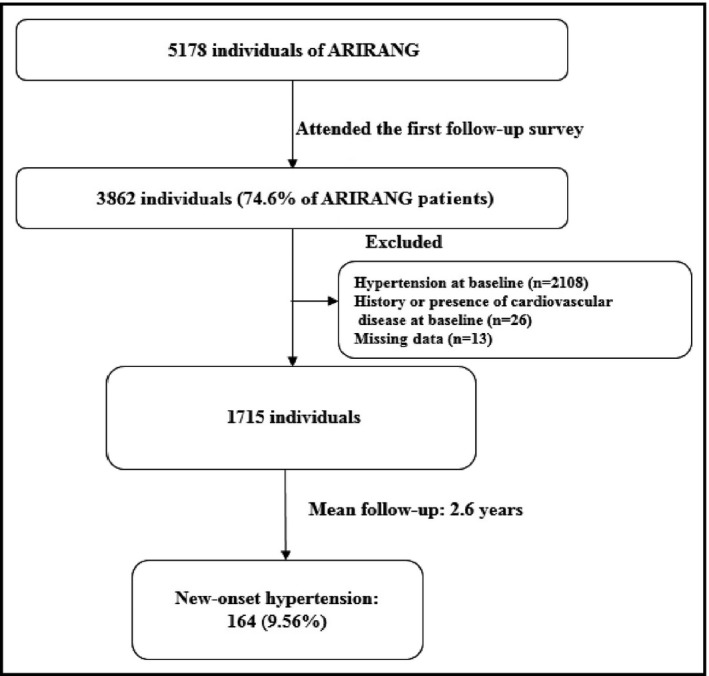
Description of the study population. ARIRANG indicates Atherosclerosis Risk in Rural Areas in the Korean General Population.
2.2. Data collection and measurements
At baseline and during the follow‐up survey, participants underwent a comprehensive health examination, which included medical history and a lifestyle questionnaire. Body weight and height were measured while participants wore light indoor clothing without shoes. Body mass index was calculated as the ratio of weight (in kilograms) to the square of height (in meters). Waist circumference was assessed using a tape measure (SECA‐200; SECA, Hamburg, Germany), as previously described.23 Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured on the right arm using a standard mercury sphygmomanometer (Baumanometer, Copiague, NY, USA) after the participant had relaxed for 5 minutes. Two measurements were performed at 5‐minute intervals, and the average of these two blood pressure readings was used in the study. The proper cuff size was selected for each participant according to mid‐arm circumference. Mean arterial pressure (MAP) represents the average arterial pressure during a single cardiac cycle and was calculated using the formula (SBP+2XDBP)/3. The reference range of MAP is 70 to 105 mm Hg. Baseline data on smoking, drinking, and regular exercise were collected with a self‐reported questionnaire (yes/no); the protocol has been described elsewhere.23
Daily energy intake was determined using a recall questionnaire and was represented in kcals/d. Education level was divided into elementary, middle school, high school, and college. Marital status was determined using the response of either “single” or “married” on a self‐reported questionnaire (yes/no). The monthly income of the study participants was categorized into low‐ (<1 million Korean won), medium‐ (1–2 million Korean won), and high‐ (>2 million Korean won) income groups.
After the participants had fasted for more than 12 hours or overnight, venous blood samples were drawn. Fasting blood glucose, triglycerides, total cholesterol, high‐density lipoprotein (HDL) cholesterol, and low‐density lipoprotein (LDL) cholesterol concentrations were measured using enzymatic methods by a chemistry analyzer (ADVIA 1650; Siemens, Tarrytown, NY, USA). The fasting insulin was analyzed by a double‐antibody radioimmunoassay using a commercial kit (Biosource Europe SA, Nivelles, Belgium). Insulin resistance was determined by Matthews and colleagues25 using a formula calculated as fasting plasma glucose (milligrams per deciliter)×fasting insulin (milli‐international units per milliliter))/22.5 homeostasis model assessment of insulin resistance. High‐sensitivity C‐reactive protein was measured using the Denka Seiken (Tokyo, Japan) assay.
