Abstract
In the absence of left ventricular hypertrophy, importance of fragmented QRS complex (fQRS) in individuals with hypertension is unknown. The authors aimed to evaluate the relationship between blood pressure levels and fQRS in the absence of left ventricular hypertrophy. A total of 548 never‐treated patients who underwent 24‐hour ambulatory blood pressure monitoring were enrolled. The frequency of fQRS was significantly higher in patients with hypertension than normotension (36.4% vs 17.6%, P<.05). Multivariate logistic regression analysis revealed that systolic blood pressure is significantly associated with presence of fQRS on electrocardiography (odds ratio, 0.931; 95% CI, 0.910–0.9521 [P<.001]) even after adjusting for other confounding factors. Receiver operating characteristic analysis revealed a cutoff value of 147.65 mm Hg for systolic blood pressure to predict presence of fQRS (sensitivity: 51%, specificity: 99%, area under the curve=0.764; 95% CI, 0.717–0.811 [P<.001]). fQRS may be a sign of increased blood pressure and may predict higher fibrotic burden in patients with hypertension.
Keywords: fibrosis, fragmented QRS, hypertension
1. INTRODUCTION
There is a strong correlation between increased blood pressure (BP) levels and cardiovascular events.1 Despite the estimated prevalence of hypertension of ≈30% to 45% of the general population, the presence of difficulties in the diagnosis and treatment causes an inability to control and monitor the condition.2 Electrocardiography (ECG) plays an important role in the monitoring of patients with hypertension; however, ECG abnormalities seem to mainly be the sign of left ventricular hypertrophy (LVH) and increased left ventricular mass caused by chronic pressure overload.3 BP elevation is an important cause of fibrotic burden in myocardium, and chronic pressure overload related to collagen synthesis is the main reason for fibrosis in individuals with hypertension.4 However, progression from early‐stage fibrosis to manifest LVH as end organ damage is likely a lengthy process. A narrow fragmented QRS complex (fQRS) as a ventricular conduction abnormality is a sign of myocardial fibrosis and is associated with adverse outcomes in various cardiovascular diseases.5, 6, 7 Therefore, presence of fQRS on ECG, as an indicator of myocardial fibrosis, may be a sign of increased BP even in the absence of manifest LVH. In the present study, we aimed to investigate whether increased BP levels have a positive correlation with the presence of fQRS on ECG in the absence of LVH and to demonstrate the usefulness of fQRS before the development of LVH.
2. METHODS
2.1. Study participants
In all, 614 consecutive patients who were never treated and referred to our outpatient clinic for suspicion of hypertension and underwent 24‐hour ambulatory BP monitoring (ABPM) between July 2015 and July 2016 were enrolled. Patients with LVH (n=24), previous myocardial infarction and/or coronary artery bypass graft surgery (n=13), complete or incomplete bundle branch block and QRS duration ≥120 ms (n=11), left ventricular ejection fraction <50% (n=10), and moderate to severe valvular heart disease (n=8) were excluded. As a result, the remaining 548 patients were included in the study. All individuals were evaluated with a detailed anamnesis, physical examination, laboratory analysis, echocardiography, and ECG. LVH was defined according to electrocardiographic modified Sokolow‐Lyon index and/or an increased left ventricular mass of >95 g/m2 for women and >115 g/m2 for men detected by echocardiography.2 Coronary artery disease (CAD) was defined as angiographically detected obstructive or nonobstructive atherosclerotic lesions and/or ischemia detected by noninvasive tests. Diabetes mellitus was defined as at least two fasting plasma glucose values ≥126 mg/dL or blood glucose ≥200 mg/dL at any time or treatment with antidiabetic drugs. Smoking was defined as the current regular use of cigarettes.
The study protocol complied with Declaration of Helsinki and was approved by the local ethics committee.
