Abstract
Although brachial‐ankle pulse wave velocity (baPWV) has been widely used as an index of arterial stiffness, no consensus exists about whether baPWV can reflect central aortic stiffness. The authors investigated the association between baPWV and invasively measured aortic pulse pressure (APP) in a total of 109 consecutive patients (mean age, 62.3 ± 11.3 years; 67.9% men). Most patients (91%) had obstructive coronary artery disease, and mean baPWV and APP values were 1535 ± 303 cm/s and 66.8 ± 22.5 mm Hg, respectively. In univariate analysis, there was a significant linear correlation between baPWV and APP (r = .635, P < .001). The correlation between baPWV and APP remained significant even after controlling for potential confounders (β = 0.574, P < .001; R 2 = .469). Arterial stiffness measured by baPWV showed a strong positive correlation with invasively measured APP, independent of clinical confounders. Therefore, baPWV can be a good marker of central aortic stiffness.
Keywords: aortic pulse pressure, aortic stiffness, correlation, pulse wave velocity
1. INTRODUCTION
Aortic pulse pressure (APP), defined as the difference between systolic blood pressure (BP) and reduced diastolic BP of the aorta, is a reliable measure of arterial stiffness of central elastic arteries.1, 2 Previous studies have recognized APP as a surrogate maker of increased risk for cardiovascular morbidity and mortality.3, 4 It has been shown that APP is strongly related to future cardiovascular events and a better predictor of target organ damage compared with peripheral BP.5, 6, 7 Although cardiac catheterization is considered the gold standard to obtain APP,8, 9 it is unsuitable for routine screening of large populations because of its invasiveness, cost, and technical skill.10 Pulse wave velocity (PWV) is an alternative and noninvasive way to measure arterial stiffness, being widely used in research and clinical fields.11 Previous studies have shown that PWV is associated with increased morbidity and mortality in various populations, such as individuals with hypertension, diabetes mellitus, and stroke.12, 13, 14 Although carotid‐femoral PWV has been considered the standard noninvasive measure of arterial stiffness, the measurement of carotid‐femoral PWV is time‐consuming and requires considerable operator training as well as the exposure and palpation of the femoral artery.15 Practically, recently developed brachial‐ankle PWV (baPWV) is more feasible than carotid‐femoral PWV because it can be simply measured by brachial and tibial arterial wave analyses without exposing the femoral site.16 More importantly, the value of baPWV has been proven in many clinical studies17, 18 and meta‐analysis.19 Despite the promising results of baPWV, the correlation between baPWV and APP has not yet been fully clarified. Some previous studies evaluated the association of central BP and PWV; however, these studies have limitations because they had a small sample size or the central BP was measured noninvasively.20, 21 Therefore, the aim of this study was to investigate the association between baPWV and invasively measured APP, and to evaluate whether baPWV can reliably reflect central aortic stiffness.
2. MATERIALS AND METHODS
2.1. Study population
Between April 2013 and October 2013, 133 consecutive patients who received invasive coronary angiography and baPWV measurement on the same day at Boramae Medical Center (Seoul, Korea) were prospectively recruited. Our study excluded 24 patients with acute myocardial infarction, unstable vital signs, ongoing chest pain, pericardial effusion, impaired left ventricular (LV) systolic function (LV ejection fraction <50%), regional wall motion abnormality, significant valvular heart disease (greater than mild degree of regurgitation or stenosis), peripheral artery disease (ankle brachial index <0.9 or >1.4), and nonsinus rhythm on electrocardiography. After such exclusion, 109 patients were finally analyzed in this study. The study protocol was approved by the institutional review board of Boramae Medical Center (Seoul, Korea), and informed consent was obtained from all study participants.