2.3. Sleep duration
The study assessed sleep hours through a face‐to‐face interview. Sleep duration was assessed using a questionnaire conducted with the help of trained interviewers, who inquired, “What was your average daily sleep duration during the past year?” We divided the participants’ responses into four groups according to sleep duration: (1) <6 hours; (2) 6 to 7.9 hours; (3) 8 to 9.9 hours; and (4) 10 hours or more. We found that 6 to 7.9 hours was the median of sleep duration in the study sample; therefore, we chose 6 to 7.9 hours as a reference category.
2.4. End point definition
The study end point was the development of hypertension after 2.6 years of follow‐up based on the definition by the Eighth Joint National Committee (JNC 8) guidelines26 Hypertension was defined as an SBP of at least 140 mm Hg or a DBP of at least 90 mm Hg or current use of antihypertensive drugs.
2.5. Statistical analysis
Study results are expressed as the mean±(standard deviation) or frequency. To compare new‐onset hypertension and sleep duration (h/d), we conducted a two‐sample t test, analysis of variance, and chi‐square test (Fisher exact test) as applicable. We evaluated the association of baseline sleep duration with the incidence of new cases of hypertension at the follow‐up visits over 2.6 years. The 6 to 7.9 hour sleep duration was used as a reference category. Multivariable logistic regression was used to evaluate the independent association of baseline sleep duration with incident hypertension. We used four models (1 [crude], model 2, model 3, and model 4) with a continuous degree of adjustment. The study adjusted for several variables in order to determine the independent association of sleep duration on incident hypertension: age, sex, education, smoking, alcohol status, income, regular exercise, obesity, HDL‐C, trigylcerides, glucose, and MAP. The results were expressed as odds ratios (ORs) with 95% confidence intervals (CIs). P values <.05 were considered statistically significant, and all statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
3. Results
Baseline anthropometrical and biochemical characteristics and socioeconomic factors of the participants based on the new onset of hypertension during an average of 2.6 years are depicted in Table 1. The incidence of hypertension after 2.6 years of follow‐up was 9.56% (n=164). Participants who developed hypertension had a significantly higher baseline age, were more frequently male and current drinkers, and exhibited higher weight, waist circumference, SBP, DBP, MAP, triglycerides, total cholesterol, and LDL cholesterol levels than those who did not. The participants who developed hypertension had significantly lower income than those who were normotensive.
Table 1.
Baseline characteristics of study population by incident hypertension
| Incident Hypertension | P Value | ||
|---|---|---|---|
| No | Yes | ||
| No. (%) | 1551 (90.44) | 164 (9.56) | |
| Age, y | 53.31±8.18 | 55.79±8.00 | <.001 |
| Male sex | 552 (35.6) | 77 (47.0) | .005 |
| Weight, kg | 59.83±9.15 | 64.12±9.80 | <.001 |
| WC, cm | 80.59±8.44 | 85.80±7.70 | <.001 |
| BMI, kg/m2 | 23.69±2.87 | 25.10±2.71 | <.001 |
| SBP, mm Hg | 117.23±11.02 | 122.47±9.99 | <.001 |