2.2. BP measurements
Twenty‐four‐hour ABPM was performed in all patients. Measurements were made by an oscillometric device (Contec ABPM 50). The cuff was placed on the nondominant arm and the patients were asked to relax their arm when measurements were initiated. ABPM measurements were performed on working days and all patients were encouraged to continue their routine daily activities. BP levels were automatically recorded every 30 minutes during a 24‐hour period. If >20% of the BP recordings were invalid, 24‐hour ABPM was repeated. The 24‐hour averages of systolic BP (SBP) and diastolic BP (DBP) levels were calculated for each patient by ABPM recordings. Although hypertension by 24‐hour ABPM is defined as mean SBP ≥130 mm Hg or DBP ≥80 mm Hg,2 we divided patients into three groups according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure definition,8 to clearly demonstrate the correlation between BP levels and fQRS. The patients with mean SBP ≥140 mm Hg or DBP ≥90 mm Hg were assigned as group 1, SBP 120 to 139 mm Hg or DBP 80 to 89 mm Hg were assigned as group 2, and SBP <120 mm Hg and DBP <80 mm Hg were assigned as group 3 (normotension).8
2.3. ECG and fQRS
The 12‐lead ECG of each patient was analyzed blindly by two independent cardiologists. In case of disagreement regarding the presence of fQRS, the final decision was achieved by consensus. fQRS was defined as presence of various rSR′ patterns or notching of the S or R wave in the absence of typical bundle branch block in two contiguous leads corresponding to a major coronary artery territory with a QRS duration of <120 ms9 (Figure 1).
Figure 1.
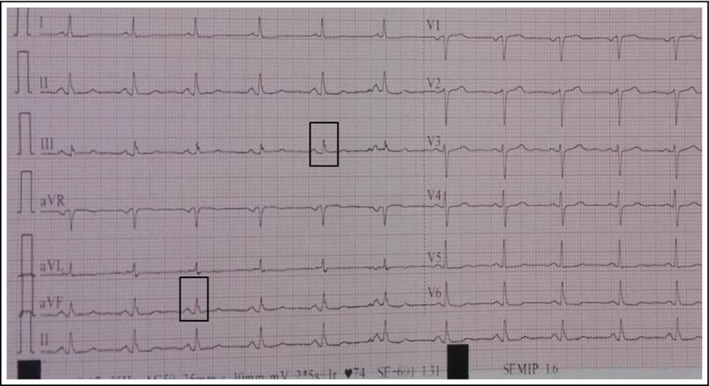
An example of fragmented QRS in our patients
SPSS (IBM) version 22.0 was used for statistical analysis. Continuous and categorical variables were expressed as mean±SD and percentages, respectively. Differences in patient groups were examined with analysis of variance and independent sample t test for continuous variables and chi‐square test for categorical variables. Multivariate logistic regression (stepwise backward elimination) analysis was applied to identify independent predictors of fQRS. All variables with P<.05 in univariate analysis were included in the model. Receiver operating characteristic analysis of SBP was performed to calculate the optimal cutoff value for predicting the fQRS. P<.05 was considered statistically significant.
3. RESULTS
The mean age of the study participants was 49.00±5.0 years, with 58% men and a frequency of fQRS of 27.9%. Among 548 patients who underwent 24‐hour ABPM, there were 217 patients in group 1, 172 patients in group 2, and 159 patients in group 3. The baseline characteristics and laboratory and BP findings of the groups are presented in Table 1. No significant differences were found between the groups in terms of sex, CAD, diabetes mellitus, and cigarette smoking. Group 1 patients were older, had the highest high‐density lipoprotein and hemoglobin levels, and the lowest low‐density lipoprotein levels. SBP and DBP levels were significantly higher in group 1 (P<.05 vs the other groups).
Table 1.
