Abstract
Plasma homocysteine (Hcy) levels are associated with elevated blood pressure. However, the causal association between Hcy levels and the risk of hypertension remains ambiguous. Taking the study design effect into consideration, this study aimed to investigate this issue through a cross‐sectional and longitudinal analysis. Data were obtained from the Beijing Health Management Cohort study, which conducted routine health check‐ups from 2012 to 2017. Multivariate logistic regression was used for the cross‐sectional analysis, and a quadratic inference function approach was performed for the longitudinal analysis. A total of 30 376 subjects (mean age = 50.0 years) were included in the cross‐sectional analysis, and a subgroup of 3913 subjects without hypertension at baseline was included in the longitudinal analysis. After adjusting for potential confounders, the risk of hypertension increased with Hcy levels in the cross‐sectional analysis using the traditional definition of hypertension (OR = 1.262, 95% CI: 1.155‐1.378, Q2 vs Q1; OR = 1.458, 95% CI: 1.335‐1.593, Q3 vs Q1; OR = 1.520, 95% CI: 1.388‐1.664, Q4 vs Q1) and the 2017 hypertension definition (OR = 1.159, 95% CI: 1.067‐1.259, Q2 vs Q1; OR = 1.328, 95% CI: 1.221‐1.445, Q3 vs Q1; OR = 1.328, 95% CI: 1.217‐1.449, Q4 vs Q1). The longitudinal analysis showed that hypertension risk increased in the third quartile of Hcy (OR = 1.268, 95% CI: 1.030‐1.560, Q3 vs Q1). Elevated total plasma Hcy may be used as a predictive biomarker for hypertension. Attention should be paid to gender‐specific mechanisms when issuing precise precautions.
Keywords: cross‐sectional analysis, gender differences, hypertension, longitudinal analysis, plasma homocysteine
1. INTRODUCTION
Hypertension contributes to the burden of heart disease, stroke, and kidney failure, as well as premature mortality and morbidity. The worldwide burden of hypertension has increased by almost 30% from 1990 to 2010.1, 2 Among Chinese people aged 35‐75 years, nearly half have hypertension.3 A previous study using a nationally representative sample in mainland China proposed that 23.2% (an estimated 244.5 million) of the adult population had hypertension, and another 41.3% (435.3 million) had prehypertension,4 indicating that China may be facing serious issues with regard to the prevention of hypertension. Hypertension rarely causes symptoms in its early stages, and many patients go undiagnosed. The identification of early biomarkers such as plasma homocysteine (Hcy)5, 6, 7 is of pivotal importance.
Plasma Hcy is a sulfur amino acid that is not included in the structure of a protein. It is formed during the metabolism of methionine from diet or endogenous protein degradation.8 Converted by transsulfuration into cysteine, Hcy is cleared mainly through the kidneys. Published studies have shown that higher plasma Hcy levels increase the risk of diseases such as acute myocardial infarction, thrombosis, atherosclerosis,9 and elevated blood pressure.10 Elevated Hcy levels can be inherited and/or acquired.8, 11 Multiple studies have demonstrated that elevated levels of Hcy may cause changes in the vascular endothelium, mainly mediated by the toxic effect of oxidized forms of this amino acid.12
Recent studies have shown that, among women, the concentration of Hcy is 90% lower than the concentration found among men.13 A prospective nested case‐control study14 highlighted that elevated plasma Hcy levels at baseline were associated with an increased risk of hypertension for men, in accordance with the results found by Yücel et al15 A cross‐sectional study revealed that Hcy was not associated with masked hypertension and/or high blood pressure levels in women. In contrast to the findings above, elevated plasma Hcy levels were not found as a significant risk factor for hypertension in women in a 2‐year follow‐up study.16 However, another study demonstrated that Hcy was an independent predictor in female patients with hypertension.17
A recent meta‐analysis showed that the study design (eg, cross‐sectional or cohort) contributed to the high heterogeneity of the existing results.18 To date, only a limited number of prospective studies have been performed on this topic. For example, Borges et al19 reported that elevated plasma Hcy levels did not play a causal role in blood pressure. However, this study was conducted from 2004 to 2005, and the time period was too short for a longitudinal analysis. Zhong et al20 suggested that Hcy may further increase the risk of poor outcomes among patients with hypertension, but the sample size in their study was relatively small to detect these effects. The present findings have not verified the hypothesis that Hcy plays a causal role in increasing the risk of hypertension. To the best of our knowledge, no study has addressed the comparison between the cross‐sectional and longitudinal relationships and between Hcy and hypertension in a general large‐scale population‐based study in China.
We hypothesized that (a) increased plasma Hcy levels may be associated with the risk of hypertension specified by gender and (b) the study design may influence the results. Thus, the aim of the present study was to cross‐sectionally and longitudinally explore whether plasma Hcy levels are associated with hypertension in the Chinese population.
2. MATERIAL AND METHODS
2.1. Study sample
The Beijing Health Management Cohort (BHMC) study is a large prospective dynamic cohort study. The study design was illustrated in former research.21 Data on a total of 45 816 individuals were retrieved from the BHMC study, with data collected annually from January 2012 to December 2017 from health check‐ups. Adequate measurements of variables such as biochemical indices and relevant demographic characteristics were missing for 15 440 respondents. Finally, 30 376 participants were enrolled in the cross‐sectional analysis.
For the longitudinal analysis, of the subjects who were included in the primary study (n = 30 376), we excluded 26 470 subjects for the following reasons: 13 750 subjects were excluded because only one measurement was made during the 6‐year follow‐up period, and 12 720 subjects were excluded due to a prior diagnosis of hypertension, a history of hypertensive diseases, or a history of taking antihypertensive drugs at baseline. Ultimately, 3913 subjects (1422 women and 2491 men) with 10 963 measurements (participants had more than one examination) were included in the longitudinal analysis.
The study followed the guidelines of the Declaration of Helsinki, and written informed consent was obtained from each subject. Approval for these experiments was obtained from the Ethics Committee of Capital Medical University (approval number: 2015SY33).