| DBP, mm Hg | 73.82±7.39 | 75.32±6.91 | .014 |
| MAP, mm Hg | 88.30±7.66 | 91.03±7.09 | <.001 |
| Triglycerides, mg/dL | 127.39±84.06 | 143.88±84.43 | .017 |
| Total cholesterol, mg/dL | 196.90±35.78 | 202.83±34.51 | .043 |
| HDL cholesterol, mg/dL | 46.72±10.78 | 45.61±11.41 | .214 |
| LDL cholesterol, mg/dL | 115.26±30.86 | 120.43±28.45 | .040 |
| FBG, mg/dL | 92.95±18.90 | 95.31±14.96 | .122 |
| HOMA‐IR, U | 1.88±1.16 | 2.06±1.27 | .066 |
| hs‐CRP, mg/L | 1.68±4.59 | 2.48±7.79 | .202 |
| Regular exercise, % | 455/1549 (29.4) | 39/163 (23.9) | .171 |
| Current smoker, % | 253/1549 (16.3) | 29/162 (17.9) | .689 |
| Current drinker, % | 581/1546 (37.6) | 76/162 (46.9) | .025 |
| Daily energy intake, kcal | 2060.62±221.49 | 2086.59±239.51 | .185 |
| Education levels | .121 | ||
| Elementary | 697/1542 (45.2) | 86/164 (52.4) | |
| Middle school | 293/1542 (19.0) | 34/164 (20.7) | |
| High school | 357/1542 (23.2) | 31/164 (18.9) | |
| College | 195/1542 (12.6) | 13/164 (7.9) | |
| Sleep duration, h/d | .100 | ||
| <6 | 143 (9.2) | 25 (15.2) | |
| 6–7.9 | 851 (54.9) | 82 (50.0) | |
| 8–9.9 | 492 (31.7) | 51 (31.1) | |
| ≥10 | 65 (4.2) | 6 (3.7) | |
| Married | .378 | ||
| Yes | 1378/1545 (89.2) | 142/164 (86.6) | |
| No | 167/1545 (10.8) | 22/164 (13.4) | |
| Monthly income, ×104 KRW | .041 | ||
| <100 (low) | 519/1391 (37.3) | 72/151 (47.7) | |
| 100–200 (medium) | 397/1391 (28.5) | 38/151 (25.2) | |
| >200 (high) | 475/1391 (34.1) | 41/151 (27.2) | |
Data are expressed as number (percentage) or mean±standard deviation. BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; MAP, mean arterial pressure; SBP, systolic blood pressure; WC, waist circumference.
The baseline characteristics of the clinical data are shown in Table 2 based on the category of sleep duration. The participants were divided into four groups according to the sleep duration (<6 h/d, 6–7.9 h/d, 8–9.9 h/d, and ≥10 h/d). Participants who slept for <6 h/d were older than those in all other groups. Triglycerides, total cholesterol, LDL cholesterol, and fasting blood glucose were significantly higher in the participants who slept <6 h/d compared with the participants who slept 6 to 7.9 h/d. However, HDL cholesterol and high‐sensitivity C‐reactive protein levels were significantly higher in short sleepers compared with the reference category. Regular exercise, being a current drinker, and total energy intake were found to be significantly lower or less frequent in participants who got <6 hours of sleep each night compared with the participants who slept 6 to 7.9 h/d. Higher‐income participants who experienced fewer sleeping hours were also more likely to be hypertensive compared with the participants who slept 6 to 7.9 h/d. A sleep duration ≥10 h/d was associated with older age, high triglycerides, high cholesterol, low HDL cholesterol, high LDL cholesterol, and high fasting blood glucose levels, lower exercise, and an excessive alcohol intake compared with a sleep duration of 6 to 7.9 h/d. Participants with either a low sleep duration or a high sleep duration made up a smaller percentage of college students compared with the participants who slept 6 to 7.9 h/d.
Table 2.
