Abstract
Increased arterial stiffness is an important determinant of cardiovascular risk, able to predict morbidity and mortality, and closely associated with ageing and blood pressure. The aims of this study were: (1) To determine the age‐dependent reference pulse wave velocity (PWV), and compare it with values from hypertensive patients, and (2) to evaluate the impact of isolated and untreated hypertension on arterial stiffness. A total of 1079 patients were enrolled and divided into a control group (NT) of asymptomatic normotensive patients and a group of asymptomatic hypertensive patients (HT). Blood pressure, carotid‐femoral PWV, and body mass index were measured in each subject, whose blood was drawn for laboratory tests. Aortic mean PWV in the NT group was 6.85 ± 1.66 m/s, which increased linearly (R 2 = 0.62; P < .05) with age. In patients over 50 years of age, PWV was significantly higher than in younger patients (8.35 vs 5.92 m/s, respectively, P < .001). This significant difference persisted when observing male and female patients separately. In the hypertensive group, mean PWV value was 8.04 ± 1.8 m/s (range 4.5‐15.8 m/s) and increased (R 2 = 0.243; P < .05) with age. The PWV increase in HT was significantly higher (0.93 m/s per decade, P < .001) than in NT (0.44 m/s per decade). Our study provides normal values of PVW per decade, and shows that these values increase with age, especially after 50 years of age, particularly in HT patients. This stiffness growth rate may be responsible for increased cardiovascular risk in both groups.
Keywords: arterial hypertension, arterial stiffness, epidemiology, population based study, pulse wave velocity, South America
1. INTRODUCTION
It is currently accepted that increased central arterial stiffness (AS) is an important determinant of cardiovascular (CV) risk.1 Reported epidemiological studies showed that increased AS predicts mortality and morbidity, independently of other CV risk factors.1, 2, 3 Similarly, the association of aortic stiffness with ageing and blood pressure (BP) has been well established. Furthermore, previous studies reported the association between pulse wave velocity (PWV) and BP independent risk factors, such as dyslipidemia,4 smoking,5 heart rate,6 and diabetes.7, 8
Ever since the publication of the pioneer work by John W. Fischer, the role of high BP in CV risk and disease has been widely investigated.9 Accordingly, the association of AS with the pathogenesis of systemic hypertension was analyzed.10 Moreover, the prognostic value of PWV has been associated to arterial ageing independently of traditional risk factors other than arterial hypertension.11 This raised the question on the need to find a relationship between normal PWV values and age (ie, arterial ageing), evaluated in normotensive populations and then in hypertensive patients with no other CV risk factors, to explain the consequences of hypertension on AS. Currently, PWV reference values, according to age used in clinical practice, come from a European population published by the Reference Values for Arterial Stiffness Collaboration Group.12 Our group recently reported PWV normal values related to each decade of age in healthy normotensive patients with no family history of hypertension.13
However, the impact of isolated hypertension on aortic elasticity in non‐treated patients has not been specifically analyzed.
Consequently, the purpose of this research was to evaluate the effects of isolated and untreated hypertension on AS in a representative sample a city from Argentina. Thus, the specific aims of this work were:
To determine normal PWV values for each decade of life in a large group of normotensive patients without parental history of hypertension or other CV risk factors
To determine PWV values in a subgroup of patients with hypertension as an isolated risk factor
To compare these values of PWV with healthy normotensive patients from this community‐based study
2. METHODS
This epidemiological study is a part of a project that started in 2010 in Tandil, Buenos Aires Province, aimed at investigating the prevalence of CV risk factors in a well‐characterized population. Preliminary results have been recently reported.13, 14 This work is a descriptive case‐control, observational, and cross‐sectional population‐based study and was divided into 2 well‐differentiated analysis: (1) the quantification of normal values of PWV corresponding to each decade of age in a normotensive population of normotensive parents, and (2) to investigate the effects of isolated arterial hypertension on PWV in non‐treated patients.
Tandil city is located 360 km to the west of Buenos Aires. According to the National Institute of Statistics and Census report of 2010, the population was 123 871 people.15 Ethnically, the population is a mix of European immigration influx and native population.
Patients included in this investigation were evaluated in the Rodriguez Larreta Hospital and in the Tandil Institute of Cardiology. The research protocol was evaluated and approved by the local Institutional Ethics and Research Committee. The study was carried out according to the Declaration of Helsinki and the Good Clinical Practice Guidelines. A written consent was signed by all patients included in this research, as requested by the Good Clinical Practice Guidelines.
From March 2010 to December 2014, 1079 consecutive patients were enrolled. The recruited patients were divided into a control group (healthy normotensive patients) and a hypertensive group (with systemic hypertension as their unique risk factor). During their routine checkup, we measured the carotid‐femoral PWV in both groups.
2.1. Normotensive group (NT)
We included asymptomatic non‐smoking patients, with normal BP values, without diabetes, dyslipidemia, and no history of hypertension in first‐degree relatives. They met all the following criteria.
