Abstract
Blood pressure control in patients with type 2 diabetes and hypertension is poor. It is unclear how effectively general practitioners assess and treat such patients. T2Target included hypertensive patients with type 2 diabetes mellitus who had undergone ambulatory blood pressure monitoring within the past 3 months. Recordings were analyzed by the general practitioner and an independent center and the conclusions were compared. Nighttime hypertension was reported less frequently by the general practitioner in comparison with central assessment (43.9% vs 77.9%, P<.001), as were masked (4.0% vs 13.1%, P<.001) and isolated office (4.4% vs 8.8%, P<.001) hypertension. A total of 13.9% of patients were deemed to have controlled blood pressure (ambulatory blood pressure monitoring). For the 784 patients with uncontrolled blood pressure, 40.7% underwent no change to their antihypertensive treatment. Cardiovascular risk was underestimated, with 11.1% deemed to be at very high risk, in contrast to the 97.0% of patients by central assessment. In conclusion, blood pressure control in hypertensive patients with type 2 diabetes mellitus is poor and not accurately assessed by office‐based general practitioners, despite the use of ambulatory blood pressure monitoring.
Keywords: ambulatory, antihypertensive drugs, cardiovascular risk, comorbidities, diabetes, masked, white coat
1. Introduction
Hypertension is highly prevalent in patients with type 2 diabetes mellitus (T2DM) and is reported in approximately 70% to 80% of cases.1, 2 This comorbidity significantly increases the patient's risk of death and of experiencing an adverse cardiovascular event such as myocardial infarction (MI) or stroke.3, 4 Despite the demonstrated benefits of lowering blood pressure (BP) in patients with T2DM,5 more emphasis is generally placed on treating hyperglycemia than on treating hypertension.6
Many studies have identified poor BP control in patients with T2DM.6 In Germany in particular, an analysis of six population‐based studies found that only 22.9% of diabetic patients with hypertension had a BP <140/90 mm Hg (the guideline‐recommended target at that time).7, 8 A further issue of concern is the additional comorbidities that are prevalent in patients with T2DM. Heart disease, cerebrovascular disease, and kidney disease are all common in these patients, and are all compounded by the presence of hypertension. Therefore, BP lowering is of great importance in the management of T2DM.
It is evident that the treatment of hypertension in patients with T2DM is inadequate; however, the reasons for such poor BP control require further study. The availability of ambulatory BP monitoring (ABPM) to primary care physicians provides the means by which reliable BP measurements can be obtained. However, the extent to which these values are utilized when determining treatment strategies is unknown. Moreover, the accuracy of a general physician's assessment of cardiovascular risk for individual patients is unclear.
To address these concerns, we analyzed data from the T2Target registry. This was a German registry that gathered information on patients with both T2DM and hypertension who were attending an appointment with a general practice physician. The data were evaluated in order to determine how physicians assessed BP control and cardiovascular risk in their patients. Furthermore, the data were reevaluated in a central, independent review, and the conclusions were used to establish the accuracy of the diagnoses made in the general practice clinic.
2. Methods
2.1. Study design and patients
The German T2Target registry was established across multiple physician offices in Germany. A total of 306 physicians were included. Of these, 184 were general practitioners, 121 were internal medicine physicians, and one was a diabetologist. An average of three patients were recruited per physician. Patients with T2DM who were being treated for hypertension were enrolled from December 2013 to June 2014. Patients were excluded from the registry if they had diagnosed secondary hypertension. All included patients provided written informed consent, and the study was approved by the responsible ethics committee.
2.2. Documentation
Patient characteristics including demographics, risk factors, and current treatment regimens were recorded on a case report form at the baseline visit. Patients were required to have ABPM measurements not older than 3 months available, with an office BP measurement obtained on the same day. Quality criteria for the ABPM were the availability of at least 14 valid measurements during the day (6:01 am–7 pm) and at least seven during the night (7:01 pm–6 am). All measurements were made with a properly validated device. Any changes made to hypertension treatment during the physician visit were documented, including dose alterations and changes in drug(s) prescribed.