Baseline characteristics of patient data according to sleep duration
| <6 h/d (n=168) | 6–7.9 h/d (n=933) | 8–9.9 h/d (n=543) | ≥10 h/d (n=71) | P Value | |
|---|---|---|---|---|---|
| Incident cases | 25 (14.9) | 82 (8.8) | 51 (9.4) | 6 (8.5) | .100 |
| Age, y | 56.36±8.12 | 53.02±8.23 | 53.42±7.91 | 54.80±8.49 | <.001 |
| Male sex | 49 (29.2) | 342 (36.7) | 211 (38.9) | 27 (38.0) | .152 |
| Weight, kg | 58.88±9.13 | 60.22±9.12 | 60.58±9.75 | 61.01±8.42 | .183 |
| WC, cm | 81.26±8.08 | 80.70±8.41 | 81.61±8.78 | 81.68±8.59 | .223 |
| BMI, kg/m2 | 23.97±3.02 | 23.75±2.77 | 23.85±3.08 | 24.25±2.43 | .447 |
| SBP, mm Hg | 117.84±11.24 | 117.67±11.18 | 118.07±10.68 | 115.70±11.19 | .399 |
| DBP, mm Hg | 73.98±7.03 | 74.08±7.39 | 73.85±7.42 | 73.63±7.24 | .924 |
| MAP, mm Hg | 88.60±7.58 | 88.61±7.72 | 88.59±7.53 | 87.66±7.78 | .792 |
| Triglycerides, mg/dL | 129.26±129.74 | 125.23±75.65 | 131.32±77.06 | 158.86±100.15 | .010 |
| Total cholesterol, mg/dL | 203.28±36.32 | 195.29±35.03 | 198.05±36.12 | 207.17±36.66 | .004 |
| HDL‐C, mg/dL | 48.43±10.91 | 46.29±10.89 | 46.82±10.71 | 44.54±10.67 | .039 |
| LDL‐C, mg/dL | 118.60±30.90 | 114.24±30.31 | 116.33±31.10 | 124.31±29.58 | .024 |
| FBG, mg/dL | 94.80±23.54 | 92.93±16.63 | 92.39±14.94 | 98.42±40.83 | .044 |
| HOMA‐IR, U | 1.97±1.31 | 1.88±1.08 | 1.86±1.04 | 2.20±2.25 | .111 |
| hs‐CRP, mg/L | 1.900±3.681 | 1.506±3.132 | 2.178±7.512 | 1.515±2.254 | .087 |
| Regular exercise | 25/167 (15.0) | 307/931 (33.0) | 148/543 (27.3) | 14/71 (19.7) | <.001 |
| Current smoker | 23/168 (13.7) | 150/931 (16.1) | 98/541 (18.1) | 11/71 (15.5) | .513 |
| Current drinker | 52/168 (31.0) | 345/931 (37.1) | 231/538 (42.9) | 29/71 (40.8) | .027 |
| Energy intake (calorie) | 2001.19±202.08 | 2068.67±227.64 | 2075.23±218.85 | 2044.29±227.55 | .001 |
| Education levels | <.001 | ||||
| Elementary | 105/167 (62.9) | 376/930 (40.4) | 260/539 (48.2) | 42/70 (60.0) | |
| Middle school | 29/167 (17.4) | 180/930 (19.4) | 108/539 (20.0) | 10/70 (14.3) | |
| High school | 23/167 (13.8) | 234/930 (25.2) | 117/539 (21.7) | 14/70 (20.0) | |
| College | 10/167 (6.0) | 140/930 (15.1) | 54/539 (10.0) | 4/70 (5.7) | |
| Married | .376 | ||||
| Yes | 143/168 (85.1) | 836/929 (90.0) | 478/541 (88.4) | 63/71 (88.7) | |
| No | 25/168 (14.9) | 93/929 (10.0) | 63/541 (11.6) | 8/71 (11.3) | |
| Monthly income, ×104 KRW | <.001 | ||||
| <100 (low) | 92/154 (59.7) | 270/849 (31.8) | 195/476 (41.0) | 34/63 (54.0) | |
| 100–200 (medium) | 33/154 (21.4) | 255/849 (30.0) | 134/476 (28.2) | 13/63 (20.6) | |
| >200 (high) | 29/154 (18.8) | 324/849 (38.2) | 147/476 (30.9) | 16/63 (25.4) |
Data are expressed as number (percentage) or mean±standard deviation. BMI, body mass index; DBP, diastolic blood pressure; FBG, fasting blood glucose; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostasis model assessment of insulin resistance; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; MAP, mean arterial pressure; SBP, systolic blood pressure; WC, waist circumference.