2.2. Definition of hypertension
Blood pressure (BP) was measured by a trained nurse on the right arm of each participant (after at least 5 minutes of rest) during the check‐up, using the standard classification criteria. In the 30 minutes preceding the measurements, the participants were required to avoid smoking or the consumption of caffeine. Three readings of systolic and diastolic BP were recorded for each participant, and an average of the three measurements was used. Following the 2017 American College of Cardiology (ACC), American Heart Association (AHA) High Blood Pressure Guideline,22 hypertension was defined as a systolic BP ≥130 mm Hg, a diastolic BP ≥80 mm Hg, and/or the use of antihypertensive medicine within 2 weeks before data collection. The traditional definition of hypertension, a systolic BP ≥140 mm Hg, a diastolic BP ≥90 mm Hg, and/or the use of antihypertensive medicine within 2 weeks was used in the sensitivity analysis.
2.3. Measurements of the variables
Data on medications, biochemical indices, and demographic characteristics were obtained from all subjects who underwent successive standardized physical examinations. Participants were required to remove their shoes to measure their weight and height, and body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Lifestyle factors such as smoking and alcohol consumption were recorded using a practical and structured questionnaire. Blood samples were collected in the morning from an antecubital vein into tubes containing ethylenediaminetetraacetic acid. Blood platelets (PLT), fasting plasma glucose (FPG), triglycerides (TG), high‐density lipoprotein (HDL), low‐density lipoprotein (LDL), total cholesterol (TC), white blood cell count (WBC), red blood cell count (RBC), hemoglobin (HGB), red blood cell specific volume (HCT), creatinine (Cr), erythrocyte mean corpuscular volume (MCV), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and hypersensitive C‐reactive protein (hs‐CRP) were measured using an autoanalyzer (Sysmex SE‐9000, Kobe, Japan) in the same laboratory. The estimated glomerular filtration rate (eGFR) was calculated using the Chinese Chronic Kidney Disease Epidemiology Collaboration Equation 23
2.4. Statistical analysis
The original continuous Hcy value was categorized into four levels: ≤P 25 for quartile 1 (Q1), >P 25 and ≤P 50 for quartile 2 (Q2), >P 50 and ≤P 75 for quartile 3 (Q3), and >P 75 for quartile 4 (Q4).
Descriptive analyses of the participants’ general characteristics were performed by gender, and levels of plasma Hcy. Continuous variables are shown as the means ± standard deviation, and categorical variables are presented as numbers and percentages. Normality assumptions of the continuous variables were tested. Student's t test or Wilcoxon test was used for continuous variables to detect any statistically significant differences between genders. Quartile groups were tested using ANOVAs and χ2 tests when appropriate.
Multivariate logistic regression was conducted to assess the odds ratios (ORs) for cross‐sectional associations between Hcy and hypertension for each quartile compared to the reference group (the lowest quartile: Q1).
The quadratic inference function (QIF) approach was used to assess the relationship between Hcy and hypertension in the longitudinal analysis. A previous study showed that the QIF method is more acceptable for correlated data because of its advantages over generalized estimating Equation.24 First, the QIF method requires fewer model assumptions.25 Second, this technique constructs more estimating functions than the number of parameters. QIF does not need to estimate the parameters in a given correlation structure, especially when the working correlation is misspecified.26 Finally, the QIF estimators are robust with a bounded influence function against unduly large outliers or contaminated data points.27
To better clarify the relationship between hypertension and Hcy, several confounding factors were adjusted for multiple regression and the QIF models. Model 1 was a univariate model. In Model 2, gender, age, eGFR, BMI, FPG, TG, HDL, the ratio of AST to ALT (AST/ALT), HCT, PLT, WBC, RBC, TC, HGB, and MCV were adjusted. In Model 3, we adjusted for smoking and drinking status, as well as the variables in Model 2.
All analyses were performed using SAS software (version 9.4; SAS Institute Inc, Cary, North Carolina, USA). Two‐sided P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Cross‐sectional characteristics
Table 1 summarizes the cross‐sectional characteristics of the participants. The mean age of all the participants was 50 years old, and 32.02% (9727/30 376) were females. No significant differences were observed in smoking prevalence by gender. Compared to men, women had higher levels of HDL, AST/ALT, and PLT (all Ps < 0.001) and lower stages of other clinical and demographic characteristics. We also analyzed plasma Hcy concentrations and its correlation with BP (Figures S1 and S2).
Table 1.
Characteristics of the study participants in the cross‐sectional analyses
| Variables | Total (n = 30 376) | Male (n = 20 649) | Female (n = 9727) | P‐value |
|---|---|---|---|---|
| Hcy (mg/dL) | 11.76 ± 7.63 | 13.24 ± 8.53 | 8.64 ± 3.68 | <0.0001b |
| SBP (mm Hg) | 123.87 ± 16.30 | 127.25 ± 15.18 | 116.67 ± 16.28 | <0.0001b |
| DBP (mm Hg) | 72.37 ± 10.92 | 74.45 ± 10.75 | 67.92 ± 9.90 | <0.0001b |