Inclusion criteria:
Asymptomatic patients aged between 10 and 87 years with no history of cardiovascular, pulmonary or renal disease
Normal BP at the time of examination (BP ≤ 140/90 mm Hg in adults and BP ˂ 90th percentile in patients ˂16 years of age)16
Total blood cholesterol levels <5.172 mmol/L
Serum triglycerides levels <1.694 mmol/L
Glycemia <6.11 mmol/L
Non‐smoking history
Patient with normotensive first‐degree relatives with no history of CV disease before 65 years of age
Exclusion criteria:
Patients with hypertension at the time of the examination (systolic BP ≥ 140 mm Hg and/or diastolic BP ≥ 90 mm Hg).17 High BP states in children and adolescents were determined according to sex, age, and body height, following the criteria set by the report of the American Pediatrics Association and the European Society of Hypertension.16
History or symptoms of CV disease
History of diabetes
Record of serum creatinine levels >132.6 μmol/L
History of smoking
Record of lipid profile with one or more of the following conditions: serum triglycerides ≥1.694 mmol/L, total blood cholesterol ≥5.172 mmol/L
Patients with body mass index (BMI) ≥ 30 kg/m2
2.2. Hypertensive group (HT)
In this cohort, asymptomatic non‐smoking patients that were aged 10‐98 years were included. Their only risk factor was hypertension.
Inclusion criteria:
Patients with confirmed diagnosis of hypertension (detected during three separate visits); not undergoing pharmacological treatment; and no history of CV, pulmonary, or renal disease. In this population, hypertension was the only CV risk factor. Hypertension was defined as BP values >140/90 mm Hg in adults and BP ≥95th percentile in patients ˂16 years old).16
Total blood cholesterol levels <5.172 mmol/L
Serum triglycerides levels <1.694 mmol/L
Glycemia <6.11 mmol/L
Non‐smoking patients
Patient with normotensive first‐degree relatives with no history of CV disease before 65 years of age
Exclusion criteria:
Patients under treatment for hypertension at the time of the examination
History or symptoms of CV disease (stroke, acute coronary syndrome, cardiac ischemic disease, or any revascularization procedures)
Diabetes mellitus, glycaemia >6.11 mmol/L or under treatment
History of sedentarism
Serum creatinine levels >132.6 μmol/L
History of smoking
BMI > 30 kg/m2
Lipid profile with one or more of the following conditions: serum triglycerides ≥1.694 mmol/L, total blood cholesterol ≥5.172 mmol/L
2.3. Blood pressure measurements
BP was measured three times after the patient remained seated for at least 10 minutes. All measurements were performed using a digital automatic BP monitor (Omron model 705IT).
2.4. Pulse wave velocity measurement
All measurements were performed in a quiet room with stable room temperature (22 ± 1°C). The patient remained in a supine position for at least 10 minutes. Aortic PWV was determined by recording the carotid and femoral waveforms using a previously validated technology (Arteriometer, Model V100).13, 14, 18, 19 The hardware uses two high‐fidelity silicon piezoresistive pressure sensors (Motorola MPX 2050, Motorola Inc.) connected to an amplifier. The signal was acquired in a PC computer. During data acquisition, both pressure sensors were simultaneously placed in the left carotid and left femoral arteries. Data were continuously recorded, while pressure waves were monitored on the computer screen using specific software as previously described. This software works in a Windows environment, acquiring an online digitized pressure wave that allows several PWV measurements along a single continuous record, which includes at least 10 cardiac cycles. The distance between sensors is needed for the online PWV calculation. PWV value for each patient was expressed as the mean and standard deviation. To ensure a reliable measurement, special care was taken in monitoring that the standard deviations of measurements were less than 10%. Two physicians acquired all data and calculated PWV; one of them operated the arteriometer to record pressure waves and the other one operated the computer. When technical mistakes or low signal quality were detected, the procedure was repeated. All measurements were recorded by duplicate in all patients.20 Finally, in accordance with European recommendations,12, 17, 20, 21 PWV values were corrected by multiplying the carotid‐femoral distance by 0.8. To compare the normal values calculated from our data, we considered, as suggested by the European recommendations, 10 m/s as the normal upper PWV limit value.
2.5. Laboratory measurements
Venous blood samples were obtained from all patients using standard techniques and processed to determine glycemia, serum triglycerides, total blood cholesterol, and creatinine.
2.6. Data analysis
Measured and calculated values were expressed as mean value ± SD. The normo‐ and hypertensive groups were categorized per decade of age (Group 1: 10‐19 years, Group 2: 20‐29 years, Group 3: 30‐39 years, Group 4: 40‐49 years, Group 5: 50‐59 years, Group 6: 60‐69 years, group 7: ≥70 years old). Differences among groups were tested by means of ANOVA and Bonferroni's post‐test. A Pearson's correlation analysis was performed to examine the age‐related increases of aortic stiffness. To evaluate possible structural or hemodynamic arterial adaptations, we performed a multiple regression analysis in a sub‐group of 400 normotensive patients where PWV, mean BP (MBP), and heart rate (HR) were considered dependent variables related to age, the independent variable. The Multiple Linear Regression identity formula was: PWV = −0.744+ (0.0704·Age) + (0.00643·HR) + (0.0531·MBP). The statistical software that was used was IBM SPSS 20.0. A value of P < .05 was considered statistically significant.