2.3. Physician assessment
Physicians completed the case report form using both office BP and ABPM values. They noted the presence of BP control as well as isolated systolic or diastolic hypertension, masked hypertension, or isolated office hypertension. Several risk factors were assessed and entered into the case report form, but not further validated; thus, this information solely relies on the physician's assessment. Overall risk assessment was requested, categorizing patients as being at low, intermediate, high, or very high cardiovascular risk.
2.4. Central committee assessment
BP control was determined by a central assessment committee, employing the 2012 Joint Task Force of the European Society of Cardiology guidelines.9 These values were used because specific ABPM target values for patients with T2DM are not currently available. The mean ABPM was considered controlled below 130/80 mm Hg, with daytime control defined as <135/85 mm Hg and nighttime control defined as <120/70 mm Hg. For the patient's BP to be considered under control, each of the mean, daytime, and nighttime control criteria had to be satisfied. Isolated systolic hypertension was defined as >130/<80 mm Hg and isolated diastolic hypertension was defined as <130/>80 mm Hg, using mean ABPM readings. Masked hypertension was defined as at least one of the ABPM criteria for control being exceeded (mean, daytime, or nighttime), with an office BP <140/90 mm Hg. Isolated office hypertension was defined as all of the ABPM criteria for control being satisfied, with an office BP >140/90 mm Hg.
Cardiovascular risk assessment was performed according to the 2013 European Society of Hypertension/European Society of Cardiology guidelines on the management of arterial hypertension3 based on the presence of risk factors, end organ damage, diabetes, and cardiovascular events. Based on this risk estimate, patients were judged to have low, intermediate, intermediate to high, high, high to very high, or very high cardiovascular risk. To match the physician estimate, patients with moderate to high risk were counted as high, and patients with high to very high risk were counted as very high.
2.5. Statistical analysis
Office and ABPM values were analyzed using descriptive statistics. Continuous variables are given as means±standard deviations, and statistical significance was calculated using a t test. Categorical variables are given as absolute values or percentages, with statistical significance determined using Fisher exact test.
3. Results
3.1. Patient characteristics
Of the 960 patients with T2DM, established hypertension, and completed case report forms, ABPM data were available for 919 (the analysis population). The mean age of these patients was 64.4±12.3 years, and 51.0% were women (Table 1). The most common risk factors were dyslipidemia (63.2%), high waist circumference (53.1%), older age (men: >55 years, women: >65 years; 50.9%), family history of cardiovascular disease (39.4%), and heart disease (20.5%), while 3.3% had no additional risk factors.
Table 1.
Patient Characteristics (n=919)
| Mean±SD or No. (%) | |
|---|---|
| Age, y | 64.4±12.3 |
| Female sex | 469 (51.0) |
| Risk factorsa | |
| None | 30 (3.3) |
| Dyslipidemia | 581 (63.2) |
| High waist circumference | 488 (53.1) |
| Age (men >55 y, women >65 y) | 468 (50.9) |
| Family history of cardiovascular disease | 362 (39.4) |
| Heart disease | 188 (20.5) |
| Atherosclerotic plaques | 179 (19.5) |
| Smoker | 177 (19.3) |
| Left ventricular hypertrophy | 143 (15.6) |
| Microalbuminuria | 140 (15.2) |
| Elevated serum creatinine | 104 (11.3) |
| Low creatinine clearance | 93 (10.1) |
| Kidney disease | 71 (7.7) |
| Peripheral artery disease | 58 (6.3) |
| Cerebrovascular disease | 41 (4.5) |
| Elevated pulse wave velocity | 27 (2.9) |
| Advanced retinopathy | 26 (2.8) |
| Decreased ankle‐brachial index | 17 (1.8) |
Multiple nominations possible. Abbreviation: SD, standard deviation.
The mean office systolic BP/diastolic BP values were 151.7/87.3 mm Hg (Table 2), with 18.5% of patients achieving a value <140/85 mm Hg. ABPM assessment showed a mean BP of 138.6/79.3 mm Hg. Readings were higher during the day (141.3/81.8 mm Hg) than during the night (131.1/72.6 mm Hg).