Table 3 shows the ORs (95% CIs) for new onset of hypertension according to baseline sleep duration. The reference group slept for approximately 6 to 7.9 hours. In the unadjusted model (model 1), the participants who slept <6 h/d were significantly more likely to develop hypertension compared with participants in the reference category. The unadjusted OR in participants who slept <6 h/d was 1.991 (95% CI, 1.201–3.275). Participants with a long sleep duration did not show any significant difference in OR, 1.129 (95% CI, 0.470–2.709) for developing hypertension compared with those who slept 6 to 7.9 hours. In model 2, after adjusting for age and sex, the association between sleep duration and incident hypertension was similar to that of the crude model (model 1). Participants who slept for <6 h/d were found to be at risk for developing hypertension. After adjustment for age, sex, education, smoking, alcohol status, income, regular exercise, and obesity (model 3), a slight reduction in OR was observed in participants who slept <6 hours daily. Model 4 adjusted for the biochemical parameters and metabolic factors along with those of model 2 and model 3. The results continued to be significant for incident hypertension in participants who slept <6 h/d compared with participants who slept for 6 to 7.9 h/d. The corresponding OR for developing hypertension after adjustment for several variables in model 4 was 1.712 (95% CI, 1.014–2.890) compared with the participants with a sleep duration of 6 to 7.9 hours. In contrast, participants who had long sleeping duration did not have increased risk of hypertension (both unadjusted and adjusted models).
Table 3.
ORs (95% confidence intervals) for new‐onset hypertension according to baseline sleep duration
| Incident Hypertension | ||||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |
| Sleep duration, h | ||||
| <6 | 1.991 (1.210–3.275)a | 1.854 (1.115–3.082)a | 1.761 (1.048–2.958)a | 1.712 (1.014–2.890)a |
| 6–7.9 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 8–9.9 | 1.148 (0.779–1.692) | 1.103 (0.746–1.629) | 1.036 (0.698–1.537) | 1.053 (0.708–1.565) |
| ≥10 | 1.129 (0.470–2.709) | 1.070 (0.443–2.583) | 0.965 (0.396–2.352) | 0.940 (0.383–2.309) |
Model 1: Crude odds ratio (OR).
Model 2: Adjusted OR: adjusted for age and sex.
Model 3: Adjusted OR: adjusted for age, sex, education, smoking, alcohol status, income, regular exercise, and obesity.
Model 4: Adjusted OR: adjusted for age, sex, education, smoking, alcohol status, income, regular exercise, obesity, high‐density lipoprotein cholesterol, triglyceride, glucose, and mean arterial pressure.
P<.05 compare with the reference.
4. Discussion
Short sleep duration (sleeping <6 hours) was significantly associated with new onset of hypertension over 2.6 years of follow‐up in a middle‐aged and elderly Korean population. This prospective study therefore suggests that a short sleep duration is an important positive predictor for the development of hypertension. However, participants who achieved long sleeping duration (≥10 hours) did not show any significant difference in incident hypertension compared with the study participants who slept for 6 to 7.9 h/d.
In this prospective study, the overall incidence of hypertension was 9.56% after an average of 2.6 years of follow‐up. We observed an independent association with an OR of 1.712 (95% CI, 1.014–2.890) for new‐onset hypertension in participants who slept <6 h/d compared with those who slept 6 to 7.9 h/d. Previously published studies that have defined the association between objective sleep duration and risk of hypertension reported an OR of 1.66 (95% CI, 1.35–2.04) and 1.56 (95% CI, 1.14–2.11), which were quite similar to that found in our study. However, these previous studies used a cross‐sectional design.16, 27
Epidemiological studies and a few meta‐analyses have reported the relationship between short sleep duration and risk of hypertension.17, 18, 20, 22 These studies differed by sample size, follow‐up period, ethnic background, criteria of sleep duration, and method of hypertension assessment. In a recent systematic and meta‐analysis of longitudinal studies, Guo and colleagues18 reported that short sleep duration was significantly associated with the development of hypertension; the relative risk in the study was 1.23 (95% CI, 1.06–1.42). Meng and colleagues28 found a similar observation and reported a relative risk of 1.21 (95% CI, 1.05–1.40) for incident hypertension in participants with a short sleep duration. The biological mechanism underlying the link between short sleep duration and hypertension remains unknown. The association between sleep deprivation and hypertension may be mediated by an increase in sympathetic nervous activity.29 Sleep deprivation has also been reported to compromise insulin sensitivity and further imbalances the secretion of hunger and satiety hormones (leptin and ghrelin), which lead to obesity and type 2 diabetes, known risk factors for hypertension.30 Other reports have also suggested that the involvement of endothelial dysfunction and an unhealthy lifestyle could elevate the risk of hypertension in people with sleep deprivation.31
It has been reported that sleep quality also plays a significant role in the pathophysiology of incident hypertension. The quality of sleep was calculated by Pittsburgh Sleep Quality Index (PSQI) and recently researchers are interested in measuring sleep quality.32 Previously published studies have reported that poor sleep quality in individuals was associated with higher prevalence of hypertension.33, 34 Lu and colleagues35 highlighted the additive interaction of poor sleep quality and short sleep duration on the prevalence of hypertension in adult Chinese men. A follow‐up study is needed to understand the true relationship between sleep quality and risk of future development of hypertension. However, our prospective study did not evaluate sleep quality and its association with incident hypertension.
