Abstract
The authors evaluated a new algorithm for detecting atrial fibrillation (AF) using a home blood pressure monitor. Three serial blood pressure values were measured by the monitor in 16 patients with AF and 20 patients with sinus rhythm. The authors defined “monitor AF in irregular pulse peak (IPP) 25” as follows: (1) IPP: |interval of pulse peak − the average of the interval of the pulse peak| ≥ the average of the interval of the pulse peak ×25%; (2) irregular heart beat: beats of IPP ≥ total pulse ×20%; and (3) monitor AF: two or more irregular heart beats of the three blood pressure measurements. Cutoff IPP values were set at 20% (IPP20) and 15% (IPP15). The monitor's AF specificity was 1.0 in IPP25, IPP20, and IPP15, and its sensitivity was 0.88 in IPP25, 0.94 in IPP20, and 1.0 in IPP15. The new algorithm had high diagnostic accuracy for detecting AF and a low false‐positive rate.
Keywords: atrial fibrillation, blood pressure monitor, hypertension
1. INTRODUCTION
Atrial fibrillation (AF) is not only a major risk factor for stroke, it also causes heart failure and cardiovascular death.1, 2, 3, 4 AF is a common disease and the prevalence of AF increases with age.2, 5, 6, 7 Patients with paroxysmal AF have a stroke risk that is similar to that of patients with persistent AF.8 However, paroxysmal AF is often not detected by standard 12‐lead electrocardiography (ECG), as was shown both in patients who have undergone pacemaker implantation9 and those with cryptogenic stroke who have received an implantable loop recorder.10 Pulse self‐examination is useful to detect AF,11 but paroxysmal AF is often asymptomatic.12
Hypertension is an important risk factor for the onset of AF.13 The causes of the onset of AF are an increase in the stress of the left atrium and autonomic nervous abnormality.14, 15 An increase in the afterload caused by hypertension causes left atrial hypertrophy and fibrosis, which are related to AF. It has been shown that a reduction in blood pressure (BP) results in a decrease in the incidence of AF.16
Two studies revealed that home BP had a higher predictive value for cardiovascular events than office BP.17, 18 Home BP monitors are now widely used, and the Japanese Society of Hypertension (JSH) guidelines recommend the use of a home BP monitor for the control of hypertension.19 The detection of AF by a home BP monitor would provide a significant contribution to community health.
Huang and colleagues20 compared irregular heart beat and ECG tracings using four BP monitors in 2009. After their report, several reports about the detection of paroxysmal AF by home BP monitors have been published. The sensitivity values were near or equal to 100%, but the specificity values were 80% to 90%,21, 22, 23, 24 and the agreement between pulse rate and heart rate was not examined simultaneously. In the present study, we confirmed the correlation between the interval of the pulse wave and QRS wave, and we evaluated a new algorithm for the detection of AF by a home BP monitor.
2. METHODS
2.1. Patients
We enrolled 16 patients with AF and 20 outpatients with sinus rhythm. Findings from the ECG showed AF during all three BP measurements in the patients with AF and showed sinus rhythm during all three measurements in the 20 patients with sinus rhythm.
2.2. Pulse wave analysis and ECG
For each patient, three BP measurements were taken after the patient had rested for ≥5 minutes in the lying position and with at least 30 seconds between measurements, using a validated home BP monitor (UA‐1020, A&D).25 The three BPs were measured by cuff inflation to a point over the patient’s systolic BP, then cuff pressure was reduced gradually. The pulse wave was extracted by change of pressure after cuff deflation during each of three BP measurements. The interval of pulse waves were analyzed automatically by the UA‐1020 during the decompression phase (Figure 1). We defined “monitor AF in irregular pulse peak (IPP) 25” as follows (Figure 2): (1) IPP: |interval of pulse peak–the average of the interval of the pulse peak| ≥ the average of the interval of the pulse peak ×25%; (2) irregular heart beat (IHB): beats of IPP ≥ total pulse ×20%; and (3) the “monitor AF (IPP25):” two or more IHB of the three BP measurements. We also confirmed the diagnostic accuracy when the cutoff IPP values were set at 20% (IPP20) and 15% (IPP15).