2.2. Clinical data collection
Demographic characteristics were collected, including age, height (cm), weight (kg), body mass index (kg/m2), and traditional risk factors including hypertension, diabetes mellitus, dyslipidemia, and smoking status. Hypertension was defined by a history of hypertension or antihypertensive medications. Diabetes mellitus was defined by a history of diabetes mellitus or antidiabetic medications. Dyslipidemia was defined as a history of dyslipidemia or antidyslipidemic medications. Patients who smoked regularly within 12 months were considered current smokers. Information on concomitant vasoactive medications, including calcium channel blockers, β‐blockers, renin‐angiotensin system blockers, and statins, was collected. Stable angina referred to chest discomfort that occurred reproducibly at a certain level of exertion and was relieved with rest or nitroglycerin.22 Unstable angina was diagnosed if at least one of the following features was shown: (1) resting chest pain, usually lasting more than 10 minutes; (2) severe and new‐onset chest pain; and (3) crescendo‐pattern chest discomfort.23 Venous blood samples for laboratory tests were collected after an overnight fasting of 8 hours, and white blood cell count, hemoglobin, glucose, uric acid, total cholesterol, low‐ and high‐density lipoprotein cholesterol, triglyceride, C‐reactive protein, and serum creatinine were measured. Estimated glomerular filtration rate was calculated by the Modification of Diet in Renal Disease equation.24
2.3. Transthoracic echocardiography
Transthoracic echocardiography was performed using a 2.5‐MHz probe with commercially available ultrasound systems (Sequoia [Siemens Medical Solutions] or Vivid 7 [GE Medical Systems]). LV ejection fraction was calculated using Simpson's biplane method. LV mass was calculated with a validated formula and indexed to the body surface area.25 Peak early transmitral filling velocity during early diastole (E) was imaged at the tip of the mitral leaflet from an apical four‐chamber view, and color‐coded tissue Doppler imaging was applied to the apical four‐chamber view to determine mean early (e') velocity at the septal mitral annulus. E/e' was calculated as an index of LV filling pressure. The left atrial volume index was calculated using the biplane method and indexed to body surface area. All measurements represented the average of three consecutive cardiac cycles. Two experienced cardiosonographers performed echocardiography. Correlation coefficients for interobserver agreements were 0.96 and 0.92 for e' and E/e', respectively, in our laboratory.26
2.4. Aortic pressure measurement
Central aortic pressure measurements were made in the ascending aorta using a 5F fluid‐filled pigtail catheter with the patient in the supine position before invasive coronary angiography. On the day of invasive aortic pressure measurement, smoking, alcohol drinking, and caffeine consumption were prohibited. Three consecutive beats at full expiration were averaged in each case. Pressure tracing was recorded using a hemodynamic monitoring system (Horizon XVu‐hemodynamic monitoring system, Mennen Medical), and systolic BP and diastolic BP values of the central aorta were obtained. Pulse pressure (PP) was calculated as the difference between the peak systolic pressure and the end‐diastole pressure. The mean arterial aortic pressure was calculated as 1/3 systolic BP + 2/3 diastolic BP. Invasive coronary angiography was performed according to standardized technique. Obstructive coronary artery disease (CAD) was defined when a major epicardial coronary artery or a branch artery sized ≥2 mm in luminal diameter had >50% diameter stenosis on invasive coronary angiography. The number of arteries with stenosis ≥50% was counted, and the extent of CAD was defined as one‐, two‐, or three‐vessel disease.
2.5. baPWV measurement
The baPWV values were measured noninvasively using an automated wave form analyzer (VP‐1000, Colin Co. Ltd,).27 After approximately 5 minutes of rest in a temperature‐controlled and quiet environment, patients were studied in the supine position. All of the regular medications were permitted, but smoking, alcohol drinking, and caffeine consumption were prohibited on the day of examination. Briefly, volume plethysmography was used to measure the arterial pulse wave at the brachial and tibial arteries, simultaneously recording BP, heart rate, and heart sound. The time delay (Qt, seconds) was measured by the transmission time between the respective rise (foot) in the brachial and tibial pressure wave (foot‐to‐foot duration), and the distance was automatically calculated from the patient's height and a fixed regression equation. L1 was the distance from the heart to the ankle and L2 was the distance from the heart to the arm; each were calculated as: L1 = 0.2195 × H – 2.0734, L2 = 0.5643 × H – 18.381. Then, PWV was calculated as (L1–L2)/Qt. When measurements were obtained from both extremities, the average value of the left and right measurements was chosen for analysis. In order to get precise brachial BP data, all clothing that covered the location of cuff placement was removed. The appropriate cuff size was selected based on the patient's arm circumference. The brachial artery was palpated in the antecubital fossa to locate the point to place the cuff. The lower end of the cuff was 2 to 3 cm above the antecubital fossa. A minimum of two readings was taken at intervals of at least 1 minute, and the average of those readings was used as the patient's BP. If there was a > 5 mm Hg difference between the first and second readings, an additional reading was obtained, and then the average of these multiple readings was used. All the measurements were made by the same experienced operator blinded to all of the clinical data. The intraobserver coefficient of variation for baPWV measurement was 0.949 (95% confidence interval [CI], 0.911–0.971) in our laboratory and Bland‐Altman plot is shown in Figure S1.