| Age (y) | 49.95 ± 12.76 | 51.04 ± 12.65 | 47.63 ± 12.69 | <0.0001b |
| Egfr | 107.50 ± 40.92 | 119.84 ± 38.78 | 81.27 ± 32.05 | <0.0001b |
| BMI (kg/m2) | 25.62 ± 3.75 | 26.30 ± 3.19 | 24.14 ± 4.43 | <0.0001a |
| FPG (mmol/L) | 5.60 ± 1.38 | 5.75 ± 1.49 | 5.28 ± 1.03 | <0.0001b |
| TG (mmol/L) | 1.67 ± 1.59 | 1.87 ± 1.77 | 1.25 ± 1.00 | <0.0001b |
| HDL (mmol/L) | 1.30 ± 0.35 | 1.22 ± 0.30 | 1.49 ± 0.38 | <0.0001b |
| AST/ALT | 1.19 ± 0.89 | 1.11 ± 0.96 | 1.37 ± 0.71 | <0.0001b |
| HCT (%) | 43.49 ± 3.99 | 45.23 ± 3.12 | 39.80 ± 3.01 | <0.0001b |
| PLT (109/L) | 227.36 ± 54.24 | 217.61 ± 49.94 | 248.06 ± 57.15 | <0.0001b |
| WBC (1012/L) | 6.20 ± 1.57 | 6.28 ± 1.58 | 5.81 ± 1.46 | <0.0001b |
| RBC (1012/L) | 4.77 ± 0.45 | 4.94 ± 0.39 | 4.41 ± 0.33 | <0.0001b |
| TC (mmol/L) | 4.79 ± 0.95 | 4.77 ± 0.97 | 4.83 ± 0.90 | <0.0001b |
| HGB (g/L) | 147.81 ± 15.72 | 154.91 ± 11.76 | 132.70 ± 11.96 | <0.0001b |
| MCV (fl) | 91.27 ± 4.95 | 91.69 ± 4.60 | 90.37 ± 5.50 | <0.0001b |
| Smoker (n, %) | 109 (0.36) | 86 (0.28) | 23 (0.08) | 0.0144c |
| Alcohol user (n, %) | 301 (0.99) | 214 (0.70) | 87 (0.89) | 0.2439c |
ALT, alanine aminotransferase (mmol/L); AST, aspartate aminotransferase (mmol/L); BMI, body mass index (kg/m2); DBP, diastolic blood pressure (mm Hg); Drinking, any alcoholic drinks once a week; eGFR, the estimated glomerular filtration rate; FPG, fasting plasma glucose (mmol/L); HCT, red blood cell specific volume (%); Hcy, homocysteine(mg/dL); HDL, high‐density lipoprotein (mmol/L); HGB, hemoglobin (g/L); MCV, erythrocyte mean corpuscular volume (fl); PLT, blood platelet(109/L); RBC, red blood cell (1012/L); SBP, systolic blood pressure (mm Hg); Smoking, any tobacco usage; TC, total cholesterol (mmol/L); TG, triglyceride (mmol/L) WBC, white blood cell (1012/L).
The result of student's t test.
The result of Kruskal‐Wallis rank test.
The result of χ 2 test.
3.2. Cross‐sectional association between Hcy levels and hypertension
The results of the cross‐sectional analyses from both the univariate and the multivariate regressions are described in Table 2. The prevalence of hypertension at different Hcy levels shows the increasing trends in the different age groups (Figure S3).
Table 2.
Cross‐sectional association between Hcy and hypertension (n = 30 376)
| Total | The new definition of hypertension | The traditional definition of hypertension | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |
| Q1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q2 | 1.633 (1.530, 1.742) | 1.160 (1.068, 1.261) | 1.159 (1.067, 1.259) | 1.632 (1.521, 1.751) | 1.262 (1.155, 1.378) | 1.262 (1.155, 1.378) |
| Q3 | 2.166 (2.030, 2.311) | 1.329 (1.222, 1.446) | 1.328 (1.221, 1.445) | 2.104 (1.962, 2.255) | 1.460 (1.336, 1.594) | 1.458 (1.335, 1.593) |
| Q4 | 2.407 (2.254, 2.571) | 1.331 (1.219, 1.452) | 1.328 (1.217, 1.449) | 2.335 (2.178, 2.504) | 1.521 (1.389, 1.666) | 1.520 (1.388, 1.664) |
| Male | ||||||
| Q1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q2 | 1.148 (1.047, 1.259) | 1.145 (1.027, 1.277) | 1.143 (1.025, 1.275) | 1.200 (1.092, 1.320) | 1.225 (1.094, 1.371) | 1.223 (1.092, 1.368) |
| Q3 | 1.337 (1.222, 1.462) | 1.353 (1.217, 1.503) | 1.351 (1.215, 1.501) | 1.399 (1.276, 1.533) | 1.447 (1.298, 1.613) | 1.442 (1.294, 1.608) |
| Q4 | 1.352 (1.238, 1.477) | 1.368 (1.232, 1.520) | 1.365 (1.229, 1.516) | 1.450 (1.325, 1.588) | 1.534 (1.377, 1.710) | 1.531 (1.374, 1.706) |
| Female | ||||||
| Q1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Q2 | 1.372 (1.236, 1.523) | 1.191 (1.041, 1.362) | 1.195 (1.045, 1.367) | 1.488 (1.324, 1.672) | 1.303 (1.122, 1.514) | 1.298 (1.118, 1.508) |
| Q3 | 1.652 (1.464, 1.865) | 1.262 (1.080, 1.475) | 1.269 (1.086, 1.484) | 1.807 (1.582, 2.063) | 1.435 (1.210, 1.703) | 1.426 (1.203, 1.691) |
| Q4 | 1.658 (1.414, 1.944) | 1.105 (0.896, 1.363) | 1.109 (0.899, 1.369) | 1.903 (1.603, 2.259) | 1.291 (1.029, 1.619) | 1.286 (1.026, 1.613) |
Q1‐Q4 represent 4 levels of the original continuous serum Hcy using the 3 quartiles (P25, P50 and P75) as cut‐off values. Model 1: univariate model; Model 2: adjusted for gender, age, eGFR, BMI, FPG, TG, HDL, AST_ALT, HCT, PLT, WBC, RBC, TC, HGB, HCT, and MCV. Model 3: adjusted for variables in Model 2 and smoking and drinking.
A statistically significant association between Hcy levels and hypertension was observed (Model 1) for the whole sample (both men and women). The results did not vary appreciably in Model 2, and a statistically significant association was discovered in Model 3 (OR = 1.262, 95% CI: 1.155‐1.378, P = 0.0005, Q2 vs Q1; OR = 1.458, 95% CI: 1.335‐1.593, P < 0.0001, Q3 vs Q1; OR = 1.520, 95% CI: 1.388‐1.664, P < 0.0001, Q4 vs Q1), when using the traditional definition of hypertension. The same result was detected when using the new definition of hypertension, and a statistically significant association was discovered in Model 3 (OR = 1.159, 95% CI: 1.067‐1.259, P < 0.0001, Q2 vs Q1; OR = 1.328, 95% CI: 1.221‐1.445, P < 0.0001, Q3 vs Q1; OR = 1.328, 95% CI: 1.217‐1.449, P < 0.0001, Q4 vs Q1). All of the different gender populations followed the same association pattern except for females using the new definition of hypertension. No statistically significant differences were found in Model 2 (OR = 1.105, 95% CI: 0.896‐1.363, P = 0.3514, Q4 vs Q1) and Model 3 (OR = 1.109, 95% CI: 0.899‐1.369, P = 0.3334, Q4 vs Q1) in women.