Baseline demographic and clinical characteristics of the study groups
| Group 3 (n=159) | Group 2 (n=172) | Group 1 (n=217) | P Value | Group1 vs group 2 | Group 1 vs group 3 | Group 2 vs group 3 | |
|---|---|---|---|---|---|---|---|
| Age, y | 47±4.3 | 49±4.4 | 51±4.5 | .000 | .000 | .000 | .018 |
| Male sex, No. (%) | 100.00 (62.9) | 98.00 (57.0) | 120.00 (55.3) | .319 | .983 | .362 | .617 |
| CAD, No. (%) | 23.00 (14.5) | 26.00 (15.1) | 37.00 (17.1) | .768 | .939 | .872 | .998 |
| DM, No. (%) | 28.00 (17.6) | 31.00 (18.0) | 44.00 (20.3) | .769 | .923 | .885 | 1.000 |
| Smokers, No. (%) | 32.00 (20.1) | 33.00 (19.2) | 45.00 (20.7) | .930 | .974 | .998 | .995 |
| Fragmented QRS, No. (%) | 28.00 (17.6) | 46.00 (26.7) | 79.00 (36.4) | .000 | .118 | .000 | .130 |
| SBP, mm Hg | 116.5±1.6 | 128.9±4.2 | 147.7±8.0 | .000 | .000 | .000 | .000 |
| DBP, mm Hg | 76.3±2.3 | 84.2±2.0 | 92.5±3.8 | .000 | .000 | .000 | .000 |
| Hemoglobin, mg/dL | 12.8±1.4 | 13.0±1.4 | 13.4±1.5 | .000 | .031 | .000 | .506 |
| WBC count, 103/mm3 | 7.3±0.9 | 7.2±1.0 | 7.1±0.9 | .181 | .639 | .176 | .854 |
| Creatinine, mg/dL | 0.8±0.1 | 0.8±0.1 | 0.8±0.1 | .602 | .904 | .689 | .973 |
| LDL, mg/dL | 110±17.7 | 104±20.1 | 96±24.1 | .000 | .001 | .000 | .027 |
| Triglycerides, mg/dL | 132±23.5 | 127±29.0 | 132±41.8 | .221 | .323 | .997 | .238 |
| HDL, mg/dL | 36±5.7 | 36±6.0 | 40±7.1 | .000 | .000 | .000 | .442 |
Abbreviations: CAD, coronary artery disease; DBP, diastolic blood pressure; DM, diabetes mellitus; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure; WBC, white blood cell.
The frequency of fQRS was higher in group 2 than group 3, but this difference was not statistically significant (26.7% vs 17.6%, P=.130). However, the frequency of fQRS was significantly higher in group 1 than group 3 (36.4% vs 17.6%, P<.05).
When classifying patients based on presence or absence of fQRS, patients with fQRS were older and had a higher incidence of CAD and diabetes compared with patients without fQRS. The clinical characteristics of patients with and without fQRS are presented in Table 2.
Table 2.
Characteristics of patients with and without fragmented QRS
| Fragmented QRS (+; n=153) | Fragmented QRS (−; n=395) | P value | |
|---|---|---|---|
| Age, y | 52±4.2 | 48±4.1 | .000 |
| Male sex, No. (%) | 96.00 (62.7) | 222.00 (56.2) | .164 |
| CAD, No. (%) | 61.00 (39.9) | 25.00 (6.3) | .000 |
| DM, No. (%) | 59.00 (38.6) | 44.00 (11.1) | .000 |
| Smokers, No. (%) | 59.00 (38.6) | 51.00 (12.9) | .000 |
| SBP, mm Hg | 142.8±16.0 | 128.9±11.3 | .000 |
| DBP, mm Hg | 90.0±7.6 | 83.3±6.3 | .000 |
| LVEF, % | 62±2.0 | 62±2.4 | .569 |
| Hemoglobin, mg/dL | 13.7±1.6 | 12.9±1.4 | .000 |
| WBC count, 103/mm3 | 7.3±0.9 | 7.1±0.9 | .179 |
| Creatinine, mg/dL | 0.9±0.1 | 0.8±0.1 | .000 |
| LDL, mg/dL | 103±23.6 | 103±21.2 | .893 |
| Triglycerides, mg/dL | 138±39.6 | 127±30.2 | .002 |
| HDL, mg/dL | 36±6.2 | 38±6.7 | .003 |
CAD, coronary artery disease; DBP, diastolic blood pressure; DM, diabetes mellitus; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; WBC, white blood cell.