2.6. Statistical analysis
Continuous variables are presented as mean ± standard deviation, and categorical variables are expressed as percentages. Univariate associations between two continuous variables were assessed using bivariate Pearson's correlation analysis. Scatter plots were used for the demonstration of linear correlations between two continuous variables. Multivariable linear regression analysis was subsequently applied to examine independent relationships between baPWV and APP. Age, height, heart rate, estimated glomerular filtration rate, e' velocity, and left atrial volume index were controlled in this multivariable analyses. To examine multicollinearity in a linear regression model, the variance inflation factor was used. All VIF values were less than three in our multivariable analysis, suggesting that there was no significant multicollinearity problem. For comparison of the correlation coefficients between APP and brachial PP, a Fisher r‐to‐z transformation was used. A P value <.05 was considered statistically significant. All statistical analyses were conducted using SPSS version 20.0 (IBM).
3. RESULTS
3.1. Baseline clinical characteristics
The baseline characteristics of the total 109 patients are shown in Table 1. The mean age was 62.3 ± 11.3 years, and there were 67.9% men. A total of 66.1%, 23.9%, and 40.4% of patients had hypertension, diabetes mellitus, and dyslipidemia, respectively, and 29.4% of the patients were current smokers. About half of the patients (51.4%) were diagnosed with unstable angina. Blood test results and echocardiographic findings were within the normal range. A total of 26.6%, 20.2%, 53.2%, and 56.0% of patients took calcium channel blockers, β‐blockers, renin‐angiotensin system blockers, and statins, respectively. Invasive coronary angiography revealed that 91.7% of patients had obstructive CAD, where 21.1%, 33.9%, and 36.7% had one‐vessel, two‐vessel, and three‐vessel disease, respectively.
Table 1.
Clinical characteristics of study patients
| Characteristic | Value (n = 109) |
|---|---|
| Age, y | 62.3 ± 11.3 |
| Male sex | 74 (67.9) |
| Height, cm | 162 ± 8 |
| Weight, kg | 65.4 ± 10.9 |
| Body mass index, kg/m2 | 24.5 ± 3.0 |
| Brachial SBP, mm Hg | 123 ± 17 |
| Brachial DBP, mm Hg | 71 ± 9 |
| Heart rate, beats per min | 67.0 ± 12.9 |
| Traditional risk factors | |
| Hypertension | 72 (66.1) |
| Diabetes mellitus | 26 (23.9) |
| Dyslipidemia | 44 (40.4) |
| Current smoking | 32 (29.4) |
| Clinical diagnosis | |
| Stable angina | 53 (48.6) |
| Unstable angina | 56 (51.4) |
| Laboratory findings | |
| White blood cell count, per µL | 7112 ± 2231 |
| Hemoglobin, g/dL | 13.4 ± 62.3 |
| Uric acid, mg/dL | 5.44 ± 1.51 |
| Total cholesterol, mg/dL | 160 ± 44 |
| Low‐density lipoprotein cholesterol, mg/dL | 100 ± 46 |
| High‐density lipoprotein cholesterol, mg/dL | 47.3 ± 13.6 |
| Triglyceride, mg/dL | 121 ± 64 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 82.5 ± 19.7 |
| C‐reactive protein, mg/dL | 0.32 ± 0.88 |
| Echocardiographic findings | |
| Left ventricular ejection fraction, % | 65.3 ± 6.9 |
| Left ventricular mass index, g/m2 | 99.4 ± 24.5 |
| e' velocity, cm/s | 6.08 ± 1.81 |
| E/e' | 11.3 ± 4.5 |
| Left atrial volume index, mL/m2 | 29.6 ± 11.2 |
| Tricuspid regurgitation maximal velocity, m/s | 2.35 ± 0.35 |
| Concomitant medications | |
| Calcium channel blocker | 29 (26.0) |
| β‐Blocker | 45 (41.3) |
| Renin‐angiotensin system blocker | 58 (53.2) |
| Statin | 61 (56.0) |
| Findings of coronary angiography | |
| Insignificant | 9 (8.3) |
| One‐vessel disease | 23 (21.1) |
| Two‐vessel disease | 37 (33.9) |
| Three‐vessel disease | 40 (36.7) |
Values are expressed as number (percentage) or mean ± standard deviation.