3.3. Longitudinal association between Hcy and hypertension
Table 3 shows the characteristics of Hcy and BP together with the potential confounding factors at every measurement. The mean follow‐up period was 3.80 years (minimum = 2 years; maximum = 8 years). The distribution of BP and other potential confounding factors is shown in Table 4.
Table 3.
Characteristics of study populations for longitudinal analysis by Hcy levels
| Variables | Q1 (4.40‐7.60 mg/dL) | Q2 (7.61‐9.70 mg/dL) | Q3 (9.71‐12.60 mg/dL) | Q4 (12.61‐44.70 mg/dL) | F‐statistic/chi‐square | P value |
|---|---|---|---|---|---|---|
| Age (y) | 44.46 ± 9.46 | 46.20 ± 9.92 | 47.67 ± 10.39 | 47.41 ± 11.20 | 148.8300 | <.0001 |
| SBP (mm Hg) | 115.58 ± 14.37 | 119.69 ± 14.72 | 121.27 ± 14.43 | 123.20 ± 14.26 | 396.1135 | <.0001 |
| DBP (mm Hg) | 68.05 ± 9.75 | 70.77 ± 10.08 | 71.63 ± 10.13 | 72.87 ± 10.09 | 342.8079 | <.0001 |
| eGFR | 95.54 ± 30.03 | 104.15 ± 32.94 | 108.78 ± 34.24 | 116.52 ± 35.18 | 615.8940 | <.0001 |
| BMI (kg/m2) | 24.25 ± 3.36 | 24.86 ± 3.38 | 25.50 ± 5.85 | 25.66 ± 3.24 | 264.1564 | <.0001 |
| FPG (mmol/L) | 5.38 ± 1.28 | 5.47 ± 1.30 | 5.41 ± 1.08 | 5.42 ± 1.08 | 40.7914 | <.0001 |
| TG (mmol/L) | 1.38 ± 1.92 | 1.57 ± 1.51 | 1.64 ± 1.34 | 1.74 ± 1.52 | 301.1583 | <.0001 |
| HDL (mmol/L) | 1.42 ± 0.38 | 1.34 ± 0.37 | 1.29 ± 0.34 | 1.25 ± 0.32 | 339.6817 | <.0001 |
| AST/ALT | 1.23 ± 1.04 | 1.15 ± 0.76 | 1.12 ± 1.55 | 1.10 ± 0.80 | 116.6284 | <.0001 |
| HCT (%) | 41.41 ± 4.11 | 43.21 ± 4.10 | 44.09 ± 3.88 | 45.08 ± 3.47 | 1184.5767 | <.0001 |
| PLT (109/L) | 241.32 ± 55.54 | 230.61 ± 55.15 | 223.66 ± 52.94 | 223.12 ± 49.53 | 205.8349 | <.0001 |
| WBC (1012/L) | 5.99 ± 1.53 | 6.09 ± 1.59 | 6.08 ± 1.48 | 6.35 ± 1.65 | 70.2250 | <.0001 |
| RBC (1012/L) | 4.56 ± 0.43 | 4.75 ± 0.44 | 4.85 ± 0.44 | 4.92 ± 0.42 | 1047.1519 | <.0001 |
| TC (mmol/L) | 4.87 ± 0.97 | 4.89 ± 0.90 | 4.84 ± 0.44 | 4.85 ± 0.91 | 4.4282 | 0.2188 |
| HGB (g/L) | 138.78 ± 16.27 | 146.42 ± 16.15 | 150.14 ± 15.22 | 154.48 ± 13.54 | 1396.2364 | <.0001 |
| MCV (fl) | 90.99 ± 16.27 | 90.94 ± 5.04 | 91.20 ± 4.58 | 91.72 ± 4.82 | 20.8424 | 0.0001 |
| Smoker (n, %) | 60 (0.92) | 86 (1.22) | 167 (2.5) | 111 (1.66) | 9.8579 | 0.0198 |
| Alcohol user (n, %) | 140 (2.15) | 252 (3.59) | 405 (6.07) | 309 (4.63) | 11.4073 | 0.0097 |
Q1‐Q4 represent 4 levels of the original continuous serum Hcy using the 3 quartiles (P25, P50, and P75) as cut‐off values.
ALT, alanine aminotransferase (mmol/L); AST, aspartate aminotransferase (mmol/L); BMI, body mass index (kg/m2); eGFR, the estimated glomerular filtration rate; FPG, fasting plasma glucose (mmol/L); HCT, red blood cell specific volume (%); HDL, high‐density lipoprotein (mmol/L); HGB, hemoglobin (g/L); MCV, erythrocyte mean corpuscular volume (fl); PLT, blood platelet(109/L); RBC, red blood cell (1012/L); TC, total cholesterol (mmol/L); TG, triglyceride (mmol/L); WBC, white blood cell (1012/L).
Table 4.