Multivariate logistic regression analysis revealed that SBP is independently associated with presence of fQRS on ECG (odds ratio [OR], 0.931; 95% CI, 0.910–0.952 [P<.001]) even after adjusting for other confounding factors. The other factors that were associated with presence of fQRS included age (OR, 0.816; 95% CI, 0.764–0.872 [P<.001]), CAD (OR, 7.903; 95% CI, 3.886–16.075 [P<.001]), diabetes mellitus (OR, 3.750; 95% CI, 1.969–7.142 [P<.001]), cigarette smoking (OR, 3.025; CI, 1.592–5.747 [P<.001]), and creatinine (OR, 0.082; 95% CI, 0.010–0.662 [P=.019]) (Table 3).
Table 3.
Predictors of fragmented QRS in multivariate analysis
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| P value | Odd ratio | 95% CI | P value | Odd ratio | 95% CI | |
| Age | .000 | 0.776 | 0.735–0.819 | .000 | 0.816 | 0.764–0.872 |
| SBP | .000 | 0.926 | 0.91–0.941 | .000 | 0.931 | 0.910–0.952 |
| CAD | .000 | 9.813 | 5.843–16.480 | .000 | 7.903 | 3.886–16.075 |
| DM | .000 | 5.007 | 3.187–7.868 | .000 | 3.750 | 1.969–7.142 |
| Smoking | .000 | 4.234 | 2.730–6.565 | .001 | 3.025 | 1.592–5.747 |
| Creatinine | .000 | 0.012 | 0.002–0.059 | .019 | 0.082 | 0.010–0.662 |
CAD, coronary artery disease; DM, diabetes mellitus; SBP, systolic blood pressure.
In receiver operating characteristic analysis, an SBP cutoff value of 147.65 mm Hg was determined to predict presence of fQRS on ECG (sensitivity: 51%, specificity: 99%, area under the curve=0.764; 95% CI, 0.717–0.811 [P<.001]) (Figure 2).
Figure 2.
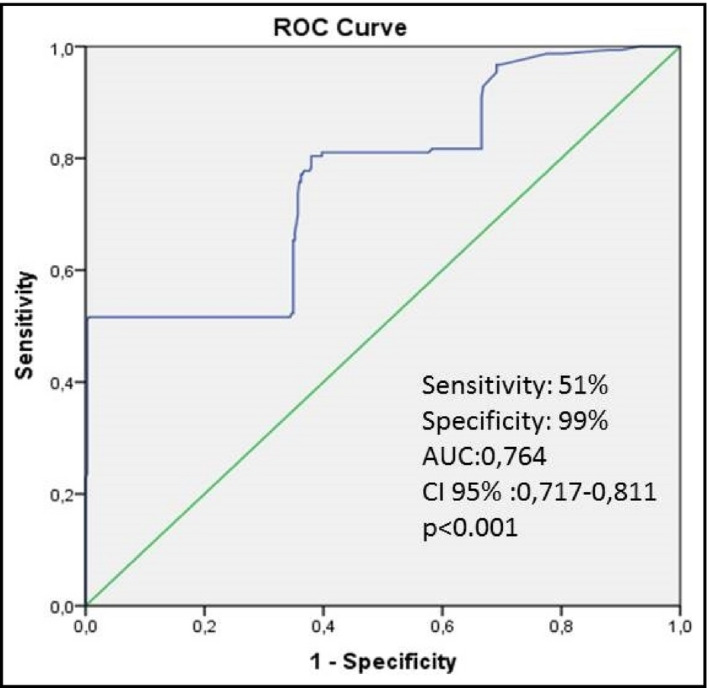
Receiver operating characteristic (ROC) curve analysis of systolic blood pressure for predicting the presence of fragmented QRS. AUC indicates area under the ROC curve
4. DISCUSSION
The results of our study revealed that higher SBP is associated with presence of fQRS on ECG in individuals with hypertension, even in the absence of LVH. fQRS is a sign of inhomogeneous ventricular conduction caused by myocardial fibrosis and scar.6, 9 Presence of fQRS is an independent predictor of adverse outcomes in various cardiovascular diseases.6, 7, 10, 11 However, its importance in patients with hypertension is not well described, particularly in patients without LVH. It has been shown that the frequency of fQRS is significantly higher in patients with LVH compared with absence of LVH in individuals with hypertension.12 This finding may indicate the higher fibrotic burden in patients with LVH. Chronic pressure overload leads to pathological fibrosis in hypertensive hearts because of excessive accumulation of collagen fibers and connective tissue matrix within the myocardium, and this accumulation proceeds to LVH.4, 13 However, progression from the onset of fibrosis to LVH is not a short process, and absence of LVH does not exclude the presence of higher fibrotic burden within myocardium. Although hypertension can cause many ECG abnormalities, LVH is the main prognostic factor and it is the cornerstone of cardiovascular