3. RESULTS
Carotid‐femoral PWV values calculated in NT and HT groups are shown in box plots of Figure 1. Male and female patients were distributed in each age group to achieve a homogeneous amount of each gender in both the NT and HT subgroups.
Figure 1.
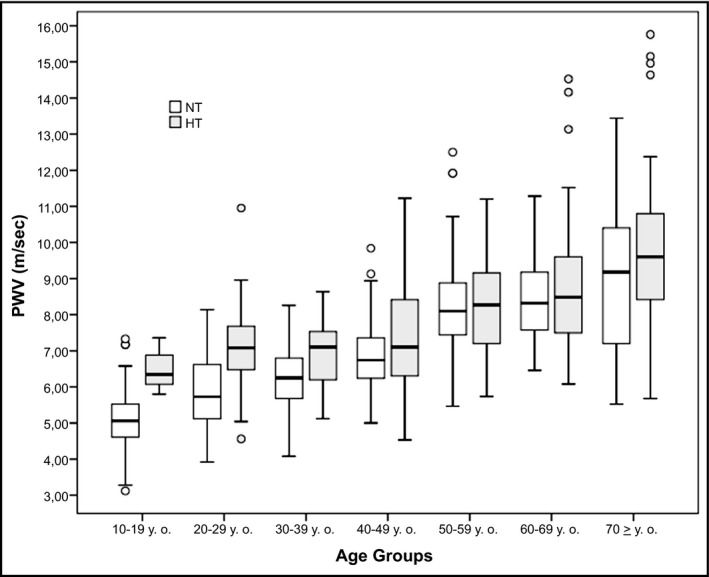
Box plot showing the carotid‐femoral pulse wave velocity (PWV) for each age group in healthy (NT) and hypertensive patients (HT)
3.1. NT group
Of the 780 healthy patients that were included in this group (Table 1), mean age was 40.0 ± 18.5 years (range 10‐87 years), 414 were male (53.1%), and 366 were female (46.9%). Aortic mean PWV in the healthy group was 6.85 ± 1.66 m/s (range 3.12‐13.44 m/s). Only 29 of the 780 healthy patients (3.72%) exceeded the PWV cut‐off point of 10 m/s, established by expert consensus.
Table 1.
Clinical and hemodynamic variables of the analyzed population
| Variable | Healthy normotensive group (n = 780) | Hypertensive group (n = 299) | P value |
|---|---|---|---|
| Age (years) | 39.8 ± 18.5 | 50.8 ± 19 | .001 |
| BMI (kg/m2) | 24.1 ± 3.8 | 25.5 ± 3.4 | .08 |
| Waist (cm) | 87.7 ± 14.4 | 91.7 ± 13 | .08 |
| SBP (mm Hg) | 121.0 ± 11.6 | 148 ± 13 | .0001 |
| DBP (mm Hg) | 74.8 ± 8.6 | 85.4 ± 9 | .001 |
| PP (mm Hg) | 46.1 ± 8.4 | 62.6 ± 9 | .001 |
| Total serum cholesterol (mmol/L) | 43.68 ± 5.9 | 44.2 ± 7.5 | ns |
| Serum triglycerides (mmol/L) | 1.43 ± 0.23 | 1.47 ± 22 | ns |
| Glycemia (mmol/L) | 4.6 ± 0.53 | 4.78 ± 0.72 | .41 |
BMI, body mass index; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; SBP, systolic blood pressure.
Values are mean ± SD. Statistical significance was determined by a Student's t test.
Figure 2 shows the mean values of PWV, range, and the 95% confidence intervals in patients divided into 7 age groups. An age‐related increase (R 2 = 0.62; P < .05) in PWV values was observed.
Figure 2.