Distribution of blood pressure and other potential confounding factors
| Variables | First measurement (N = 3913) | Second measurement (N = 3913) | Third measurement (N = 1942) | Fourth measurement (N = 906) | Fifth measurement (N = 275) | Sixth measurement (N = 18) | Seventh measurement (N = 11) | Eighth measurement (N = 3) |
|---|---|---|---|---|---|---|---|---|
| Hcy (mmol/L) | 11.94 ± 8.05 | 11.26 ± 7.21 | 11.71 ± 6.87 | 10.04 ± 6.16 | 10.15 ± 6.13 | 10.98 ± 5.24 | 11.14 ± 6.04 | 10.30 ± 4.18 |
| SBP (mm Hg) | 120.02 ± 14.80 | 119.84 ± 14.54 | 119.93 ± 14.95 | 119.84 ± 14.42 | 119.68 ± 14.86 | 118.82 ± 12.44 | 122.27 ± 13.36 | 131.67 ± 8.5 |
| DBP (mm Hg) | 71.01 ± 10.22 | 70.81 ± 10.13 | 70.49 ± 10.11 | 70.73 ± 10.14 | 71.18 ± 10.20 | 70.64 ± 10.06 | 73.54 ± 10.73 | 79.66 ± 8.50 |
| eGFR | 107.13 ± 32.38 | 106.59 ± 35.17 | 102.93 ± 37.61 | 105.76 ± 29.25 | 108.73 ± 27.14 | 111.41 ± 27.96 | 110.42 ± 27.30 | 113.84 ± 39.40 |
| BMI (kg/m2) | 24.98 ± 3.38 | 25.04 ± 3.33 | 25.19 ± 6.77 | 25.21 ± 3.32 | 24.91 ± 3.10 | 25.21 ± 2.68 | 25.06 ± 1.83 | 24.16 ± 1.10 |
| FPG (mmol/L) | 5.43 ± 1.19 | 5.43 ± 1.19 | 5.4 ± 1.17 | 5.43 ± 1.24 | 5.41 ± 1.27 | 5.19 ± 0.46 | 5.17 ± 0.42 | 5.24 ± 0.50 |
| TG (mmol/L) | 1.58 ± 1.49 | 1.62 ± 1.83 | 1.57 ± 1.51 | 1.5 ± 1.14 | 1.52 ± 1.22 | 2.09 ± 2.53 | 1.42 ± 0.90 | 1.25 ± 0.49 |
| HDL (mmol/L) | 1.27 ± 0.34 | 1.34 ± 0.36 | 1.35 ± 0.37 | 1.39 ± 0.38 | 1.41 ± 0.39 | 1.41 ± 0.49 | 1.55 ± 0.41 | 1.59 ± 0.45 |
| AST/ALT | 1.07 ± 0.79 | 1.14 ± 0.97 | 1.2 ± 0.51 | 1.31 ± 0.50 | 1.47 ± 1.00 | 1.51 ± 0.54 | 1.53 ± 0.41 | 1.3 ± 0.26 |
| HCT (%) | 43.13 ± 4.16 | 43.95 ± 4.07 | 43.31 ± 4.14 | 42.99 ± 4.07 | 42.89 ± 3.90 | 42.53 ± 3.39 | 43.04 ± 3.77 | 44.86 ± 5.33 |
| PLT (109/L) | 226.96 ± 52.57 | 230.55 ± 54.21 | 231.95 ± 54.7 | 232.66 ± 55.49 | 229.51 ± 56.14 | 238.18 ± 41.35 | 248.45 ± 41.21 | 244.67 ± 30.98 |
| WBC (1012/L) | 6.22 ± 1.62 | 6.15 ± 1.57 | 6.02 ± 1.50 | 5.99 ± 1.51 | 5.74 ± 1.38 | 6.33 ± 1.43 | 6.44 ± 1.51 | 6.18 ± 0.94 |
| RBC (1012/L) | 4.73 ± 0.46 | 4.79 ± 0.45 | 4.81 ± 0.45 | 4.79 ± 0.46 | 4.78 ± 0.42 | 4.78 ± 0.43 | 4.8 ± 0.49 | 4.9 ± 0.66 |
| TC (mmol/L) | 4.93 ± 0.93 | 4.83 ± 0.94 | 4.83 ± 0.89 | 4.87 ± 0.91 | 4.83 ± 0.91 | 4.94 ± 0.85 | 4.65 ± 1.01 | 4.79 ± 0.75 |
| HGB (g/L) | 146.4 ± 16.29 | 148.3 ± 16.13 | 147.87 ± 16.86 | 146.75 ± 16.63 | 149.04 ± 16.29 | 147.24 ± 12.48 | 148 ± 15.56 | 156 ± 24.02 |
| MCV (fl) | 91.41 ± 5.01 | 91.9 ± 4.95 | 90.19 ± 5.05 | 89.98 ± 5.13 | 89.75 ± 4.88 | 89.22 ± 4.66 | 89.88 ± 5.73 | 91.66 ± 4.36 |
| Gender (male, n, %) | 2491 (63.66) | 2491 (63.66) | 1224 (63.62) | 558 (61.59) | 189 (68.73) | 11 (61.11) | 6 (54.55) | 2 (66.67) |
| Hypertension (n, %) | 0 (0) | 635 (16.23) | 497 (25.83) | 259 (28.59) | 62 (22.55) | 9 (50) | 5 (45.45) | 1 (33.33) |
| Smoking (n, %) | 0 (0) | 2 (0.05) | 3 (0.16) | 0 (0) | 1 (0.36) | 0 (0) | 1 (9.09) | 0 (0) |
| Drinking (n, %) | 1 (0.03) | 10 (0.26) | 11 (0.57) | 2 (0.22) | 3 (1.09) | 2 (11.11) | 0 (0) | 1 (33.33) |
ALT, alanine aminotransferase (mmol/L); AST, aspartate aminotransferase (mmol/L); BMI, body mass index (kg/m2); eGFR, the estimated glomerular filtration rate; FPG, fasting plasma glucose (mmol/L); HCT, red blood cell specific volume (%); HDL, high‐density lipoprotein (mmol/L); HGB, hemoglobin (g/L); MCV, erythrocyte mean corpuscular volume (fl); PLT, blood platelet(109/L); RBC, red blood cell (1012/L); TC, total cholesterol (mmol/L); TG, triglyceride (mmol/L); WBC, white blood cell (1012/L).
A statistically significant association between Hcy level and hypertension was observed in Model 1. The results did not vary appreciably in Model 2, which was further adjusted for age and gender, and a statistically significant association was discovered in Model 3 (OR = 1.268, 95% CI: 1.030‐1.560, P = 0.0249, Q3 vs Q1), which was further adjusted for the clinical indices, and Model 4 (OR = 1.265, 95% CI: 1.028‐1.557, P = 0.0263, Q3 vs Q1), which was adjusted for life customs and used the new definition of hypertension. The same result was detected when using the traditional definition of hypertension. The association between Hcy and hypertension in the longitudinal analyses is shown in Figure 1.
Figure 1.