Measurements of hemodynamic parameters and baPWV of the study patients are shown in Table 2. Mean values of central APP and baPWV were 66.8 ± 22.5 mm Hg and 1535 ± 303 cm/s, respectively.
Table 2.
Measures of central aortic pressures and brachial‐ankle pulse wave velocity
| Measure | Value (n = 109) |
|---|---|
| Measures of central hemodynamics | |
| Central aortic systolic BP, mm Hg | 143 ± 26 |
| Central aortic diastolic BP, mm Hg | 75.7 ± 14.2 |
| Central aortic pulse pressure, mm Hg | 66.8 ± 22.6 |
| Central aortic mean BP, mm Hg | 98.0 ± 15.6 |
| Brachial‐ankle pulse wave velocity, cm/s | 1535 ± 303 |
Abbreviation: BP, blood pressure.
Values are expressed as mean ± standard deviation.
3.2. Correlation between APP and baPWV
The linear associations of baPWV values with various demographic parameters, laboratory findings, and echocardiographic/hemodynamic measurements are shown in Table 3. Results showed that baPWV had significant correlations with age, height, estimated glomerular filtration rate, and diastolic indices including e', E/e', left atrial volume index, and BP profiles. Among these factors, APP showed the strongest correlation with baPWV (r = .635 [95% CI, 0.508–0.735]; R 2 = .404, P < .001). Although there was a significant correlation between baPWV and brachial PP (r = .441 [95% CI, 0.278–0.581]; R 2 = .210, P < .001), the correlation power was stronger between baPWV and APP, which met statistical significance (P = .044; Figure). Additionally, we performed analysis using the higher value of the left and right baPWV measurements (baPWVmax) instead of using the average value. The linear associations of baPWVmax values with various parameters are shown in Table S1. APP showed strong correlation with baPWVmax (r = .582 [95% CI, 0.442–0.694]; R 2 = .339, P < .001), which was stronger than the correlation between baPWVmax and brachial PP (r = .458 [95% CI, 0.281–0.604]; R 2 = .209, P < .001) (Figure S2).
Table 3.
Simple linear correlations between baPWV and various clinical parameters
| Parameter | Correlation coefficient (95% confidence interval) | |
|---|---|---|
| With baPWV | With central aortic pulse pressure | |
| baPWV | – | 0.635* (0.508–0.735) |
| Central aortic pulse pressure | 0.635* (0.508–0.735) | – |
| Age | 0.577** (0.436–0.670) | 0.511** (0.357–0.638) |
| Brachial pulse pressure | 0.441** (0.276–0.581) | 0.699** (0.578–0.790) |
| E/e' | 0.371** (0.197–0.523) | 0.368** (0.193–0.520) |
| Central aortic SBP | 0.369** (0.194–0.521) | 0.754** (0.659–0.825) |
| Brachial SBP | 0.305** (0.124–0.466) | 0.678** (0.551–0.774) |
| Left ventricular mass index | 0.302** (0.121–0.464) | 0.153 (−0.043 to 0.337) |
| Left atrial volume index | 0.226* (0.082–0.463) | 0.186 (−0.015 to 0.373) |
| Tricuspid regurgitation maximal velocity | 0.204 (−0.033 to 0.419) | 0.318** (0.090–0.515) |
| Left ventricular ejection fraction | 0.055 (−0.135‐0.241) | 0.312** (0.132–0.472) |
| Heart rate, beats per min | −0.002 (−0.190 to 0.186) | −0.053 (−0.243 to 0.137) |
| Brachial DBP | −0.097 (−0.280 to 0.093) | 0.124 (−0.081 to 0.319) |
| Central aortic DBP | −0.174 (−0.351 to 0.015) | −0.085 (−0.269 to 0.105) |
| White blood cell count | −0.188 (−0.363 to 0.000) | −0.325** (−0.484 to 0.146) |
| Height | −0.258* (−0.426 to 0.074) | −0.310** (−0.471 to 0.129) |
| eGFR | −0.317** (−0.477 to 0.137) | −0.351** (−0.476 to 0.123) |
| e' velocity | −0.369** (−0.521 to 0.194) | −0.349** (−0.504 to 0.172) |
Abbreviations: DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
Parameters were arranged by the direction of association and effect size for the correlation with brachial‐ankle pulse wave velocity (baPWV) (from the most positive association to most negative association).