Figure 1.
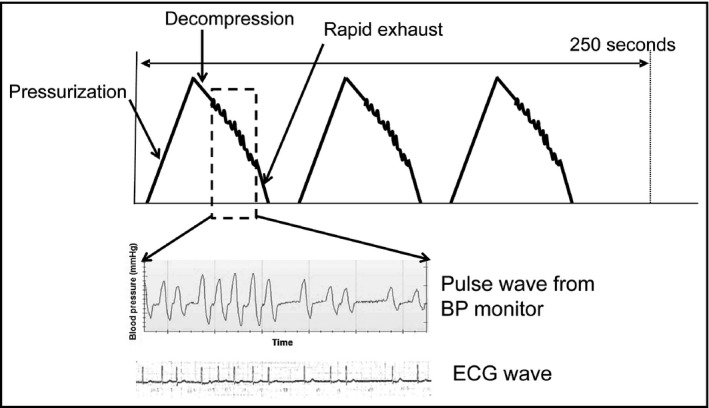
The mechanism of pulse wave analysis and the concordance between the interval of the pulse wave and QRS wave on electrocardiography (ECG). Pulse waves from the blood pressure (BP) monitor were obtained (three dotted rectangles)
Figure 2.
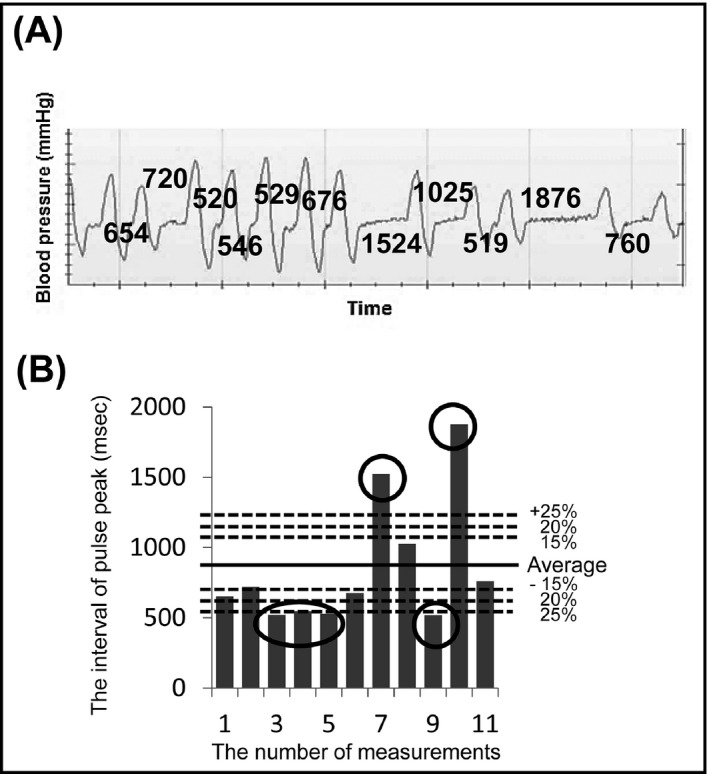
The atrial fibrillation detection algorithm and an example of irregular heart beat (IHB). A, The pulse peak record. The numbers indicate the interval of the pulse wave (ms). B, An example of IHB. The six encircled bars were judged as irregular pulse peak (IPP). The six beats of IPP were more than 20%×11 beats
Simultaneously during the deflation phase of each BP measurement (when the AF detector of the device was operating), the ECG was recorded continuously in the II lead (Figure 1). We confirmed the concordance between the interval of the pulse wave and QRS wave in the ECG in five patients with AF (total 100 beats).
2.3. Ethical issues
The internal review board of the Jichi Medical University School of Medicine approved this study. Written informed consent for the study was obtained individually from all of the patients.