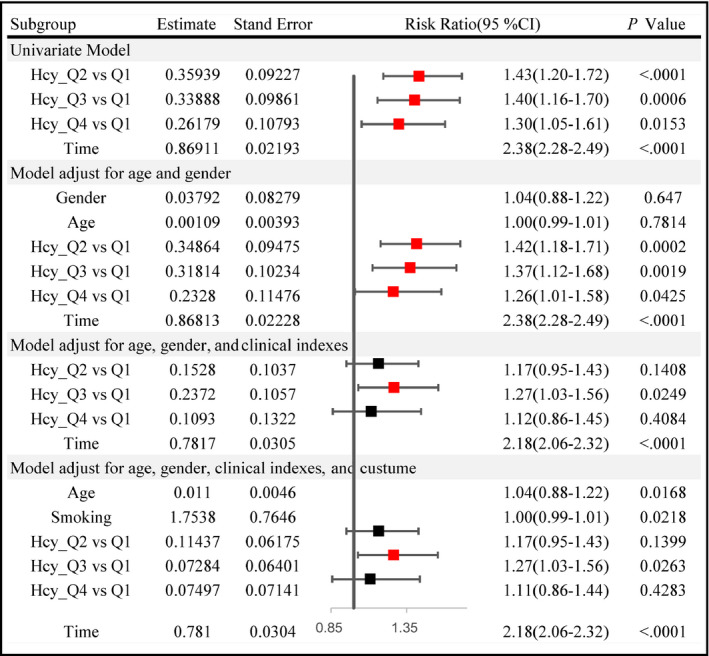
The association between Hcy and hypertension in the longitudinal analyses
4. DISCUSSION
Based on a well‐designed epidemiological cohort with a large sample size, the present study provides powerful evidence of a significant association between Hcy and hypertension, using both cross‐sectional and longitudinal analyses. Furthermore, elevated plasma Hcy levels may be an independent predictor of hypertension, regardless of the study design (cross‐sectional or longitudinal) and gender effect.
The relationship between Hcy and BP has been proposed by several researchers.28, 29, 30 It has previously been suggested that high Hcy levels may damage vascular endothelial cells and affect the anticoagulant effect of endothelium cells, leading to the proliferation of smooth muscle cells.31 Plasma Hcy levels have been identified as a potential biomarker for endothelial dysfunction32 and have been linked to severe diseases associated with endothelial injury.33
The gender‐specific association between Hcy and the risk of hypertension has not been illustrated clearly by existing studies.34, 35 Some researchers have confirmed that the interactions of Hcy stratified by gender and BP need to be considered in the prediction of the overall risk of stroke and hypertension.36 However, the Framingham Heart Study found no major association between the baseline plasma Hcy level and hypertension incidence or longitudinal BP progression, after adjustment for age, gender, and other important confounding factors.37 In 2002, a cross‐sectional study38 based on the third national health and nutrition survey in the United States supported the relationship between Hcy and BP levels, and this correlation seems to be more pronounced in women. A Danish study39 in the same year showed that the interaction between Hcy and hypertension was influenced by renal function. The association between elevated Hcy levels with systolic BP was found only in smokers.
In a previous study in China, Wang and colleagues16 prospectively traced the BP progression of a normotensive population with different Hcy levels over a 2‐year period. The study identified a gender difference, indicating that Hcy was not a significant risk factor for women. A study in Liao‐Ning, Jiang‐Su, and Xin‐Jiang provinces found significant and positive associations of homocysteine concentrations with hypertension and BP.29, 40, 41
Race may indeed be a specific factor influencing the interaction between Hcy levels and hypertension. Differences between these studies might be explained by the participants’ ethnicities. Total plasma Hcy levels have significant gender‐dependent differences17, 42 although the conclusions of the previous studies were conflicting.43, 44, 45
Our findings were consistent with the above‐mentioned prior studies. Furthermore, our findings were also supported by several previous studies, showing that plasma Hcy levels were significantly lower among women than among men.30, 46, 47 Both conduit and resistance vessel endothelial function may be damaged by elevated Hcy levels,33 resulting in the development of hypertension.48 Female hormones, which have been established to have antioxidant effects that may reduce the risk based on Hcy, may be responsible for the observed differences.17, 34, 42
The design of the studies contributed to the different observed results. In most retrospective studies, such as case‐control studies and cross‐sectional studies, plasma Hcy levels were related to an increase in BP and hypertension.41, 44, 49 However, different conclusions were reached in large prospective studies after adjusting for confounding factors. Several studies found no major connection between plasma Hcy levels and hypertension incidence after adjusting for sociodemographic and clinical covariates.37, 50, 51 Randomization was applied to improve causal inference, showing that Hcy was more likely a marker than a cause of BP.19
In general, our findings should be taken with caution, because in the longitudinal analysis, only the third quartile (Q3 level) was statistically significant compared to the first quartile (Q1 level). This suggests that plasma Hcy levels may be a biomarker for hypertension and that the association between them may be partially causal.
Despite these important findings, there are two limitations to our study. First, attention should be paid to the gender‐specific mechanisms and possible intrinsic causal relationships; thus, a cohort study with a longer follow‐up period is needed in the future. Second, the promotion of physical activity,51 type of dietary habits,9 genetic defects of the metabolism of enzymes in Hcy and vitamin B supplements17 in the adult population may play important roles in affecting Hcy concentrations and attenuating the health effects of deleterious conditions. More variables should be collected in future studies.
5. CONCLUSIONS
Elevated total plasma Hcy may be used as a predictive biomarker for hypertension. Considering the biases in cross‐sectional studies, the present study highlights a longitudinal association between Hcy and hypertension. Attention should be paid to gender‐specific mechanisms when issuing precise precautions.
CONFLICT OF INTEREST
The authors report no specific funding in relation to this research and have no conflict of interests to disclose.
Supporting information
ACKNOWLEDGEMENTS
The authors would like to thank all of the investigators, the staff of Xiao‐Tang‐Shan Hospital, and the participants of the present study for their valuable contributions.