*P < .05.
**P < .001.
Figure 1.
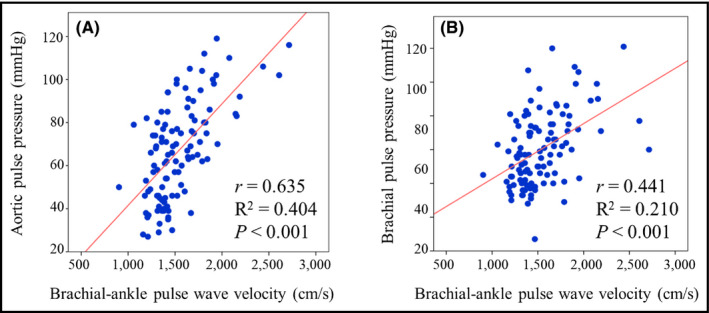
Linear correlation (A) between brachial‐ankle pulse wave velocity and invasively measured aortic pulse pressure, and (B) between brachial‐ankle pulse wave velocity and brachial pulse pressure
Table 4.
Independent association between PWV and central aortic pulse pressure
| Factor | β | 95% CI | P Value | VIF |
|---|---|---|---|---|
| baPWV | 0.574 | 0.027–0.055 | <.001 | 1.531 |
| Age | 0.024 | −0.474 to 0.573 | .851 | 2.495 |
| Height | −0.156 | −1.004 to 0.120 | .121 | 1.531 |
| eGFR | −0.082 | −0.327 to 0.141 | .432 | 1.670 |
| Heart rate | −0.112 | −0.531 to 0.091 | .163 | 1.161 |
| e' velocity | −0.011 | −0.277 to 0.249 | .915 | 1.699 |
| LA volume index | −0.043 | −0.431 to 0.261 | .625 | 1.173 |
Abbreviations: baPWV, brachial‐ankle pulse wave velocity; CI, confidence interval; eGFR, estimated glomerular filtration rate; LA, left atrial; PWV, pulse wave velocity; VIF, variance inflation factors.
A multiple linear regression model was constructed to evaluate the independent association of baPWV and APP after including potential confounders, such as age, height, heart rate, estimated glomerular filtration rate, e' velocity, and left atrial volume index. The model showed fair explanation (R 2 = .469), while the association between baPWV and APP remained significant even after controlling for confounders (β = 0.574, P < .001).
4. DISCUSSION
The current study showed that baPWV had a good linear correlation with invasively measured APP. This correlation between baPWV and APP remained significant even after controlling for potential clinical confounders. This finding supports that baPWV can be used as a useful surrogate marker of central aortic stiffness.
APP is a composite of a forward‐traveling wave generated by LV ejection, and a backward‐traveling reflected wave arising from the site of impedance mismatch.1 Although brachial BP is widely used in routine clinical assessment and known to be associated with future cardiovascular risk,28 recent evidence suggests that APP measurements are more accurate in predicting future cardiovascular events.6, 29 As central arteries are directly connected to vital organs, such as the heart, brain, and kidney, APP represents the true load imposed on these organs and influences the local flow into these vital organs.6 Indeed, previous studies have shown that APP is a predictor of end‐stage renal disease, microvascular damage in the brain, and hypertensive heart disease.30, 31
Cardiac catheterization is the most accurate method for measuring APP; however, it is difficult to use this invasive method in our daily clinical practice. Current studies have reported that noninvasive radial artery tonometry can estimate APP. However, these methods are operator‐dependent, showing a range of errors, which limit its accuracy.8 Still, direct intra‐arterial measurement is considered the gold standard.9 In this context, our results deserve clinical attention because invasive techniques, such as cardiac catheterization, are employed for more accurate and reliable measurement of APP.
Although baPWV has been widely used as a measure of arterial stiffness, some critics have suggested that baPWV may not reflect pure central aortic stiffness because it is measured at the peripheral extremities.32 Also, it is known that baPWV is more affected by peripheral artery diseases and BP at the time of measurement, compared with APP.33 In our study, however, baPWV showed a stronger correlation with APP than brachial PP. In addition, various studies have proven the predictive value of baPWV in cardiovascular events, which implies its association with central arterial stiffness.16, 17, 18 To date, few studies have evaluated the correlation between baPWV and APP. A study conducted by Jung and colleagues20 demonstrated that baPWV was associated with APP in patients with type 2 diabetes mellitus (r = .531, P < .001). Sueta and associates21 evaluated the association of APP with PWV in patients with CAD (r = .91, P < .001). Yamashina and researchers34 studied the correlation between aortic PWV and baPWV in 41 patients and showed excellent correlations between these values (r = .87, P < .01). However, Sueta and colleagues19 used mathematical transformation to calculate APP rather than cardiac catheterization, and Yamashina and associates34 conducted their study on a small population without adjustment for confounding factors. Compared with these studies, our study showed strengths because we used invasively measured APP and performed multivariable analysis.