2.4. Statistical analysis
Data are shown as mean±SD or percentage. Student t test was used to analyze the continuous data of the patients with sinus rhythm and those with AF. Comparisons of parameters among the groups were made using the χ2 test. The association between the RR interval in ECG and the pulse wave interval was assessed using Pearson correlation coefficient and a Bland‐Altman graph. The sensitivity, specificity, and κ statistic for the AF diagnosis were assessed for the individual measurements for IHB and also for the monitor's detection of AF. SPSS version 20.0 software (IBM) was used for the statistical analysis. A probability value <.05 was considered significant.
3. RESULTS
The clinical characteristics of the patients with AF and those with sinus rhythm are summarized in Table 1. The age and sex distributions of the two groups were similar. The association between the RR interval on ECG and the pulse wave interval is illustrated in Figure 3. The correlation was very good (R = .98, P < .001) and the pulse wave interval measured by the home BP monitor reflected the RR interval correctly. Figure 4 shows the Bland‐Altman plot of the RR interval on ECG and the pulse wave interval values.
Table 1.
Patient characteristics
| Sinus rhythm (n = 20) | AF (n=16) | P Value | |
|---|---|---|---|
| Age, y | 63 ± 11 | 66 ± 10 | .36 |
| Men, % | 75 | 88 | .49 |
| Systolic BP, mm Hg | 127 ± 17 | 128 ± 12 | .82 |
| Diastolic BP, mm Hg | 77 ± 11 | 82 ± 9 | .23 |
| Pulse rate, beats per min | 65 ± 11 | 78 ± 14 | .003 |
Abbreviations: AF, atrial fibrillation; BP, blood pressure.
Figure 3.
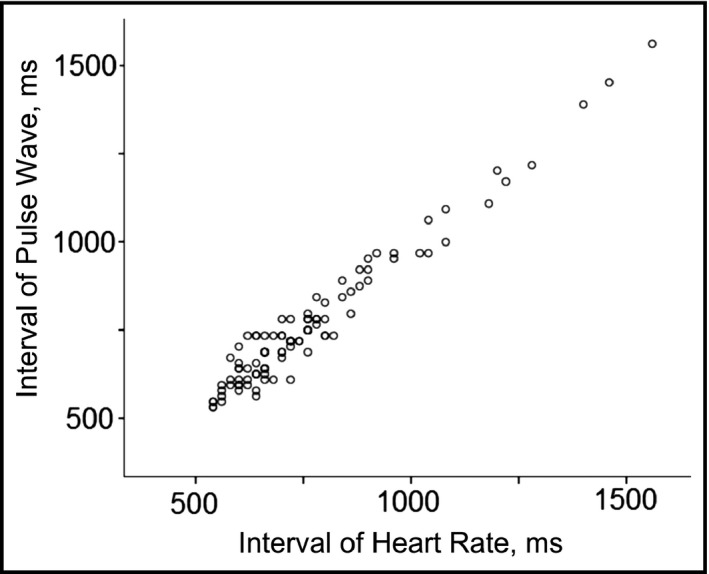
The association between the interval of the pulse wave and heart rate
Figure 4.
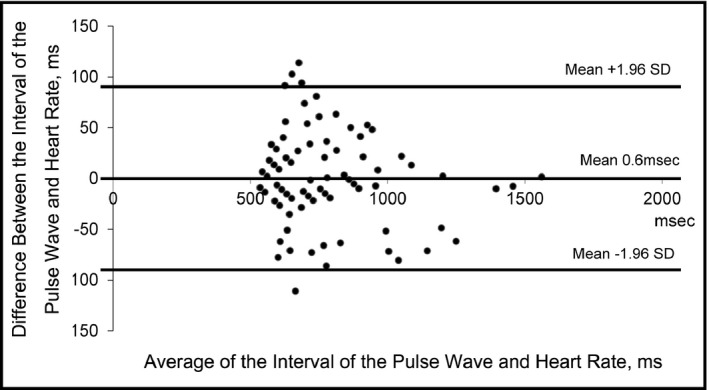
Bland‐Altman plot of the interval of the pulse wave and heart rate values
The accuracy for detecting IHBs was as follows (Table 2): the specificity was 1.0 in IPP25 and IPP20, and the sensitivity was 0.69 to 0.88 in IPP25 and 0.88 to 0.94 in IPP20. In IPP15, the specificity was 0.95 to 1.00 and the sensitivity was 1.0. One case of sinus rhythm was judged as IHB in the IPP15 setting. The second measurement was judged as IHB, but the first and third readings were not IHB. Monitor AF was defined as two or more IHBs of three BP measurements. The patient had only one IHB and thus was not classified as having monitor AF. Therefore, the specificity of the second reading in IPP15 was 0.95 (Table 2). However, all of the first and third readings in the patients with sinus rhythm were judged as sinus rhythm, and the specificities of both the first and third readings in IPP15 were 1.0. Monitor AF was purposefully defined as two or more IHBs of three BP measurements to avoid false‐positive judgment, as would have occurred otherwise in this case. The monitor's accuracy for diagnosing AF is summarized in Table 3. The specificity of the monitor‐diagnosed AF was 1.0 in IPP25, IPP20, and IPP15, and the sensitivity was 0.88 in IPP25, 0.94 in IPP20, and 1.0 in IPP15.