Table 2.
BP Assessment
| Physician Assessment, Mean±SD or No. (%) | |
|---|---|
| Office BP (n=912) | |
| Systolic, mm Hg | 151.7±19.6 |
| Diastolic, mm Hg | 87.3±11.5 |
| BP <140/85 mm Hg, % | 169 (18.5) |
| ABPM | |
| Mean (n=902) | |
| Systolic, mm Hg | 138.6±15.2 |
| Diastolic, mm Hg | 79.3±9.9 |
| BP <130/85 mm Hg, % | 252 (27.9) |
| Daytime (n=919) | |
| Systolic, mm Hg | 141.3±15.2 |
| Diastolic, mm Hg | 81.8±10.2 |
| BP <135/85 mm Hg, % | 286 (31.1) |
| Nighttime (n=894) | |
| Systolic, mm Hg | 131.1±18.3 |
| Diastolic, mm Hg | 72.6±11.1 |
| BP <120/70 mm Hg, % | 194 (21.7) |
| Controlled BPa | 127 (13.9) |
| Antihypertensive therapy at baseline | |
| No treatment reportedb | 31 (3.4) |
| Drug treatment, yes (n=888)c | |
| RAS inhibitor | 791 (89.1) |
| Diuretic | 477 (53.7) |
| β‐Blocker | 470 (52.9) |
| Calcium channel blocker | 337 (38.0) |
| Others | 62 (7.1) |
| Combinations (n=919) | |
| Monotherapy | 227 (24.7) |
| Two | 259 (28.2) |
| Three | 240 (26.1) |
| Four or more | 162 (17.6) |
Abbreviations: ABPM, ambulatory blood pressure monitoring; RAS, renin‐angiotensin system; SD, standard deviation.
Displayed all of the following: mean blood pressure (BP) <130/85 mm Hg, daytime BP <135/85 mm Hg, and nighttime BP <120/70 mm Hg.
These were regarded as missing data because of hypertension treatment being an inclusion criterion.
Multiple nominations possible and percentages were calculated using the number of patients with available data on drug treatment (n=888).
The majority of patients were receiving renin‐angiotensin system inhibitors (89.1%), followed by diuretics (53.7%) and β‐blockers (52.9%). Combination antihypertensive treatment (≥2 drugs) was reported for 71.9% of patients.
3.2. Patient characteristics according to BP control
When patients with controlled BP according to the ABPM values in the European Society of Cardiology guidelines (n=127; 13.9%) were compared with those with uncontrolled BP (n=784; 86.1%), the former had lower office systolic BP and diastolic BP values, although the means were still close to the guideline‐defined treatment targets of 140 and 85 mm Hg (Table 3). This resulted in 50.4% of the controlled and 75.4% of the uncontrolled patients having office systolic BP ≥140 and/or diastolic BP ≥85 mm Hg.
Table 3.
Controlled vs Uncontrolled Patients
| Controlled Patients,a Mean±SD or No. (%) (n=127) | Uncontrolled Patients,b Mean±SD or No. (%)( )(n=784) | P Value | |
|---|---|---|---|
| Age, y | 67.3±11.5 | 63.9±12.3 | .003 |
| Female sex | 68 (53.5) | 398 (50.1) | .774 |
| Office BP | |||
| Systolic, mm Hg | 140.5±17.3 | 153.6±19.4 | <.001 |
| Diastolic, mm Hg | 82.3±10.1 | 88.1±11.4 | <.001 |
| BP ≥140 and/or ≥85 mm Hg | 64 (50.4) | 586 (75.4) | <.001 |
| Risk factors | |||
| None | 4 (3.1) | 25 (3.1) | 1.000 |
| Dyslipidemia | 79 (62.2) | 498 (63.5) | .767 |
| High waist circumference | 72 (56.7) | 413 (52.7) | .443 |
| Age (men >55 y, women >65 y) | 69 (54.1) | 395 (50.4) | .444 |
| Family history of cardiovascular disease | 59 (46.5) | 299 (38.1) | .078 |
| Heart disease | 39 (30.7) | 148 (18.9) | .003 |
| Atherosclerotic plaques | 34 (26.8) | 144 (18.4) | .030 |
| Smoker | 22 (17.3) | 150 (19.1) | .714 |
| Left ventricular hypertrophy | 28 (22.0) | 115 (14.7) | .048 |
| Microalbuminuria | 28 (22.0) | 112 (14.3) | .033 |