risk assessment in patients with hypertension.2, 14 However, despite the fact that only a minority of patients with hypertension reach target BP levels, LVH is not a common finding and only a small proportion of individuals with hypertension have a diagnosis of LVH.2, 15 Hence, the majority of patients with hypertension without LVH requires further ECG evaluation. In this sense, we aimed to evaluate the usefulness of fQRS in this population. Our findings demonstrated that increased SBP is an indicator of presence of fQRS on ECG, which may indicate fibrosis in myocardium even in the absence of LVH. This finding also indicates the importance of higher fibrotic burden in myocardium and electrocardiographic risk assessment of persons with hypertension in the absence of LVH. The higher frequency of fQRS in the hypertensive population seems to be mainly related to LVH,12 and CAD‐related fibrosis is one of the most powerful predictors of presence of fQRS on ECG.6, 9 Hence, we excluded all patients with LVH to avoid its effect on fQRS frequency. The incidence of CAD (15.7%) was low in our study population and there were no differences in patient groups regarding the incidence of CAD. Both univariate and multivariate analyses demonstrated the independent relationship between SBP and presence of fQRS on ECG in patients without LVH. In addition, receiver operating characteristic curve analysis revealed an SBP cutoff value of 147.65 mm Hg to predict presence of fQRS on ECG with a 51% sensitivity and 99% specificity. These findings clearly demonstrate the importance and usefulness of fQRS to predict higher fibrotic burden in myocardium in patients with hypertension without LVH. Hence, fQRS may be used in the monitoring of all patients with hypertension with or without LVH.
Besides the prognostic value of fQRS in various cardiovascular diseases, it may not be associated with adverse events in patients without a known cardiac disease.16 Therefore, in the absence of a known cardiac disease, fQRS seems to be a benign ECG variant. Our study is the first to report the importance of fQRS in individuals with hypertension in the absence of LVH. Higher fibrotic burden defined via fQRS in patients without LVH may indicate that it should not be identified as a benign variant even in the absence of a known cardiac disease.
5. STUDY LIMITATIONS
Our study has some limitations. First, there is a lack of data regarding localization and number of leads with fQRS. However, fQRS is an indicator of fibrosis independent of localization and number of leads with fQRS. Second, there are no data regarding medications in the study groups. The effects of medical therapy on the emergence of fQRS are unknown. Nevertheless, all patients with hypertension and prehypertension were newly diagnosed and were not taking antihypertensive treatment. Finally, the absence of confirmation of fibrosis and fibrotic burden via magnetic resonance imaging is another limitation.