Table 2.
Accuracy of the monitor for detecting IHB
| Reading | IHB diagnosis +/‐ | Sensitivity | Specificity | κ | |
| IPP 25% | First | 14/22 | 0.88 | 1.00 | 0.89 |
| Second | 11/25 | 0.69 | 1.00 | 0.71 | |
| Third | 14/22 | 0.88 | 1.00 | 0.89 | |
| IPP 20% | First | 14/22 | 0.88 | 1.00 | 0.89 |
| Second | 14/22 | 0.88 | 1.00 | 0.89 | |
| Third | 15/21 | 0.94 | 1.00 | 0.94 | |
| IPP 15% | First | 16/20 | 1.00 | 1.00 | 1.00 |
| Second | 17/19 | 1.00 | 0.95 | 0.94 | |
| Third | 16/20 | 1.00 | 1.00 | 1.00 |
Abbreviations: IHB, irregular heart beat; IPP, irregular pulse peak.
Table 3.
Accuracy of the monitor for diagnosing AF
| Monitor detection AF +/‐ | Sensitivity | Specificity | κ | |
|---|---|---|---|---|
| IPP 25% | 14/22 | 0.88 | 1.00 | 0.89 |
| IPP 20% | 15/21 | 0.94 | 1.00 | 0.94 |
| IPP 15% | 16/20 | 1.00 | 1.00 | 1.00 |
Abbreviations: AF, atrial fibrillation; IPP, irregular pulse peak.
4. DISCUSSION
The judgment of IHD at 100% specificity was obtained by IPP15, and the detection of AF at 100% specificity was obtained by both IPP20 and IPP15. The frequency of paroxysmal AF varies, and the detection rate increased in accord with the length of the observation period.9, 10 Self‐measured home BP can be taken every day and over a long term, and thus the longer a home BP monitor is used, the more likely it is that paroxysmal AF will be detected.
A stroke event is often the first event of AF,26 and screening and anticoagulation therapy for asymptomatic AF is useful to prevent stroke.27 Pulse palpation is useful to detect AF,28 but most instances of paroxysmal AF may not be detected. Pulse self‐examination is a useful technique to detect paroxysmal AF,11 but it might be inaccurate compared with home BP monitoring.
Hypertension is a major risk factor for the onset of AF,13 and BP reduction is useful to suppress AF.16 Home BP values are a better predictor of mortality and cardiovascular events compared with office BP,29, 30 and thus the control of home BP is important. In Japan, roughly 40 million home BP devices are being used,31 and thus when the AF detection algorithm described in the present study is adapted, more individuals with asymptomatic AF will be identified. Hypertension is also a major risk for stroke in patients with AF,32 and patients with asymptomatic AF with hypertension have a high stroke risk. Increased BP is associated with cardiovascular events in patients with AF.33 Thus, the necessary control of home BP could be greatly aided by the use of a home BP monitor.