A few cross‐sectional and longitudinal studies have demonstrated that short sleep duration is not associated with a greater risk of hypertension. In other words, short sleep duration may not be a risk factor for hypertension in population‐based studies. Bansil and colleagues36 and Hall and colleagues37 reported an insignificant association between short sleep duration and hypertension from cross‐sectional studies. The ORs in their studies were 1.03 (95% CI, 0.91–1.18) and 1.10 (95% CI, 0.75–1.63), respectively.36, 37 Longitudinal studies conducted by Lopez‐Garcia and colleagues21 and Knutson and colleagues21, 22 also found an insignificant relationship between short sleep duration and incident hypertension in a Spanish and a US population, respectively. Nonetheless, none of the longitudinal studies thus far have demonstrated an association between long sleep duration and incident hypertension, although cross‐sectional studies involving 90 356 participants in a meta‐analysis by Wang and colleagues20 showed that there was a significant association between long sleep duration and risk of hypertension. Previous studies have also identified independent, U‐shaped relationships between sleep duration and risk of type 2 diabetes8 and cardiovascular outcome.38 Similarly, Fang and colleagues39 identified a U‐shaped relationship between hours of sleep and hypertension in a cross‐sectional study. However, our prospective study did not report a U‐shaped association between sleep duration and incident hypertension; the adjusted OR for participants with long sleeping hours and incident hypertension was 0.940 (95% CI, 0.383–2.309) compared with participants who slept for 6 to 7.9 hours. The reason for this finding is unknown; therefore, more prospective studies are needed to verify our results with other sleep assessment tools.
To our knowledge, only one prospective study has been reported in the Korean population, and it produced similar findings to our study results; short sleep duration was a significant risk factor for new‐onset hypertension.40 However, the study had different sleep duration categories, and the report focused on genetic associations. The novelty of this study was a population‐based longitudinal study with large sample size and adjusted possible confounding factors including socioeconomic factors, energy intake, cholesterol profiles, and mean arterial blood pressure.
4.1. Study Limitations
Some limitations of this study need to be considered. First, the information in our study about sleep duration was based on self‐reported questionnaires that mean the information could not obtain the objective assessment of sleep duration related to sleep quality. Nonetheless, questionnaire‐based measures of sleep have demonstrated good agreement against quantitative sleep assessments.17, 41 Second, our data did not provide information about depressive symptoms, which can affect sleep duration.42 Third, we did not report information regarding insomnia or sleep‐disordered breathing, which are both important confounding factors of hypertension. Fourth, our cohort follow‐up period was only 2.6 years; therefore, our findings should be verified by a longer prospective study. Finally, we did not exclude participants who were currently taking medications (neuroleptic drugs, glucocorticoids) that might have disturbed their sleep patterns.43
5. Conclusions
Our study suggests that short sleep duration is independently associated with the development of hypertension among middle‐aged and elderly Korean adults. The study highlights the importance of sleep duration as a risk predictor of incident hypertension and encourages clinicians and researchers to promote healthy sleep habits in society.