| Elevated serum creatinine | 24 (18.9) | 79 (10.1) | .006 |
| Low creatinine clearance | 20 (15.7) | 73 (9.3) | .038 |
| Kidney disease | 20 (15.7) | 51 (6.5) | .001 |
| Peripheral artery disease | 14 (11.0) | 44 (5.6) | .029 |
| Cerebrovascular disease | 9 (7.1) | 32 (4.1) | .161 |
| Elevated pulse wave velocity | 5 (3.9) | 22 (2.8) | .410 |
| Advanced retinopathy | 7 (5.5) | 19 (2.4) | .077 |
| Decreased ankle‐brachial index | 3 (2.4) | 14 (1.8) | .720 |
| Antihypertensive therapy at baseline | |||
| No treatment reported | 1 (0.8) | 30 (3.8) | .109 |
| Drug treatment yes | 126 (99.2) | 754 (96.2) | .109 |
| RAS inhibitor | 115 (91.3) | 669 (88.7) | .444 |
| Diuretic | 83 (65.9) | 393 (52.1) | .005 |
| β‐Blocker | 70 (55.6) | 397 (52.7) | .564 |
| Calcium channel blocker | 46 (36.5) | 289 (38.3) | .766 |
| Others | 4 (3.2) | 58 (7.7) | .087 |
| Combinations | |||
| Monotherapy | 25 (19.8) | 197 (26.1) | .150 |
| Two | 32 (25.4) | 225 (29.8) | .342 |
| Three | 48 (38.1) | 192 (25.5) | .005 |
| Four or more | 21 (16.7) | 140 (18.6) | .709 |
Eight patients did not have sufficient information to allow assessment of control.
Displayed all of the following: mean blood pressure (BP) <130/85 mm Hg, daytime BP <135/85 mm Hg, and nighttime BP <120/70 mm Hg.
At least one of the mean, daytime, and nighttime BP values was above the defined value for control.
Abbreviations: RAS, renin‐angiotensin system; SD, standard deviation.
Many of the documented comorbidities were more prevalent in the patients with controlled BP compared with those with uncontrolled BP. Notable differences were seen for heart disease (30.7% vs 18.9%, P=.003) and kidney disease (15.7% vs 6.5%, P=.001).
The type of antihypertensive therapy the patients were being treated with at the baseline visit did not vary greatly depending on BP control (Table 3). The only exception was the treatment with diuretics, which were more commonly used by the patients with controlled BP (65.9% vs 52.1%, P=.005). The numbers of patients being treated with monotherapy were similar, but more of the patients with controlled compared with uncontrolled BP were receiving three or more antihypertensive agents (38.1% vs 25.5%, P=.005).
During the physician visit, a higher proportion of the patients with controlled BP had no change made to their antihypertensive treatment (73.3% vs 40.7% for uncontrolled BP, P<.001; Table 4). Furthermore, 5.2% of the controlled group had their dosage reduced. For the patients with uncontrolled BP, 29.9% had a dose increase and 26.8% had a further antihypertensive drug added to their treatment regimen.
Table 4.
Action Taken in Controlled vs Uncontrolled Patients
| Controlled Patients,a No. (%) (n=127) | Uncontrolled Patients,b No. (%) (n=784) | P Value | |
|---|---|---|---|
| Dose unchanged and no drug added | 99 (73.3) | 322 (40.7) | <.001 |
| Dose increased | 10 (7.4) | 237 (29.9) | <.001 |
| Dose decreased | 7 (5.2) | 9 (1.1) | .003 |
| Addition of antihypertensive drug | 11 (8.1) | 212 (26.8) | <.001 |
| RAS inhibitor | 6/11 (54.5) | 88/212 (41.5) | .533 |
| Diuretic | 8/11 (72.7) | 60/212 (28.3) | .004 |
| β‐Blocker | 1/11 (9.1) | 25/212 (11.8) | 1.000 |
| Calcium channel blocker | 2/11 (18.2) | 75/212 (35.4) | .338 |
| Others | 0/11 (0.0) | 19/212 (9.0) | .605 |
Eight patients did not have sufficient information to allow assessment of control.