4.1. Clinical implications
Considering the prognostic value of APP in future cardiovascular events,5, 6, 7 a noninvasively measured indicator of APP could provide valuable information on the prediction of patients' risk to clinicians. Our results showed that baPWV may be a good candidate for such purpose. In our study, it was found that baPWV can be a reliable marker for central aortic stiffness, which was measured by invasive method. With its simplicity and reproducibility,35 baPWV may be a good method to estimate APP in clinical practice, especially in the mass screening of large populations.
4.2. Study limitations
This study has several limitations. First, patients undergoing elective invasive coronary angiography were enrolled, which may have been associated with potential selection bias. Second, not all confounders were controlled as a result of the relatively small study sample size. Third, APP and baPWV were not measured simultaneously. However, we tried to minimize variability of various factors that could influence test results by performing both measurements on the same working day. Finally, clinical outcomes were not evaluated in our study. Large‐scale studies with long‐term follow‐up are needed to solve these issues.
5. CONCLUSIONS
This study demonstrated a linear correlation between baPWV and invasively measured APP, with strong correlation power, in patients undergoing invasive coronary angiography. Our findings suggest that baPWV can be a good surrogate marker of central aortic stiffness. Further large‐scale studies with a longitudinal clinical follow‐up design are needed to confirm our results.
Supporting information
Kang J, Kim HL, Lim WH, et al. Relationship between brachial‐ankle pulse wave velocity and invasively measured aortic pulse pressure. J Clin Hypertens. 2018;20:462–468. 10.1111/jch.13200
Jeehoon Kang and Hack‐Lyoung Kim contributed equally to this work.
REFERENCES
- 1. Dart AM, Kingwell BA. Pulse pressure––a review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001;37:975‐984. [DOI] [PubMed] [Google Scholar]
- 2. Safar ME, Levy BI, Struijker‐Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864‐2869. [DOI] [PubMed] [Google Scholar]
- 3. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55:1318‐1327. [DOI] [PubMed] [Google Scholar]
- 4. Nichols WW, Denardo SJ, Wilkinson IB, McEniery CM, Cockcroft J, O'Rourke MF. Effects of arterial stiffness, pulse wave velocity, and wave reflections on the central aortic pressure waveform. J Clin Hypertens (Greenwich). 2008;10:295‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown DW, Giles WH, Croft JB. Left ventricular hypertrophy as a predictor of coronary heart disease mortality and the effect of hypertension. Am Heart J. 2000;140:848‐856. [DOI] [PubMed] [Google Scholar]
- 6. Pini R, Cavallini MC, Palmieri V, et al. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: the ICARe Dicomano Study. J Am Coll Cardiol. 2008;51:2432‐2439. [DOI] [PubMed] [Google Scholar]
- 7. Kollias A, Lagou S, Zeniodi ME, Boubouchairopoulou N, Stergiou GS. Association of central versus brachial blood pressure with target‐organ damage: systematic review and meta‐analysis. Hypertension. 2016;67:183‐190. [DOI] [PubMed] [Google Scholar]
- 8. Nelson MR, Stepanek J, Cevette M, Covalciuc M, Hurst RT, Tajik AJ. Noninvasive measurement of central vascular pressures with arterial tonometry: clinical revival of the pulse pressure waveform? Mayo Clin Proc. 2010;85:460‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Bortel LM, Duprez D, Starmans‐Kool MJ, et al. Clinical applications of arterial stiffness, Task Force III: recommendations for user procedures. Am J Hypertens. 2002;15:445‐452. [DOI] [PubMed] [Google Scholar]
- 10. McEniery CM, Cockcroft JR, Roman MJ, Franklin SS, Wilkinson IB. Central blood pressure: current evidence and clinical importance. Eur Heart J. 2014;35:1719‐1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cavalcante JL, Lima JA, Redheuil A, Al‐Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57:1511‐1522. [DOI] [PubMed] [Google Scholar]
- 12. Najjar SS, Scuteri A, Shetty V, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51:1377‐1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laurent S, Katsahian S, Fassot C, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203‐1206. [DOI] [PubMed] [Google Scholar]