Disclosures
The authors of this manuscript have no conflicts of interest to disclose.
Acknowledgments
We are very grateful to all the participants in the KoGES‐ARIRANG study for their continuing interest and participation in the study. This study was supported in part by a grant from the Korea Centers for Disease Control and Prevention (2005‐E71013‐00, 2006‐E71002‐00, 2007‐E71013‐00, 2008‐E71004‐00, 2009‐E71006‐00, 2010‐E71003‐00). No additional external funding was received for this study.
Yadav D, Hyun DS, Ahn SV, Koh S-B, Kim JY. A prospective study of the association between total sleep duration and incident hypertension. J Clin Hypertens. 2017;19:550-557. 10.1111/jch.12960
Contributor Information
Sang‐Baek Koh, Email: kohhj@yonsei.ac.kr.
Jang Young Kim, Email: kimjy@yonsei.ac.kr.
References
- 1. Zheng L, Sun Z, Li J, et al. Pulse pressure and mean arterial pressure in relation to ischemic stroke among patients with uncontrolled hypertension in rural areas of China. Stroke. 2008;39:1932–1937. [DOI] [PubMed] [Google Scholar]
- 2. Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood‐pressure‐related disease, 2001. Lancet. 2008;371:1513–1518. [DOI] [PubMed] [Google Scholar]
- 3. Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321:412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lago RM, Singh PP, Nesto RW. Diabetes and hypertension. Nat Clin Pract Endocrinol Metab. 2007;3:667. [DOI] [PubMed] [Google Scholar]
- 5. Harrison W, Marshall T. The epidemiology of blood pressure in East Asia. J Hum Hypertens. 2006;20:97–99. [DOI] [PubMed] [Google Scholar]
- 6. Kim HC, Oh SM. Noncommunicable diseases: current status of major modifiable risk factors in Korea. J Prev Med Public Health. 2013;46:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring). 2008;16:643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29:657–661. [DOI] [PubMed] [Google Scholar]
- 9. Ferrie JE, Shipley MJ, Cappuccio FP, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30:1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cappuccio FP, Cooper D, D'Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta‐analysis of prospective studies. Eur Heart J. 2011;32:1484–1492. [DOI] [PubMed] [Google Scholar]
- 11. Kim JY, Yadav D, Ahn SV, et al. A prospective study of total sleep duration and incident metabolic syndrome: the ARIRANG study. Sleep Med. 2015;16:1511–1515. [DOI] [PubMed] [Google Scholar]
- 12. McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allostatic load. Metabolism. 2006;55:S20–S23. [DOI] [PubMed] [Google Scholar]
- 13. Lusardi P, Mugellini A, Preti P, Zoppi A, Derosa G, Fogari R. Effects of a restricted sleep regimen on ambulatory blood pressure monitoring in normotensive subjects. Am J Hypertens. 1996;9:503–505. [DOI] [PubMed] [Google Scholar]
- 14. Lusardi P, Zoppi A, Preti P, Pesce RM, Piazza E, Fogari R. Effects of insufficient sleep on blood pressure in hypertensive patients: a 24‐h study. Am J Hypertens. 1999;12:63–68. [DOI] [PubMed] [Google Scholar]
- 15. Kim J, Jo I. Age‐dependent association between sleep duration and hypertension in the adult Korean population. Am J Hypertens. 2010;23:1286–1291. [DOI] [PubMed] [Google Scholar]
- 16. Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–1014. [DOI] [PubMed] [Google Scholar]
- 17. Gangwisch JE, Heymsfield SB, Boden‐Albala B, et al. Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey. Hypertension. 2006;47:833–839. [DOI] [PubMed] [Google Scholar]
- 18. Guo X, Zheng L, Wang J, et al. Epidemiological evidence for the link between sleep duration and high blood pressure: a systematic review and meta‐analysis. Sleep Med. 2013;14:324–332. [DOI] [PubMed] [Google Scholar]
- 19. Song Q, Liu X, Wang X, Wu S. Age‐ and gender‐specific associations between sleep duration and incident hypertension in a Chinese population: the Kailuan study. J Hum Hypertens. 2016;30:503–507. [DOI] [PubMed] [Google Scholar]