Displayed all of the following: mean blood pressure (BP) <130/85 mm Hg, daytime BP <135/85 mmHg, and nighttime BP <120/70 mm Hg.
At least one of the mean, daytime, and nighttime BP values was above the defined value for control.
Abbreviation: RAS, renin‐angiotensin system.
3.3. Physician vs central assessment of BP control and cardiovascular risk
While differences in the assessment of combined systolic‐diastolic hypertension and isolated systolic hypertension between the physicians and the central committee were negligible, isolated diastolic hypertension (0.9% vs 5.7%, P<.001) and nighttime hypertension (43.9% vs 77.9%, P<.001) were more frequently diagnosed centrally (Figure 1A). Masked and isolated office hypertension were diagnosed by physicians in 4.0% and 4.4% of cases, respectively (Figure 1B). Central assessment, however, indicated that these conditions were substantially more prevalent (13.1% and 8.8%, P<.001 for both vs physician assessment).
Figure 1.
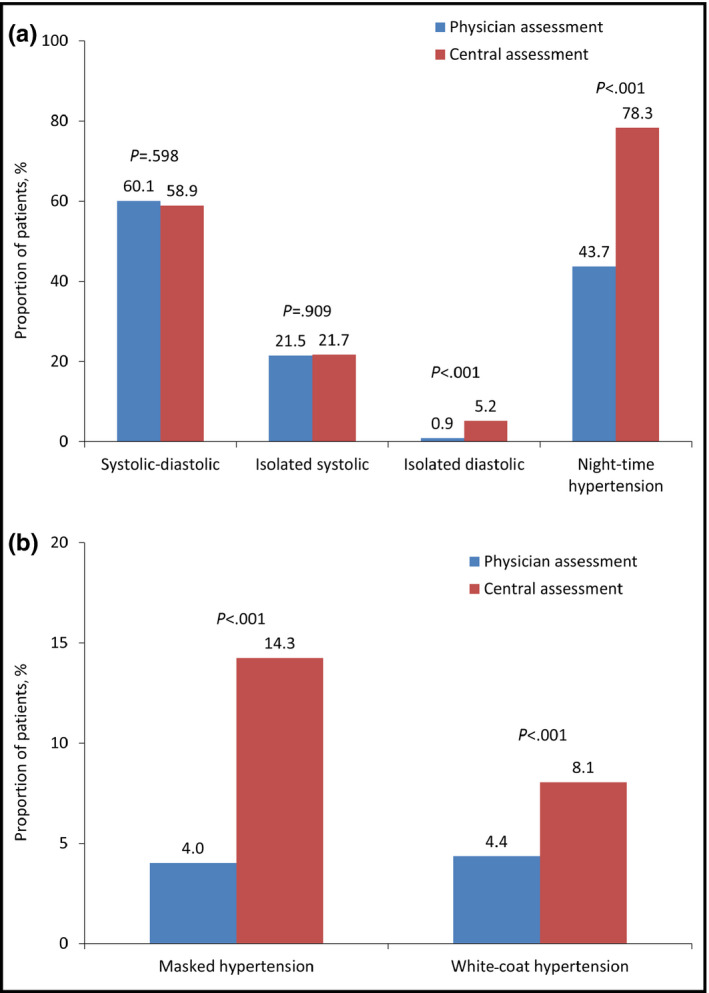
Physician vs central committee assessment of hypertension type based on ambulatory blood pressure monitoring. (A) n=866. (B) n=860.
There were significant differences in the 10‐year cardiovascular risk determined by the physicians in comparison to that by the central committee assessment (Figure 2). The physicians categorized only 11.1% of patients as being at very high risk, with greater proportions being at high (42.8%) or moderate (39.1%) risk. In stark contrast, the central committee determined that 97.0% of patients were at very high risk, with only 3.0% at high risk and none at moderate or low risk (P<.001 for all comparisons).