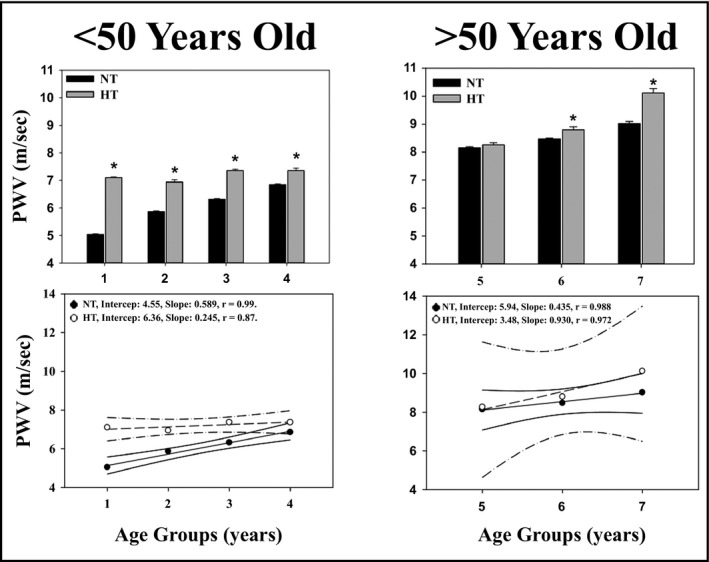
Pulse wave velocity according to age groups in normotensive and hypertensive patients. PWV vs age groups in normotensive (NT) and hypertensive (HT) patients, under (left panels) or over (right panels) 50 y of age. PWV mean values (upper panels) and correlations (lower panels) for each decade are shown. Age group (1): 10‐19 y of age; age group (2): 20‐29 y of age; (…); age group (7): 70‐79 y of age. *P < .05 with respect to the NT group
3.2. Gender study
No significant differences in terms of PWV values were found between men and women (6.81 vs 6.89 m/s, respectively). In all patients over 50 years, PWV values were significantly higher than younger patients (8.35 vs 5.92 m/s; respectively; Table 2). When gender is considered, this difference remained statistically significant in both men and women.
Table 2.
Impact of gender on PWV in healthy and hypertensive patients
| Healthy normotensive group | Hypertensive group | |
|---|---|---|
| Male (<50 y) | 5.85 ± 1.1 (3.1‐9.8) n = 265 | 7.2 ± 1.4 (5.1‐10.9) n = 84 ** |
| Male (≥50 y) | 8.4 ± 1.4 (5.5‐13.4) n = 146 * | 8.8 ± 2.0 (5.6‐15.7) n = 95 * |
| Female (<50 y) | 6.0 ± 0.9 (3.2‐9.1) n = 222 | 7.1 ± 1.3 (4.5‐11.2) n = 69 ** |
| Female (≥50 y) | 8.1 ± 1.1 (5.4‐12.3) n = 147 * | 8.6 ± 1.5 (5.7‐14.5) n = 51 *,** |
PWV [m/s] ± SD (range) of healthy and hypertensive patients, according to gender and age subgroups. Statistical significance assessed using the ANOVA test: *P < .001 with respect to patients under 50 y of age. Statistical significance assessed using the Student's t test: **P < .01 with respect to the normotensive group.
3.3. HT group
Of 299 hypertensive patients included in this group (51.0 ± 19.0 years of age, range: 14‐85 years), 179 were male (59.9%) and 120 were female (40.1%; Table 1). Mean PWV value of hypertensive patients was 8.04 ± 1.8 m/s (range: 4.5‐15.8) and was significantly higher than in NT patients (P < .001). Figure 1 (black box plot) shows the median, quartiles, and ranges of PWV in hypertensive patients divided into seven age groups. Thirty‐five of the 299 hypertensive patients (11.7%) exceeded the PWV cut‐off point of 10 m/s, established by the expert consensus.
Figure 2 shows the mean values of PWV and the 95% confidence intervals in hypertensive patients divided into 7 age groups. PWV increases linearly with age (R 2 = 0.243; P < .05).
As in the healthy group (Table 2), hypertensive patients showed no significant gender differences in terms of PWV in patients under or over 50 years of age. Moreover, no statistical significant differences regarding the baseline characteristics between males and females were found in this cohort.
3.4. Group comparison
No significant differences were found between healthy and hypertensive patients in terms of BMI, waist circumference, cholesterol, triglycerides, and glucose serum levels (Table 1). However, HT patients were older and, as expected, had significantly higher BP (P < .001) and pulse pressures (P < .01).
As shown in Figure 2, in both younger (upper left panel) or older patients (right upper panel), PWV values were always higher in HT patients than in normotensive patients. Finally, the multiple linear regression analysis showed that a linear relationship exists between PWV and age and MBP, but not with HR (see Table 3). When correcting the PWV values for MBP, mean values failed to show a significant difference between NT (0.077 ± 0.016 m/s/mm Hg, n = 780) and HT (0.078 ± 0.004 m/s/mm Hg, n = 299).
Table 3.
Multivariate correlation: statistical analysis table
| Coefficient | SE | t | P | |
|---|---|---|---|---|
| Constant | −0.7440 | 0.5670 | −1.313 | .190 |
| Age | 0.0704 | 0.0035 | 20.380 | <.001 |
| HR | 0.0064 | 0.0052 | 1.230 | .220 |
| MBP | 0.0531 | 0.0057 | 9.2450 | <.001 |
P, significance value; SE, standard error; t, Student's t test value.