The specificity of an AF detection algorithm of home BP monitor values was 80% to 90% in earlier studies,21, 22, 23, 24 and false‐positive readings were observed. In the present study, the accuracy of IHD at IPP20 was 100% in three measurements. Our goal herein was to reduce false‐positive results. When the cutoff was IPP15, sinus rhythm was judged as AF one time with three measurements. In their analysis, Stergiou and colleagues22 found that when two abnormal readings of three measurements were required to diagnose AF, the specificity increased. In this study, the specificity also benefited from defining monitor AF as two or more IHBs of three BP measurements. If the cutoff is set as IPP15, respiratory arrhythmia and premature beats might be overdiagnosed as AF. To detect AF, the cutoff level (IPP15 or IPP20) must be further investigated to obtain the best sensitivity and specificity.
5. STUDY LIMITATIONS
This study has some limitations. First, arrhythmias other than AF were not included. Further studies including arrhythmias other than AF and more cases are needed to further evaluate the AF detection algorithm. Second, the specific definitions for IPP and IHB were not validated; thus, we compared three different IPP cutoff levels (15%, 20%, and 25%). The optimal level of IPP and IHB should be investigated by further studies.
6. CONCLUSIONS
Our new algorithm for a home BP monitor had high diagnostic accuracy for detecting AF. The quality of the control of hypertension might be improved in terms of the detection of AF by using the algorithm equipped in a home BP monitor at IPP15 and IPP20 after further evaluations of sensitivity and specificity.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare related to this study.
Kabutoya T, Imai Y, Hoshide S, Kario K. Diagnostic accuracy of a new algorithm to detect atrial fibrillation in a home blood pressure monitor. J Clin Hypertens. 2017;19:1143–1147. 10.1111/jch.13076
REFERENCES
- 1. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946‐952. [DOI] [PubMed] [Google Scholar]
- 2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983‐988. [DOI] [PubMed] [Google Scholar]
- 3. Healey JS, Oldgren J, Ezekowitz M, et al. Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: a cohort study. Lancet. 2016;388:1161‐1169. [DOI] [PubMed] [Google Scholar]
- 4. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta‐analysis. BMJ. 2016;354:i4482. [DOI] [PubMed] [Google Scholar]
- 5. Majeed A, Moser K, Carroll K. Trends in the prevalence and management of atrial fibrillation in general practice in England and Wales, 1994‐1998: analysis of data from the general practice research database. Heart. 2001;86:284‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370‐2375. [DOI] [PubMed] [Google Scholar]
- 7. Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27:949‐953. [DOI] [PubMed] [Google Scholar]
- 8. Hohnloser SH, Pajitnev D, Pogue J, et al. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W Substudy. J Am Coll Cardiol. 2007;50:2156‐2161. [DOI] [PubMed] [Google Scholar]
- 9. Boriani G, Glotzer TV, Santini M, et al. Device‐detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J. 2014;35:508‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478‐2486. [DOI] [PubMed] [Google Scholar]
- 11. Munschauer FE 3rd, Sohocki D, Smith Carrow S, Priore RL. A community education program on atrial fibrillation: Implications of pulse self‐examination on awareness and behavior. J Stroke Cerebrovasc Dis. 2004;13:208‐213. [DOI] [PubMed] [Google Scholar]
- 12. Senoo K, Suzuki S, Sagara K, et al. Distribution of first‐detected atrial fibrillation patients without structural heart diseases in symptom classifications. Circ J. 2012;76:1020‐1023. [DOI] [PubMed] [Google Scholar]
- 13. Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA. 1994;271:840‐844. [PubMed] [Google Scholar]