Figure 2.
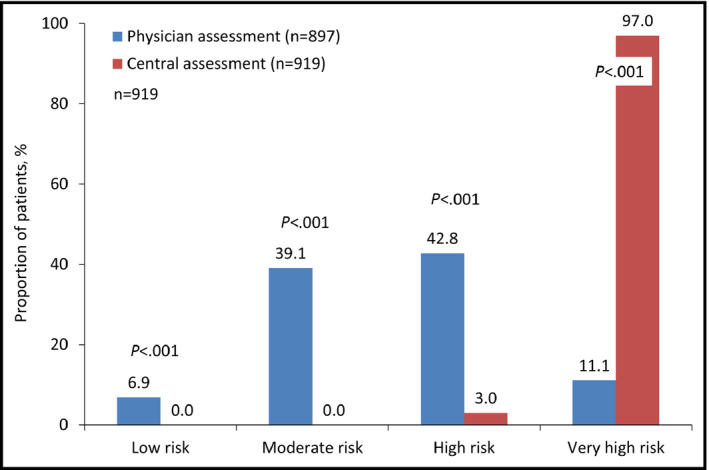
Ten‐year risk according to European Society of Hypertension/European Society of Cardiology guidelines 20133
4. Discussion
To assess the evaluation and treatment of hypertension in patients with type 2 diabetes in a real‐world clinical setting, we analyzed data from the German T2Target registry. While physicians’ identification of combined or isolated systolic hypertension appeared to be accurate, nighttime, isolated office, and masked hypertension were significantly underdiagnosed. Similarly, cardiovascular risk was often deemed to be less severe than that corresponding to expert guidelines, placing doubt on the adequacy of treatment strategies. Treatment intensification for patients with uncontrolled BP was inadequate.
It is possible that the lack of recognition of isolated diastolic hypertension is partly due to the use of ABPM values. While guidelines provide both office and ambulatory cutoffs for other forms of hypertension, there is no definition of isolated diastolic hypertension using ABPM measurements.3 The underreporting of nighttime hypertension suggests that ABPM readings taken during the night were not fully utilized by the physicians, resulting in them underestimating the presence of this condition. Similarly, masked and isolated office hypertension were diagnosed less frequently by the treating physicians than by the central committee assessment. ABPM is especially valuable for identifying such conditions, as they cannot be definitively diagnosed from office‐based readings. However, it appears that the physicians may not have evaluated all the data available to them in detail or may have drawn conclusions that were not strictly in line with the guidelines.3, 9
The proportion of patients who were deemed to have controlled hypertension according to their ABPM measurements was 13.9%. This is lower than for other studies, which have reported values of 40% and 38.3% for office BP control at <140/90 mm Hg10, 11 and 52% for <140/80 mm Hg.12 However, for studies that defined stricter control targets, the percentages were much lower at 23% (<135/85 mm Hg)10 and 11.4% and 9.8% (<130/85 mm Hg).11, 13 It should be noted that the ABPM values used as cutoffs for the definition of BP control in the present study were not specific for patients with T2DM. However, if such values were to be derived, they would likely be slightly lower than those used here, for the office BP target, which is 140/90 mm Hg in general and 140/85 mm Hg for patients with diabetes.3 Therefore, the extent of uncontrolled hypertension in patients with diabetes is likely greater with specific BP definitions for patients with diabetes than described here. Interestingly, a German analysis of patients with T2DM reported a more similar level of control to that found in the present analysis (22.9% at <140/90 mm Hg),14 suggesting that hypertension control in Germany may be particularly poor. In the present study, the patients with controlled BP were older on average, and higher percentages had comorbidities such as heart disease, left ventricular hypertrophy, and kidney disease. This is partly in contrast with other studies, which have found older age15, 16, 17 and the presence of left ventricular hypertrophy and multimorbidity to be predictive of a lack of BP control.15, 18 However, coronary heart disease has previously been associated with a greater chance of achieving BP control.16, 17 It should be noted that only low percentages of patients in these reports had T2DM, the presence of which is likely to affect BP‐lowering and treatment strategies.19 Older age and presence of comorbidities in the patients in the present analysis may have led to more intensive BP‐lowering therapy being prescribed. However, there were few significant differences apparent in terms of the medications used by the controlled and uncontrolled groups. Diuretics were used more commonly by the patients with controlled BP, and a greater proportion of patients were being treated with a combination of three different drugs. Accordingly, a slightly higher percentage of the patients with uncontrolled BP were receiving monotherapy.