- 14. Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse‐wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085‐2090. [DOI] [PubMed] [Google Scholar]
- 15. Hofmann B, Riemer M, Erbs C, et al. Carotid to femoral pulse wave velocity reflects the extent of coronary artery disease. J Clin Hypertens (Greenwich). 2014;16:629‐633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Munakata M. Brachial‐ankle pulse wave velocity in the measurement of arterial stiffness: recent evidence and clinical applications. Curr Hypertens Rev. 2014;10:49‐57. [DOI] [PubMed] [Google Scholar]
- 17. Cainzos‐Achirica M, Rampal S, Chang Y, et al. Brachial‐ankle pulse wave velocity is associated with coronary calcium in young and middle‐aged asymptomatic adults: the Kangbuk Samsung Health Study. Atherosclerosis. 2015;241:350‐356. [DOI] [PubMed] [Google Scholar]
- 18. Katakami N, Osonoi T, Takahara M, et al. Clinical utility of brachial‐ankle pulse wave velocity in the prediction of cardiovascular events in diabetic patients. Cardiovasc Diabetol. 2014;13:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vlachopoulos C, Aznaouridis K, Terentes‐Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with brachial‐ankle elasticity index: a systematic review and meta‐analysis. Hypertension. 2012;60:556‐562. [DOI] [PubMed] [Google Scholar]
- 20. Jung CH, Jung SH, Kim KJ, et al. Differential associations of central and brachial blood pressure with carotid atherosclerosis and microvascular complications in patients with type 2 diabetes. BMC Cardiovasc Disord. 2014;14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sueta D, Yamamoto E, Tanaka T, et al. Association of estimated central blood pressure measured non‐invasively with pulse wave velocity in patients with coronary artery disease. Int J Cardiol Heart Vasc. 2015;8:52‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fihn SD, Blankenship JC, Alexander KP, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64:1929‐1949. [DOI] [PubMed] [Google Scholar]
- 23. Braunwald E, Morrow DA. Unstable angina: is it time for a requiem? Circulation. 2013;127:2452‐2457. [DOI] [PubMed] [Google Scholar]
- 24. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440‐1463. [DOI] [PubMed] [Google Scholar]
- 26. Kim HL, Seo JB, Chung WY, Kim SH, Kim MA, Zo JH. Association between invasively measured central aortic pressure and left ventricular diastolic function in patients undergoing coronary angiography. Am J Hypertens. 2015;28:393‐400. [DOI] [PubMed] [Google Scholar]
- 27. Kim HL, Im MS, Seo JB, et al. The association between arterial stiffness and left ventricular filling pressure in an apparently healthy Korean population. Cardiovasc Ultrasound. 2013;11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies C . Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903‐1913. [DOI] [PubMed] [Google Scholar]
- 29. Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197‐203. [DOI] [PubMed] [Google Scholar]
- 30. Adji A, O'Rourke MF, Namasivayam M. Arterial stiffness, its assessment, prognostic value, and implications for treatment. Am J Hypertens. 2011;24:5‐17. [DOI] [PubMed] [Google Scholar]
- 31. Hashimoto J, Westerhof BE, Westerhof N, Imai Y, O'Rourke MF. Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. J Hypertens. 2008;26:1017‐1024. [DOI] [PubMed] [Google Scholar]
- 32. Sugawara J, Hayashi K, Yokoi T, et al. Brachial‐ankle pulse wave velocity: an index of central arterial stiffness? J Hum Hypertens. 2005;19:401‐406. [DOI] [PubMed] [Google Scholar]
- 33. Tsuchikura S, Shoji T, Kimoto E, et al. Brachial‐ankle pulse wave velocity as an index of central arterial stiffness. J Atheroscler Thromb. 2010;17:658‐665. [DOI] [PubMed] [Google Scholar]
- 34. Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial‐ankle pulse wave velocity measurement. Hypertens Res. 2002;25:359‐364. [DOI] [PubMed] [Google Scholar]
- 35. Chae MJ, Jung IH, Jang DH, et al. The brachial ankle pulse wave velocity is associated with the presence of significant coronary artery disease but not the extent. Korean Circ J. 2013;43:239‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