4. DISCUSSION
Pulse wave velocity is currently considered the gold standard to measure AS due to its simplicity, accuracy, reproducibility, and predictive value.21, 22, 23 Most studies provide reference PWV values that arise from retrospective analysis of patients evaluated in specialized centers. Such studies involve considerable selection bias when trying to extrapolate these data to populations from epidemiological studies.12 It is worth noting that, although there are tables with reference values of PWV for each age group, a unique PWV value (10 m/s) was established as a cut‐off value for the diagnosis of altered AS.17 Accordingly, the main contribution of this research is: (1) to provide further evidence that a single normal limit for AS is not useful, since PWV changes with age and, furthermore, this rate of change varies at ages over 50 years. No difference in PWV was observed between genders either in normotensive or hypertensive patients. These data are in accordance with Kozakova et al, who reported that no difference related to gender was observed in PWV and that, in patients over 50 years old, the PWV increasing values per year were higher than younger ages.24 Similar results were observed when patients suffer from arterial hypertension. And, (2) PWV normal values in our country (6.8 m/s) are significantly lower than the values suggested by the Consensus (10 m/s). This further supports the need to normalize PWV reference values according to ethnicity, considering the potential impact of lifestyle and CV risk profiles on AS.25, 26
Additionally, we provided further evidence suggesting the need of PWV reference values for each decade of life (since PWV showed an increase related to decades of life) and this increase was greater in patients over 50 years of age, independently of their gender. This phenomenon is also observed in HT patients.
On the other side, the contribution of risk factors (other than age and BP) to PWV has been well documented. In a systematic review with data from 77 studies, Cecelja et al showed that age and BP were consistently independently associated with PWV. They also found a non‐independent association between PWV and gender, total cholesterol, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglycerides, smoking, and body mass index. Consequently, the authors concluded that the contribution of risk factors (other than age and BP) to PWV is small or insignificant.11 More recent data were provided supporting these results.24
Our results have shown that even isolated hypertension, as a unique risk factor, has a direct impact on AS in all age groups compared to healthy patients.
This research has also several relevant aspects to discuss, for example, it represents the first Argentine record based on urban and rural population that determines the impact of isolated and untreated hypertension on PWV in many patients without other risk factors and without family history of hypertension. While this study is based on a single population, it is noteworthy that it would be unwise to extrapolate these data to all the local population, or towards other countries, due to racial or demographic differences. Nonetheless, we must consider that similarities in several demographic and socio‐cultural aspects in the general population of Argentina are present. The prevalence of hypertension reported in this study in a rural area is comparable to that reported in epidemiological population‐based studies in our country.13, 27, 28
This research also includes a comparable number of patients covering a wide range of ages (10‐87 years of age). The European registry, “Reference Values for Arterial Stiffness’ Collaboration,” reported normal values of PWV are based on 1455 records.12 These normal values are the result of a retrospective analysis of PWV obtained with different methods in 13 European high‐complexity medical centers. Additionally, this registry included several methods of measurement of PWV that required validation between these different methodologies to verify measurement accuracy. In our study, PWV was measured using a single technology of synchronous measurements that were digitized and automated for offline analysis. This methodological aspect allowed us to homogenize potential inferences to other populations and to quantify PWV reliably and consistently related to the effect of therapeutic interventions.
The third important aspect is the PWV increase‐rate observed across different age groups. As previously reported,13 in normotensive patients, PWV increases with ageing and a breaking point in the rate‐of‐increase is observed at 50 years of age. This could be due to a deterioration of arterial elasticity associated directly with age that induces an increase in stiffening of the aorta and other large arteries. This contributes, in older individuals, to a higher pulse pressure (PP), considered an indirect measure of AS and an independent risk factor for morbidity and mortality.29, 30, 31, 32 It was recently reported33 that, in coincidence with our PWV data, PP shows a similar profile related to age. This suggests that ageing induces an increase in stiffening of arteries with the consequent augmentation of PP as a biological marker and PWV as surrogate of this altered stiffness. In addition, in hypertensive patients, PWV showed a different behavior before and after the fifth decade of life. Patients under 50 years of age have a slower rate of PWV increase than older patients. This increase was greater in HT than in NT patients. This supports the idea that, within this period of life, the association between vascular ageing and hypertension leads to a greater vascular impairment, inducing a greater CV risk in accordance with previously reported data.34
Gender difference in AS is one of the most controversial and still unsolved issues in CV disease.12, 35 In our study, there was no difference in PWV values linked to gender, in accordance to large population based studies, in which gender difference in PWV was null or had no clinical significance (<0.1 m/s).24, 34, 36, 37 Moreover, in the framework of the Anglo Cardiff Collaborative Study and Multi Ethnic Study of Atherosclerosis,34 gender had no effect on the stiffness of large arteries and PWV, and Cecelja et al also reported lack of association of PWV and gender.11 Thus, it is suggested that, in healthy normotensive individuals, gender might not have a direct influence on arterial stiffening.