The finding that 18.6% of patients with uncontrolled BP were being treated with four or more antihypertensive drugs suggests the presence of a significant proportion of treatment‐resistant patients. The guidelines state that patients should be considered resistant to treatment if they have uncontrolled hypertension while taking three or more antihypertensive drugs at appropriate doses, of which one is a diuretic.3 Poor adherence to treatment could have contributed to the high number of patients who, despite being treated with multiple drugs, remained uncontrolled. Furthermore, it is not known to what extent the dosages of the individual drugs were optimal. It has been reported that patients with T2DM are more likely to require combination therapy to achieve BP control, with the condition being predictive of failure to reach an adequate level while taking treatment.3, 7, 20, 21 Therefore, additional drugs and/or higher dosages may be required for T2DM patients with uncontrolled BP. Despite this apparent undertreatment of hypertension in many patients, approximately 40% of those considered to have uncontrolled BP did not have any changes made to their therapy at the physician visit, a finding that is in agreement with previous studies.22, 23, 24
When asked to estimate the 10‐year cardiovascular risk of the patients, physicians reported much lower risk levels than those given by the central committee. According to the current guidelines, patients with diabetes are automatically at high or very high risk, even with office BP values in the high‐normal range (130–139/85–89 mm Hg)3, 9; therefore, the proportions of patients classified by the physician as being at low or moderate risk is surprising. This indicates that the presence of diabetes is not being sufficiently taken into account in these hypertensive patients.
5. Conclusions
In a large cohort of patients with T2DM and hypertension, BP control was found to be extremely poor. Furthermore, there was a large discrepancy between the level of control perceived by the office‐based physicians and the actual level of control according to guidelines. This indicates inadequate use of available measurements and/or poor adherence to or knowledge of guidelines. Physicians also greatly underestimated the cardiovascular risk level of patients. Accordingly, many patients who required treatment intensification did not receive it. Physicians clearly underestimated the cardiovascular risk level of these patients. Significant improvements need to be made to the treatment of these very high‐risk patients.
Conflict of Interest Disclosure
TM and WS received funding by Servier for designing and conducting the study. PB received funding by Servier for preparing the first version of the manuscript. AK is an employee of Servier. UL and JM have no conflicts of interest to disclose.
Authors’ Contributions
TM, UL, AK, and WS designed the study and were responsible for the acquisition of data. UL, JM, and PB designed and conducted the analyses. All authors interpreted the data. PB drafted the manuscript and the other authors revised it for important intellectual content. All authors approved the final version to be published and agree to be accountable for all aspects of the work.
Acknowledgment
The contribution of each participating physician is acknowledged. The editorial support of Katherine H. Smith (Institute for Pharmacology and Preventive Medicine) is acknowledged.
Mengden T, Ligges U, Mielke J, Bramlage P, Korzinek A, Sehnert W. Blood pressure control and cardiovascular risk in hypertensive patients with type 2 diabetes: The German T2Target registry. J Clin Hypertens. 2017;19:757–763. 10.1111/jch.13001
Funding information
The study was funded by Servier Germany GmbH, Munich, Germany
The data were previously presented at the European Society of Cardiology Congress 2015.