6. CONCLUSIONS
The present study demonstrated that fQRS is useful to indicate higher fibrotic burden in patients with hypertension even in the absence of LVH. Assessment of fQRS on ECG does not require complex methods. In the absence of LVH, it may be used in the monitoring of patients with hypertension to assess the increase in SBP and higher fibrotic burden.
CONFLICT OF INTEREST
We have no commercial, financial, or other relationships related to the subject of this article that might create any potential conflicts of interest.
Mehmet E, Yavuz K, Suleyman K, Omer S, Zulkif T, Akdeniz B. Usefulness of fragmented QRS in hypertensive patients in the absence of left ventricular hypertrophy. J Clin Hypertens. 2017;19:861–865. 10.1111/jch.13051
REFERENCES
- 1. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903‐1913. [DOI] [PubMed] [Google Scholar]
- 2. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159‐2219. [DOI] [PubMed] [Google Scholar]
- 3. Bacharova L, Schocken D, Estes EH, Strauss D. The role of ECG in the diagnosis of left ventricular hypertrophy. Curr Cardiol Rev. 2014;10:257‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Díez J. Mechanisms of cardiac fibrosis in hypertension. J Clin Hypertens (Greenwich). 2007;9:546‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pietrasik G, Zareba W. QRS fragmentation: diagnostic and prognostic significance. Cardiol J. 2012;19:114‐121. [DOI] [PubMed] [Google Scholar]
- 6. Das MK, Saha C, El Masry H, et al. Fragmented QRS on a 12‐lead ECG: a predictor of mortality and cardiac events in patients with coronary artery disease. Heart Rhythm. 2007;4:1385‐1392. [DOI] [PubMed] [Google Scholar]
- 7. Cetin MS, Ozcan Cetin EH, Canpolat U, et al. Usefulness of fragmented QRS complex to predict arrhythmic events and cardiovascular mortality in patients with noncompaction cardiomyopathy. Am J Cardiol. 2016;117:1516‐1523. [DOI] [PubMed] [Google Scholar]
- 8. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560‐2572. [DOI] [PubMed] [Google Scholar]
- 9. Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113:2495‐2501. [DOI] [PubMed] [Google Scholar]
- 10. Das MK, Maskoun W, Shen C, et al. Fragmented QRS on twelve‐lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm. 2010;7:74‐80. [DOI] [PubMed] [Google Scholar]
- 11. Jain R, Singh R, Yamini S, Das MK. Fragmented ECG as a risk marker in cardiovascular diseases. Curr Cardiol Rev. 2014;10:277‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang B, Zhen Y, Shen D, Zhang G. Significance of fragmented QRS complexes for identifying left ventricular hypertrophy in patients with hypertension. Ann Noninvasive Electrocardiol. 2015;20:175‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rossi MA. Pathologic fibrosis and connective tissue matrix in left ventricular hypertrophy due to chronic arterial hypertension in humans. J Hypertens. 1998;16:1031‐1041. [DOI] [PubMed] [Google Scholar]
- 14. Lehtonen AO, Puukka P, Varis J, et al. Prevalence and prognosis of ECG abnormalities in normotensive and hypertensive individuals. J Hypertens. 2016;34:959‐966. [DOI] [PubMed] [Google Scholar]
- 15. Cuspidi C, Rescaldani M, Sala C, Negri F, Grassi G, Mancia G. Prevalence of electrocardiographic left ventricular hypertrophy in human hypertension: an updated review. J Hypertens. 2012;30:2066‐2073. [DOI] [PubMed] [Google Scholar]
- 16. Terho HK, Tikkanen JT, Junttila JM, et al. Prevalence and prognostic significance of fragmented QRS complex in middle‐aged subjects with and without clinical or electrocardiographic evidence of cardiac disease. Am J Cardiol. 2014;114:141‐147. [DOI] [PubMed] [Google Scholar]