Tao L‐X, Yang K, Wu J, et al. Association between plasma homocysteine and hypertension: Results from a cross‐sectional and longitudinal analysis in Beijing’s adult population from 2012 to 2017. J Clin Hypertens. 2018;20:1624–1632. 10.1111/jch.13398
Lixin Tao and Kun Yang contributed equally to the work in this article.
Funding information
This work was supported by the National Natural Science Foundation of China (Serial Number: 81502886, 81530087), Beijing Natural Science Foundation (Serial Number: Z160002), Young Core Personal Project & Beijing Outstanding Talent Training Project (Serial Number: 2014000020124G150), and Program of the Beijing Municipal Science & Technology Commission (Serial Number: D141100000114003). All funding sources were independent and had no influence on the study design, the collection, analysis, and interpretation of our data, the writing of this report, or the decision to submit the article for publication.
Contributor Information
Zhao Ping, Email: pingzhao59@126.com.
Xiuhua Guo, Email: statguo@ccmu.edu.cn.
REFERENCES
- 1. Wang Y, Peng X, Nie X, et al. Burden of hypertension in China over the past decades: systematic analysis of prevalence, treatment and control of hypertension. Eur J Prev Cardiol. 2015;23(8):792‐800. [DOI] [PubMed] [Google Scholar]
- 2. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (London, England). 2012;380(9859):2224‐2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lu J, Lu Y, Wang X, et al. and control of hypertension in China: data from 1.7 million adults in a population‐based screening study. (China PEACE Million Persons Project). Lancet (London, England). 2017;390(10112):2549‐2558. [DOI] [PubMed] [Google Scholar]
- 4. Wang Z, Chen Z, Zhang L, et al. Status of hypertension in China: results from the China hypertension survey, 2012‐2015. Circulation. 2018;137(22):2344‐2356. [DOI] [PubMed] [Google Scholar]
- 5. Momin M, Fan F, Li J, et al. Associations of plasma homocysteine levels with peripheral systolic blood pressure and noninvasive central systolic blood pressure in a community‐based Chinese population. Sci Rep. 2017;7(1):6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guo S, Lin H, Pan S, Zhai X, Meng L. The differential diagnostic value of serum homocysteine for white coat hypertension. Oncotarget. 2017;8(60):101271‐101283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhi X, He J, Ma P, et al. Relationship between homocysteine and hypertension: new data add to the debate. J Clin Hypertens (Greenwich). 2017;19(11):1171‐1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fowdar JY, Lason MV, Szvetko AL, Lea RA, Griffiths LR. Investigation of homocysteine‐pathway‐related variants in essential hypertension. Int J Hypertens. 2012;2012:190923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weber GJ, Pushpakumar S, Tyagi SC, Sen U. Homocysteine and hydrogen sulfide in epigenetic, metabolic and microbiota related renovascular hypertension. Pharmacol Res. 2016;113(Pt A):300‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rodrigo R, Passalacqua W, Araya J, Orellana M, Rivera G. Homocysteine and essential hypertension. J Clin Pharmacol. 2003;43(12):1299‐1306. [DOI] [PubMed] [Google Scholar]
- 11. Mao X, Xing X, Xu R, et al. Folic acid and vitamins D and B12 correlate with homocysteine in Chinese patients with type‐2 diabetes mellitus, hypertension, or cardiovascular disease. Medicine. 2016;95(6):e2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joob B, Wiwanitkit V. Homocysteine and masked hypertension. Anatol J Cardiol. 2015;15(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Waskiewicz A, Sygnowska E, Broda G. Homocysteine concentration and the risk of death in the adult polish population. Kardiol Pol. 2012;70(9):897‐902. [PubMed] [Google Scholar]
- 14. Bowman TS, Gaziano JM, Stampfer MJ, Sesso HD. Homocysteine and risk of developing hypertension in men. J Hum Hypertens. 2006;20(8):631‐634. [DOI] [PubMed] [Google Scholar]
- 15. Yucel K, Bekci TT, Taner A, Kayrak M, Korucu EN, Unlu A. Homocysteine levels in patients with masked hypertension. Anadolu kardiyoloji dergisi . Anadolutol J Cardiol. 2014;14(4):357‐362. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Chen S, Yao T, et al. Homocysteine as a risk factor for hypertension: a 2‐year follow‐up study. PLoS One. 2014;9(10):e108223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao C, Hu J, Dong Y, et al. Gender differences in the risk factors for endothelial dysfunction in Chinese hypertensive patients: homocysteine is an independent risk factor in females. PLoS One. 2015;10(2):e0118686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhong F, Zhuang L, Wang Y, Ma Y. Homocysteine levels and risk of essential hypertension: a meta‐analysis of published epidemiological studies. Clin Exp Hypertens. 2017;39(2):160‐167. [DOI] [PubMed] [Google Scholar]
- 19. Borges MC, Hartwig FP, Oliveira IO, Horta BL. Is there a causal role for homocysteine concentration in blood pressure? A Mendelian randomization study. Am J Clin Nutr. 2016;103(1):39‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhong C, Lv L, Liu C, et al. High homocysteine and blood pressure related to poor outcome of acute ischemia stroke in Chinese population. PLoS One. 2014;9(9):e107498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu J, Zhao Z, Mu Y, et al. Gender differences in the association between serum uric acid and prediabetes: a six‐year longitudinal Cohort study. Int J Environ Res Public Health. 2018;15(7):1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2017;12(8):579.e1‐579.e73. [Google Scholar]
- 23. Zhao F, Zhang L, Lu J, et al. The chronic kidney disease epidemiology collaboration equation improves the detection of hyperfiltration in Chinese diabetic patients. Int J Clin Exp Med. 2015;8(12):22084‐22097. [PMC free article] [PubMed] [Google Scholar]
- 24. Yang K, Tao L, Mahara G, et al. An association of platelet indices with blood pressure in Beijing adults: applying quadratic inference function for a longitudinal study. Medicine. 2016;95(39):e4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qu A, Lindsay BG, Li B. Improving generalised estimating equations using quadratic inference functions. Biometrika. 2000;87(4):823‐836. [Google Scholar]