References
- 1. Fox CS, Golden SH, Anderson C, et al. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2015;132:691‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cryer MJ, Horani T, DiPette DJ. Diabetes and hypertension: a comparative review of current guidelines. J Clin Hypertens (Greenwich). 2016;18:95‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281‐1357. [DOI] [PubMed] [Google Scholar]
- 4. Chen G, McAlister FA, Walker RL, et al. Cardiovascular outcomes in framingham participants with diabetes: the importance of blood pressure. Hypertension. 2011;57:891‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703‐713. [PMC free article] [PubMed] [Google Scholar]
- 6. McLean DL, Simpson SH, McAlister FA, et al. Treatment and blood pressure control in 47,964 people with diabetes and hypertension: a systematic review of observational studies. Can J Cardiol. 2006;22:855‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206‐1252. [DOI] [PubMed] [Google Scholar]
- 8. Mancia G, De Backer G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105‐1187. [DOI] [PubMed] [Google Scholar]
- 9. Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635‐1701. [DOI] [PubMed] [Google Scholar]
- 10. Borzecki AM, Wong AT, Hickey EC, et al. Hypertension control: how well are we doing? Arch Intern Med. 2003;163:2705‐2711. [DOI] [PubMed] [Google Scholar]
- 11. Katayama S, Inaba M, Morita T, et al. Blood pressure control in Japanese hypertensives with or without type 2 diabetes mellitus. Hypertens Res. 2000;23:601‐605. [DOI] [PubMed] [Google Scholar]
- 12. Joseph F, Younis N, Sowery J, et al. Blood pressure control in diabetes: are we achieving the guideline targets? Pract Diab Int. 2003;20:276‐282. [Google Scholar]
- 13. Sequeira RP, Al Khaja KA, Damanhori AH. Evaluating the treatment of hypertension in diabetes mellitus: a need for better control? J Eval Clin Pract. 2004;10:107‐116. [DOI] [PubMed] [Google Scholar]
- 14. Ruckert IM, Schunk M, Holle R, et al. Blood pressure and lipid management fall far short in persons with type 2 diabetes: results from the DIAB‐CORE Consortium including six German population‐based studies. Cardiovasc Diabetol. 2012;11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lloyd‐Jones DM, Evans JC, Larson MG, et al. Differential control of systolic and diastolic blood pressure: factors associated with lack of blood pressure control in the community. Hypertension. 2000;36:594‐599. [DOI] [PubMed] [Google Scholar]
- 16. Labeit AM, Klotsche J, Pieper L, et al. Changes in the prevalence, treatment and control of hypertension in Germany? A clinical‐epidemiological study of 50.000 primary care patients. PLoS One. 2012;7:e52229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Knight EL, Bohn RL, Wang PS, et al. Predictors of uncontrolled hypertension in ambulatory patients. Hypertension. 2001;38:809‐814. [DOI] [PubMed] [Google Scholar]
- 18. Li YT, Wang HH, Liu KQ, et al. Medication adherence and blood pressure control among hypertensive patients with coexisting long‐term conditions in primary care settings: a cross‐sectional analysis. Medicine (Baltimore). 2016;95:e3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campo C, Segura J, Ruilope LM. Factors influencing the systolic blood pressure response to drug therapy. J Clin Hypertens (Greenwich). 2002;4:35‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lastra G, Syed S, Kurukulasuriya LR, et al. Type 2 diabetes mellitus and hypertension: an update. Endocrinol Metab Clin North Am. 2014;43:103‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mancia G, Schumacher H, Redon J, et al. Blood pressure targets recommended by guidelines and incidence of cardiovascular and renal events in the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET). Circulation. 2011;124:1727‐1736. [DOI] [PubMed] [Google Scholar]
- 22. Kerr EA, Zikmund‐Fisher BJ, Klamerus ML, et al. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. 2008;148:717‐727. [DOI] [PubMed] [Google Scholar]
- 23. Bolen SD, Samuels TA, Yeh HC, et al. Failure to intensify antihypertensive treatment by primary care providers: a cohort study in adults with diabetes mellitus and hypertension. J Gen Intern Med. 2008;23:543‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Voorham J, Haaijer‐Ruskamp FM, Wolffenbuttel BH, et al. Differential effects of comorbidity on antihypertensive and glucose‐regulating treatment in diabetes mellitus–a cohort study. PLoS One. 2012;7:e38707. [DOI] [PMC free article] [PubMed] [Google Scholar]