- 14. Maisel WH. Autonomic modulation preceding the onset of atrial fibrillation. J Am Coll Cardiol. 2003;42:1269‐1270. [DOI] [PubMed] [Google Scholar]
- 15. Sanders P, Morton JB, Davidson NC, et al. Electrical remodeling of the atria in congestive heart failure: electrophysiological and electroanatomic mapping in humans. Circulation. 2003;108:1461‐1468. [DOI] [PubMed] [Google Scholar]
- 16. Julius S, Kjeldsen SE, Weber M, et al; VALUE trial group . Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomized trial. Lancet. 2004;363:2022‐2031. [DOI] [PubMed] [Google Scholar]
- 17. Niiranen TJ, Johansson JK, Reunanen A, Jula AM. Optimal schedule for home blood pressure measurement based on prognostic data: The Finn Home Study. Hypertension. 2011;57:1081‐1086. [DOI] [PubMed] [Google Scholar]
- 18. Ohkubo T, Asayama K, Kikuya M, et al. How many times should blood pressure be measured at home for better prediction of stroke risk? Ten year follow up results from the Ohasama study. J Hypertens. 2004;22:1099‐1104. [DOI] [PubMed] [Google Scholar]
- 19. Shimamoto K, Ando K, Fujita T, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37:253‐390. [DOI] [PubMed] [Google Scholar]
- 20. Huang QF, Sheng CS, Zhang Y, Wang J, Li Y, Wang JG. Accuracy of automated oscillometric blood pressure monitors in the detection of cardiac arrhythmias. Blood Press Monit. 2009;14:91‐92. [DOI] [PubMed] [Google Scholar]
- 21. Wiesel J, Fitzig L, Herschman Y, Messineo FC. Detection of atrial fibrillation using a modified Microlife blood pressure monitor. Am J Hypertens. 2009;22:848‐852. [DOI] [PubMed] [Google Scholar]
- 22. Stergiou GS, Karpettas N, Protogerou A, Nasothimiou EG, Kyriakidis M. Diagnostic accuracy of a home blood pressure monitor to detect atrial fibrillation. J Hum Hypertens. 2009;23:654‐658. [DOI] [PubMed] [Google Scholar]
- 23. Wiesel J, Arbesfeld B, Schechter D. Comparison of the Microlife blood pressure monitor with the Omron blood pressure monitor for detecting atrial fibrillation. Am J Cardiol. 2014;114:1046‐1048. [DOI] [PubMed] [Google Scholar]
- 24. Gandolfo C, Balestrino M, Bruno C, Finocchi C, Reale N. Validation of a simple method for atrial fibrillation screening in patients with stroke. Neurol Sci. 2015;36:1675‐1678. [DOI] [PubMed] [Google Scholar]
- 25. Zeng WF, Kang YY, Liu M, Li Y, Wang JG. Validation of the A&D UA‐1020 upper‐arm blood pressure monitor for home blood pressure monitoring according to the British Hypertension Society Protocol. Blood Press Monit. 2013;18:177‐181. [DOI] [PubMed] [Google Scholar]
- 26. Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257‐e354. [DOI] [PubMed] [Google Scholar]
- 27. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994;154:1449‐1457. [PubMed] [Google Scholar]
- 28. Lowres N, Neubeck L, Redfern J, Freedman SB. Screening to identify unknown atrial fibrillation. A systematic review. Thromb Haemost. 2013;110:213‐222. [DOI] [PubMed] [Google Scholar]
- 29. Bobrie G, Chatellier G, Genes N, et al. Cardiovascular prognosis of masked hypertension_detected by blood pressure self measurement in elderly treated hypertensive patients. JAMA. 2004;291:1342‐1349. [DOI] [PubMed] [Google Scholar]
- 30. Stergiou GS, Baibas NM, Kalogeropoulos PG. Cardiovascular risk prediction based on home blood pressure measurement: the Didima study. J Hypertens. 2007;25:1590‐1596. [DOI] [PubMed] [Google Scholar]
- 31. Shirasaki O, Terada H, Niwano K, et al. The Japan Home‐health Apparatus Industrial Association: investigation of home use electronic sphygmomanometers. Blood Press Monit. 2001;6:303‐307. [DOI] [PubMed] [Google Scholar]
- 32. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864‐2870. [DOI] [PubMed] [Google Scholar]
- 33. Kodani E, Atarashi H, Inoue H, et al. Impact of blood pressure control on thromboembolism and major hemorrhage in patients with nonvalvular atrial fibrillation: a subanalysis of the J‐RHYTHM Registry. J Am Heart Assoc. 2016;5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]