Our study defined “normal PWV values” in patients with first‐degree relatives with no history of hypertension, CV disease, or sudden death before age 65.38 Several studies have shown a significant hereditary burden on several indexes of arterial function, independently of BP values, and an association between PWV and genetic polymorphisms.39, 40, 41 These associations justify the redefinition of the inclusion criteria to define a population as the reference standard. Our study population was carefully enrolled prospectively after over 5 years of work with rigorous selection criteria. Consequently, they represent a selected population that constitutes an exceptional portion of the total population. However, it constitutes the ideal population to study the main determinants of PWV (ie, age and BP) without involving confounding factors or other determinants of PWV. Despite the methodological differences between the 2 studies, it is possible to attempt to compare the reference values between our data with the reference values of the arterial measurement collaboration group. For the 10th and 50th percentiles, similar PWV values were observed in each age category, with non‐significantly lower values in the Argentinean population. However, the 90th percentiles, for patients older than 60 years, PWV values of the Argentine cohort were particularly lower (between ‐2.7 and ‐2.59 m/s, P < .05) than the European population.12 The different measurement algorithms and different devices used, in addition to the strict inclusion criteria used in our work should be considered for an adequate interpretation and comparison of the reference values with the European group.
Analyzing PWV values for our population, and considering only the cut‐off value set at 10 m/s, regardless of age, we observed that 3.7% of patients considered apparently normal had elevated PWV values. Following the same criteria, 11.7% of hypertensive patients would have abnormal values of PWV. This suggests that it is essential for clinical practice to determine the reference values for each age group with their percentiles from a population of healthy patients and our data showed that PWV could be a valuable tool to evaluate changes in AS in young patients with hypertension. A recent meta‐analysis, which included more than 17 635 patients, showed that PWV is a stronger risk factor among younger individuals,1 thus giving further support to our data. This supports the idea that age and BP are strongly associated with PWV, arterial wall dysfunction, target organ damage, and increase in CV risk.
Another important aspect of this study is related to the difference in PWV observed in young patients (<20 years of age) between healthy and hypertensive patients. This difference is quantitatively and qualitatively significant and shows an early impairment of arterial compliance in hypertensive young patients. In this regard, 3 decades ago, Simon and Levenson hypothesized that arterial damage may precede the development of hypertension.42, 43 Recent epidemiological data also supports the notion that vascular stiffness precedes the development of hypertension, rather than vice versa.44 A further support was given by findings from the Framingham Study, suggesting that AS is a precursor of, rather than the result of, arterial hypertension.44 Our data allows to hypothesize if early hypertension strongly impacts vascular elasticity. Moreover, there are studies in patients with borderline hypertension with vascular damage.45 Our results support the idea that PWV should be controlled in each decade of life, especially in young hypertensive patients.
However, our study has several limitations. First, we used a cross‐sectional design; consequently, the increase of PWV across age groups should be interpreted with caution, since it may misestimate the real age‐related change of carotid femoral PWV corresponding to every subject included in this study. Second, we used office BP measurements and it is widely known that ambulatory BP monitoring provides additional information and has been consistently shown to have a stronger correlation with CV outcomes than office BP measurements.17 Moreover, ambulatory BP monitoring provides essential information about white‐coat hypertension and masked hypertension—both conditions that could have overestimated or underestimated the diagnosis of hypertension. The overall prevalence of masked hypertension and white‐coat hypertension in population‐based studies averaged 13% and white‐coat hypertension was about 32% among hypertensive patients.17, 46 These conditions were independently associated with increases of AS.47, 48
5. CONCLUSIONS
To date, this is the first population‐based study from urban and rural individuals of Latin America to analyze the impact of hypertension as an isolated risk factor on pulse wave velocity values. Our study provides normal values of pulse wave velocity, related to decades of life in healthy, normotensive patients, without family history of hypertension. These PWV values showed an increase associated with the aging process, especially after 50 years of age, in both normo‐ and hypertensive patients. No gender‐related differences were found in either hypertensive or normotensive patients.
Additionally, relevant clinical information to daily clinical practice, by setting PWV cut‐off values for each age group with a 95% confidence interval, was also provided in this report. Moreover, our findings suggest that the PWV growth rate, after the 5th decade, expresses an increase in AS, that may be responsible for an increased CV risk, even in apparently healthy normotensive or hypertensive patients.
Finally, isolated hypertension (even as the only risk factor) could increase AS together with a marked and sustained elevation of PWV in all age groups. Although PWV values were higher in patients with hypertension, a marked increase in PWV was observed after 50 years of age, accelerating the effect of aging of the arterial wall. In our population of hypertensive patients, the observed increased PWV seems to be the result of a hemodynamic adaptative response.
CONFLICT OF INTEREST
None.
ACKNOWLEDGMENTS
This work was supported by the René Favaloro University Foundation, Buenos Aires, Argentina. Moreover, technology destine to arterial research were received from the “Asociación Marcapasos Tandil,” Tandil City, Argentina.