- 26. Westgate PM. Criterion for the simultaneous selection of a working correlation structure and either generalized estimating equations or the quadratic inference function approach. Biom J. 2014;56(3):461‐476. [DOI] [PubMed] [Google Scholar]
- 27. Lai P, Li G, Lian H. Quadratic inference functions for partially linear single‐index models with longitudinal data. J Multivariate Anal. 2013;118:115‐127. [Google Scholar]
- 28. Dominguez LJ, Galioto A, Pineo A, et al. Age, homocysteine, and oxidative stress: relation to hypertension and type 2 diabetes mellitus. J Am Coll Nutr. 2010;29(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 29. Lu H, Lu ZH, Li PG, Wang YY, Yan ZY. Elevated homocysteine and hypertension in Xinjiang Province, China. Ethn Dis. 2010;20(1):7‐10. [PubMed] [Google Scholar]
- 30. Sen U, Mishra PK, Tyagi N, Tyagi SC. Homocysteine to hydrogen sulfide or hypertension. Cell Biochem Biophys. 2010;57(2‐3):49‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang T, Tucker KL, Lee YC, et al. Methylenetetrahydrofolate reductase variants associated with hypertension and cardiovascular disease interact with dietary polyunsaturated fatty acids to modulate plasma homocysteine in puerto rican adults. J Nutr. 2011;141(4):654‐659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tsuda K. Associations among plasma total homocysteine levels, circadian blood pressure variation, and endothelial function in hypertension. Am J Hypertens. 2018;31(4):e1‐e2. [DOI] [PubMed] [Google Scholar]
- 33. Essouma M, Noubiap JJ. Therapeutic potential of folic acid supplementation for cardiovascular disease prevention through homocysteine lowering and blockade in rheumatoid arthritis patients. Biomark Res. 2015;3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pitla S, Nagalla B. Gender‐related differences in the relationship between plasma homocysteine, anthropometric and conventional biochemical coronary heart disease risk factors in middle‐aged Indians. Ann Nutr Metab. 2009;54(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 35. Naik S, Joglekar C, Bhat D, et al. Marked gender difference in plasma total homocysteine concentrations in indian adults with low vitamin B(1)(2). Int J Nutr Res. 2011;81(5):306‐316. [DOI] [PubMed] [Google Scholar]
- 36. Pang H, Han B, Fu Q, Hao L, Zong Z. Association between homocysteine and conventional predisposing factors on risk of stroke in patients with hypertension. Sci Rep. 2018;8(1):3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sundstrom J, Sullivan L, D'Agostino RB, et al. Plasma homocysteine, hypertension incidence, and blood pressure tracking: the Framingham Heart Study. Hypertension. 2003;42(6):1100‐1105. [DOI] [PubMed] [Google Scholar]
- 38. Tessari P, Vettore M, Puricelli L, Cecchet D, Coracina A, Kivanuka E. Decreased homocysteine trans‐sulfuration in hypertension with hyperhomocysteinemia: relationship with insulin resistance. J Clin Endocrinol Metab. 2018;103(1):56‐63. [DOI] [PubMed] [Google Scholar]
- 39. Veerabhadrappa P, Schutte AE. Homocysteine and Nighttime Blood Pressure Dipping‐Is There a Connection? Am J Hypertens. 2017;30(12):1151‐1152. [DOI] [PubMed] [Google Scholar]
- 40. Yang B, Fan S, Zhi X, et al. Interactions of homocysteine and conventional predisposing factors on hypertension in Chinese adults. J Clin Hypertens. 2017;19(11):1162‐1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu H, Wang B, Ban Q, et al. Association of total homocysteine with blood pressure in a general population of Chinese adults: a cross‐sectional study in Jiangsu province, China. BMJ open. 2018;8(6):e021103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Henry OR, Benghuzzi H, Taylor HA Jr, Tucci M, Butler K, Jones L. Suppression of homocysteine levels by vitamin B12 and folates: age and gender dependency in the Jackson Heart Study. Am J Med Sci. 2012;344(2):110‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gonzalez‐Gross M, Benser J, Breidenassel C, et al. Gender and age influence blood folate, vitamin B12, vitamin B6, and homocysteine levels in European adolescents: the Helena Study. Nutr Res (New York, NY). 2012;32(11):817‐826. [DOI] [PubMed] [Google Scholar]
- 44. Xiao W, Bai Y, Ye P, et al. Plasma homocysteine is associated with aortic arterial stiffness but not wave reflection in Chinese hypertensive subjects. PLoS One. 2014;9(1):e85938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dong YF, Zhan BM, Hao QY, et al. Plasma homocysteine levels are associated with circadian blood pressure variation in Chinese hypertensive adults. Am J Hypertens. 2017;30(12):1203‐1210. [DOI] [PubMed] [Google Scholar]
- 46. McMahon JA, Skeaff CM, Williams SM, Green TJ. Lowering homocysteine with B vitamins has no effect on blood pressure in older adults. J Nutr. 2007;137(5):1183‐1187. [DOI] [PubMed] [Google Scholar]
- 47. Sen U, Tyagi SC. Homocysteine and hypertension in diabetes: does PPARgamma have a regulatory role? PPAR Res. 2010;2010:806538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li WX, Liao P, Hu CY, et al. Interactions of methylenetetrahydrofolate reductase gene polymorphisms, folate, and homocysteine on blood pressure in a chinese hypertensive population. Clin Lab. 2017;63(4):817‐825. [DOI] [PubMed] [Google Scholar]
- 49. Lim U, Cassano PA. Homocysteine and blood pressure in the Third National Health and Nutrition Examination Survey, 1988‐1994. Am J Epidemiol. 2002;156(12):1105‐1113. [DOI] [PubMed] [Google Scholar]
- 50. Hu S, Ren L, Wang Y, et al. Homocysteine‐lowering therapy and early functional outcomes of ischemic patients with H‐type hypertension: a retrospective analysis of CNSR. Australas Phys Eng Sci Med. 2015;38(4):785‐791. [DOI] [PubMed] [Google Scholar]
- 51. Wang H, Xu BP, Xu RB, Walker SO, Wang G. Joint effect of maternal plasma homocysteine and prepregnancy obesity on child blood pressure: a prospective birth cohort study. Int J Obes (Lond). 2017;41(9):1447‐1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