- 20. Wang Q, Xi B, Liu M, Zhang Y, Fu M. Short sleep duration is associated with hypertension risk among adults: a systematic review and meta‐analysis. Hypertens Res. 2012;35:1012–1018. [DOI] [PubMed] [Google Scholar]
- 21. Lopez‐Garcia E, Faubel R, Guallar‐Castillon P, Leon‐Munoz L, Banegas JR, Rodriguez‐Artalejo F. Self‐reported sleep duration and hypertension in older Spanish adults. J Am Geriatr Soc. 2009;57:663–668. [DOI] [PubMed] [Google Scholar]
- 22. Knutson KL, Van Cauter E, Rathouz PJ, et al. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med. 2009;169:1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim JY, Ahn SV, Yoon JH, et al. Prospective study of serum adiponectin and incident metabolic syndrome: the ARIRANG study. Diabetes Care. 2013;36:1547–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yadav D, Lee ES, Kim HM, et al. Prospective study of serum uric acid levels and incident metabolic syndrome in a Korean rural cohort. Atherosclerosis. 2015;241:271–277. [DOI] [PubMed] [Google Scholar]
- 25. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 26. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 27. Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela‐Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meng L, Zheng Y, Hui R. The relationship of sleep duration and insomnia to risk of hypertension incidence: a meta‐analysis of prospective cohort studies. Hypertens Res. 2013;36:985–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. [DOI] [PubMed] [Google Scholar]
- 30. Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141:846–850. [DOI] [PubMed] [Google Scholar]
- 31. Sauvet F, Leftheriotis G, Gomez‐Merino D, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol (1985). 2010;108:68–75. [DOI] [PubMed] [Google Scholar]
- 32. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 33. Kara B, Tenekeci EG. Sleep quality and associated factors in older Turkish adults with hypertension: a pilot study. J Transcult Nurs 2016;doi: 10.1177/1043659615623330. [DOI] [PubMed] [Google Scholar]
- 34. Liu RQ, Qian Z, Trevathan E, et al. Poor sleep quality associated with high risk of hypertension and elevated blood pressure in China: results from a large population‐based study. Hypertens Res. 2016;39:54–59. [DOI] [PubMed] [Google Scholar]
- 35. Lu K, Chen J, Wu S, Chen J, Hu D. Interaction of sleep duration and sleep quality on hypertension prevalence in adult Chinese males. J Epidemiol. 2015;25:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bansil P, Kuklina EV, Merritt RK, Yoon PW. Associations between sleep disorders, sleep duration, quality of sleep, and hypertension: results from the National Health and Nutrition Examination Survey, 2005 to 2008. J Clin Hypertens (Greenwich). 2011;13:739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hall MH, Muldoon MF, Jennings JR, Buysse DJ, Flory JD, Manuck SB. Self‐reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–444. [DOI] [PubMed] [Google Scholar]
- 39. Fang J, Wheaton AG, Keenan NL, Greenlund KJ, Perry GS, Croft JB. Association of sleep duration and hypertension among US adults varies by age and sex. Am J Hypertens. 2012;25:335–341. [DOI] [PubMed] [Google Scholar]
- 40. Kim SJ, Lee SK, Kim SH, et al. Genetic association of short sleep duration with hypertension incidence–a 6‐year follow‐up in the Korean genome and epidemiology study. Circ J. 2012;76:907–913. [DOI] [PubMed] [Google Scholar]
- 41. Lockley SW, Skene DJ, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999;8:175–183. [DOI] [PubMed] [Google Scholar]
- 42. Mezick EJ, Hall M, Matthews KA. Are sleep and depression independent or overlapping risk factors for cardiometabolic disease? Sleep Med Rev. 2011;15:51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Foral P, Knezevich J, Dewan N, Malesker M. Medication‐induced sleep disturbances. Consult Pharm. 2011;26:414–425. [DOI] [PubMed] [Google Scholar]