Diaz A, Tringler M, Wray S, Ramirez AJ, Cabrera Fischer EI. The effects of age on pulse wave velocity in untreated hypertension. J Clin Hypertens. 2018;20:258–265. 10.1111/jch.13167
REFERENCES
- 1. Ben Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55:1318‐1327. [DOI] [PubMed] [Google Scholar]
- 3. Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilkinson IB, Mohammad NH, Tyrrell S, et al. Heart rate dependency of pulse pressure amplification and arterial stiffness. Am J Hypertens. 2002;15:24‐30. [DOI] [PubMed] [Google Scholar]
- 5. Jatoi NA, Jerrard‐Dunne P, Feely J, Mahmud A. Impact of smoking and smoking cessation on arterial stiffness and aortic wave reflection in hypertension. Hypertension. 2007;49:981‐985. [DOI] [PubMed] [Google Scholar]
- 6. Lantelme P, Mestre C, Lievre M, Gressard A, Milon H. Heart rate: an important confounder of pulse wave velocity assessment. Hypertension. 2002;39:1083‐1087. [DOI] [PubMed] [Google Scholar]
- 7. Lacy PS, O'Brien DG, Stanley AG, et al. Increased pulse wave velocity is not associated with elevated augmentation index in patients with diabetes. J Hypertens. 2004;22:1937‐1944. [DOI] [PubMed] [Google Scholar]
- 8. Olivera Alvim R, Santos PC, Musso MM, et al. Impact of diabetes mellitus on arterial stiffness in a representative sample of an urban Brazilian population. Diabetol Metab Syndr. 2013;5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fischer JW. The diagnostic value of the sphygmomanometer in examination for life insurance. JAMA. 1914;63:1752‐1754. [Google Scholar]
- 10. Mitchell GF. Arterial stiffness and hypertension. Hypertension. Chicken or egg? Hypertension. 2014;64:13‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension, a systematic review. Hypertension. 2009;54:1328‐1336. [DOI] [PubMed] [Google Scholar]
- 12. The Reference Values for Arterial Stiffness’ Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: “establishing normal and reference values.” The reference values for arterial stiffness collaboration. Eur Heart J. 2010;31:2338‐2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Díaz A, Galli CN, Tringler M, Ramírez A, Cabrera Fischer EI. Reference values of pulse wave velocity in healthy people from an urban and rural Argentinean population. Int J Hypertens. 2014;2014:653239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Díaz A, Tringler M, Galli C, Ramirez A, Fischer EI. Arterial stiffness in a rural population of Argentina: pilot study. High Blood Press Cardiovasc Prev. 2015;22:403‐409. [DOI] [PubMed] [Google Scholar]
- 15. Annual Census 2010 . Final results. http://www.censo2010.indec.gov.ar/resultadosdefinitivos.asp. Accessed July 12, 2013.
- 16. Lurbe E, Agabiti‐Rosei E, Criuckshank JK, et al. European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34:1887‐1920. [DOI] [PubMed] [Google Scholar]
- 17. Mancia G, Fagard R, Narkiewicz K, et al. “2013 ESH/ESC Guidelines for the management of arterial hypertension.” The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281‐1357. [DOI] [PubMed] [Google Scholar]
- 18. Cabrera Fischer EI, Bia D, Valtuille R, et al. Vascular access localization determines regional changes in arterial stiffness. J Vasc Access. 2009;10:192‐198. [DOI] [PubMed] [Google Scholar]
- 19. Bia D, Cabrera‐Fischer EI, Zócalo Y, et al. Vascular accesses for haemodialysis in the upper arm cause greater reduction in the carotid‐brachial stiffness than those in the forearm: study of gender differences. Int J Nephrol. 2012;2012:598512. 10.1155/2012/598512. Epub 2012 Apr 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid‐femoral pulse wave velocity. J Hypertens. 2012;30:445‐448. [DOI] [PubMed] [Google Scholar]
- 21. Huybrechts SA, Devos DG, Vermeersch SJ, et al. Carotid to femoral pulse wave velocity: a comparison of real travelled aortic path lengths determined by MRI and superficial measurements. J Hypertens. 2011;29:1577‐1582. [DOI] [PubMed] [Google Scholar]
- 22. Cavalcante JL, Lima JAC, Redheuil A, Al‐Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57:1511‐1522. [DOI] [PubMed] [Google Scholar]
- 23. Asmar RG, Benetos A, Topouchian J, et al. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Validation and clinical application studies. Hypertension. 1995;26:485‐490. [DOI] [PubMed] [Google Scholar]
- 24. Kozakova M, Morizzo C, Guarino D, et al. The impact of age and risk factors on carotid and carotid‐femoral pulse wave velocity. J Hypertens. 2015;33:1446‐1451. [DOI] [PubMed] [Google Scholar]
- 25. Santos PC, Alvim RO, Ferreira NE, et al. Ethnicity and arterial stiffness in Brazil. Am J Hypertens. 2011;24:278‐284. [DOI] [PubMed] [Google Scholar]
- 26. Markert MS, Della‐Morte D, Cabral D, et al. Ethnic differences in carotid artery diameter and stiffness: the Northern Manhattan Study. Atherosclerosis. 2011;219:827‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Díaz A, Ferrante D. Trends in prevalence of hypertension in Argentina in the last 25 years: a systematic review of observational studies. Rev Panam Salud Publica. 2015;38:496‐503. [PubMed] [Google Scholar]
- 28. Díaz A, Tringler MF. Prevalence of hypertension in rural populations from Ibero‐America and the Caribbean. Rural Remote Health. 2014;14:2591. [PubMed] [Google Scholar]
- 29. Benetos A, Safar M, Rudnichi A, et al. Pulse pressure: a predictor of long‐term cardiovascular mortality in a French male population. Hypertension. 1997;30:1410‐1415. [DOI] [PubMed] [Google Scholar]
- 30. Blacher J, Staessen JA, Girerd X, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med. 2000;160:1085‐1089. [DOI] [PubMed] [Google Scholar]
- 31. Franklin SS. Ageing and hypertension: the assessment of blood pressure indices in predicting coronary heart disease. J Hypertens 1999;17:S29‐S36; 22. [PubMed] [Google Scholar]
- 32. Winston GJ, Palmas W, Lima J, et al. Pulse pressure and subclinical cardiovascular disease in the multiethnic study of atherosclerosis. Am J Hypertens. 2013;26:636‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Butler KR Jr, Penman AD, Minor DS, Mosley TH Jr. Determinants of pulse pressure and annual rates of change in the atherosclerosis risk in communities study. J Hypertens. 2015;33:2463‐2470. [DOI] [PubMed] [Google Scholar]
- 34. McEniery CM, Yasmin IR, Hall A, Qasem IB, Wilkinson IB, Cockcroft JR, ACCT Investigators . Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo‐Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol. 2005;46:1753‐1760. [DOI] [PubMed] [Google Scholar]
- 35. Mattace‐Raso FUS. Gender differences in arterial structure and function. Are men really from Mars and women from Venus? Artery Res. 2009;3:148‐150. [Google Scholar]
- 36. Duprez DA, Jacobs DR Jr, Lutsey PL, et al. Race/ethnic and sex differences in large and small artery elasticity–results of the multi‐ethnic study of atherosclerosis (MESA). Ethn Dis. 2009;19:243‐250. [PMC free article] [PubMed] [Google Scholar]
- 37. Magalhães P, Capingana DP, Silva ABT, et al. Age‐ and gender‐specific reference values of pulse wave velocity for African adults: preliminary results. Age (Dordr). 2013;35:2345‐2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mitsumata K, Saitoh S, Ohnishi H, Akasaka H, Miura T. Effects of parental hypertension on longitudinal trends in blood pressure and plasma metabolic profile: mixed‐effects model analysis. Hypertension. 2012;60:1124‐1130. [DOI] [PubMed] [Google Scholar]
- 39. Sayed Tabatabaei F, Sayed A, van Rijn MJE, et al. Heritability of the function and structure of the arterial wall: findings of the Erasmus Rucphen Family (ERF) study. Stroke. 2005;36:2351‐2356. [DOI] [PubMed] [Google Scholar]
- 40. Mitchell GF, Parise H, Benjamin EJ, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239‐1245. [DOI] [PubMed] [Google Scholar]
- 41. Seidlerova J, Bochudb M, Staessan JA, et al. Heritability and intrafamilial aggregation of arterial characteristics. J Hypertens. 2008;26:721‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Simon A, Levenson J. La compliance artérielle joue‐t‐elle un rôle dans laphysiopathologie de l'hypertension artérielle? Presse Méd. 1986;15:2243‐2246. [PubMed] [Google Scholar]
- 43. Simon A, Levenson J. Use of arterial compliance for evaluation of hypertension. Am J Hypertens. 1991;3:97‐105. [DOI] [PubMed] [Google Scholar]
- 44. Kaess BM, Rong J, Larson MG, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Christen A, Sánchez RA, Baglivo HP, et al. Non‐invasive assessment of systemic elastic behaviour in hypertensive patients: análisis of posible determinants. Med Prog Technol. 1997;21:5‐11. [PubMed] [Google Scholar]
- 46. Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white‐coat, masked and sustained hypertension vs. true normotension: a meta‐analysis. J Hypertens. 2007;25:2193‐2198. [DOI] [PubMed] [Google Scholar]
- 47. Scuteri A, Morrell CH, Orru’ M, et al. Gender specific profiles of white coat and masked hypertension impacts on arterial structure and function in the SardiNIA study. Int J Cardiol 2016;217:92‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tientcheu D, Ayers C, Das SR, et al. Target organ complications and cardiovascular events associated with masked hypertension and white‐coat hypertension: analysis from the Dallas Heart Study. J Am Coll Cardiol. 2015;66:2159‐2169. [DOI] [PMC free article] [PubMed] [Google Scholar]


