Abstract
We aim to compare 24‐hour aortic blood pressure variability (BPV) with brachial BPV in relation to carotid damage as estimated by carotid intima‐media thickness (CIMT) and cross‐sectional area (CCSA). Four hundred and forty five individuals received brachial and aortic 24‐hour ambulatory BP monitoring with a validated device (Mobil‐O‐Graph). Systolic BPV was estimated by average real variability (ARV) and time‐weighted standard deviation (wSD). In multiple logistic regression analysis, CIMT > 900 μm was significantly and independently associated with aortic ARV (OR = 1.38; 95% CI: 1.04‐1.84), aortic wSD (OR = 1.65; 95% CI: 1.19‐2.29) and brachial ARV (OR = 1.53; 95% CI: 1.07‐2.18), but not with brachial wSD. CCSA > 90th percentile was significantly and independently associated with aortic ARV (OR = 1.50; 95% CI: 1.07‐2.10) and wSD (OR = 1.70; 95% CI: 1.12‐2.56), but not with brachial BPVs. In receiver operator characteristics curve analysis, aortic wSD identified CCSA > 90th percentile better than brachial wSD (AUC: 0.73 vs 0.68, P < .01). In conclusion, aortic 24‐hour systolic BPV showed a slightly stronger association with carotid damage than brachial BPV.
Keywords: average real variability, blood pressure variability, carotid cross‐sectional area, intima‐media thickness
1. INTRODUCTION
Carotid damage, characterized by increased carotid intima‐media thickness (CIMT) and/or enlarged carotid cross‐sectional area (CCSA), is considered a critical subclinical target organ damage (TOD) and can provide prognostic value for cardiovascular events.1, 2, 3 It was reported that hypertensives have significantly greater CIMT, when compared to normotensives.4, 5 Further investigations showed that brachial systolic blood pressure (BP) was a significant determinant of CIMT.6, 7 In 1663 hypertensives from the European Lacidipine Study on Atherosclerosis (ELSA), Mancia et al8 indicated that not only brachial systolic BP, but also brachial systolic BP fluctuation, was significantly associated with CIMT. Subsequently cross‐sectional and longitudinal studies provided more evidences that brachial blood pressure variability (BPV) either in the short‐term or in the long‐term was significantly associated with CIMT and could predict the risk of clinical events independent of mean BP level.9, 10, 11, 12 CCSA, another marker of carotid damage, was also found to be associated with brachial BPV.13 However, due to the limitation of consecutive measurement of central BP, the association of carotid damage with central (aortic, carotid) BPV is less studied.
From the pathophysiological point of view, target organs, such as the heart, kidneys, and large arteries are directly exposed to central rather than brachial BP. Therefore, it is logical to think that cardiovascular events and organ damage may ultimately more closely correlate to central rather than brachial BP. Recent investigations supported this theory and indicated that central BP was more strongly associated with cardiovascular structural and functional modifications and acted as a reliable predictor of cardiovascular events and mortalities.14, 15, 16, 17 Although central BP has preliminarily shown its superiority as a predictor of hypertensive TODs and cardiovascular events over brachial BP, whether central BPV is also superior to brachial BPV in terms of prognostic value is still unknown.
We therefore conducted this study, applying a validated oscillometry‐based BP monitor for noninvasive ambulatory blood pressure monitoring (ABPM) at both the brachial and aortic levels simultaneously, to investigate aortic BPV and its comparison with brachial BPV in terms of the association with carotid damage as estimated by CIMT and CCSA.
2. METHODS
2.1. Study design and population
The non‐invaSive Aortic ABPM For the detection of tARrget organ damage (SAFAR) study is an on‐going single‐center prospective study. The SAFAR study enrolled consecutive individuals with established hypertension (whether untreated or treated with antihypertensive drugs) or suspected hypertension, who were referred to the Cardiovascular Research Laboratory of “Laiko” Hospital for BP and global cardiovascular risk assessment and TOD evaluation. The details of the SAFAR study were described in our previous publications.18, 19 We excluded participants who met the following criteria: age < 18 years old, the absence of sinus rhythm during laboratory testing, any modification in cardiovascular disease medication in the previous month, inability to provide informed consent, or unwillingness/inability to adhere to study protocol. In total, 445 participants were included in the present analysis. Each enrolled participant was required to refrain from consuming food or any vasoactive substance or medication on the morning of the examination. A standardized structured questionnaire was used to obtain every participant's medical and family histories. Then, a series of examinations, including venous blood and urine tests, brachial and aortic ABPM, and carotid ultrasound were performed for all participants, according to the study protocol. The Ethical/Scientific Committee of the “Laiko” Hospital approved this study and all participants provided informed consent according to the declaration of Helsinki.
2.2. Brachial and aortic ABPM
Brachial and aortic ABPM was noninvasively performed using a well validated device Mobil‐O‐Graph,20, 21, 22, 23 which is a commercially available brachial cuff‐based automated oscillometric device approved by the US Food and Drug Administration (FDA) and the European Union for 24‐hour ABPM. The device was set to function under the manufacturer's inbuilt protocol, namely 4 BP recordings per hour from 08:00 to 23:59 and 2 recordings per hour from 00:00 to 07:59. In this mode of function, the device performs both brachial BP and brachial pressure waveform recording simultaneously. Aortic BP was generated by software analysis (with the application of pulse wave analysis and of a generalized transfer function) when the data were downloaded to the manufacturer's software (HMS version 4.6). The brachial mean BP and diastolic BP calibration method was used in the present study. Valid recording must meet the following criteria: (1) Valid readings were at least 70% of the expected readings; (2) at least one valid reading per hour for at least 21 hours. Hypertension was defined by 24‐hour brachial mean systolic BP ≥ 130 mm Hg or diastolic BP ≥ 80 mm Hg or taking anti‐hypertensive agents for BP control.
Affected by day‐night BP fluctuations, the crude standard deviation (SD) of 24‐hour BPs is not accurate enough to estimate the true 24‐hour BPV. Thus, in the present study, we selected average real variability (ARV)24 and time‐weighted standard deviation (wSD)25 to assess 24‐hour systolic BPV. But we still provided the corresponding results about crude SD of 24‐hour BP. ARV and wSD are 2 recently developed markers of BPV that are not affected by day‐night BP reduction. They are more accurate in estimating BPV and serve as better predictors of organ damage and cardiovascular risk than conventional crude SD of 24‐hour BP.26 ARV was computed as the average of the absolute differences between consecutive BP readings, and wSD was computed as the average of day and night SDs weighted their respective durations. We used the following formula to calculate ARV:
Where k ranges from 1 to n, BPk is one BP measurement, w is time interval between BPk and BPk−1, and n is the number of BP readings in 24 hours.
We applied the following formula to calculate wSD:
Where k ranges from 1 to n, w is corresponding time interval, BPk is one BP measurement, and n is the number of BP readings in corresponding daytime or nighttime. AT and ST stand for awaken and sleeping time in hours, respectively.
2.3. Carotid ultrasonography
All participants underwent carotid ultrasonography by a validated ultrasound system (Vivid 7 Pro). The internal (or lumen) and external (or interadventitial) diameters and CIMT of common carotid arteries were measured in end‐diastole of the cardiac cycle. CIMT was measured at plaque‐free site. The internal diameter was defined as the distance between the trailing edge of the echo produced by the intima‐lumen interface of the near wall and the leading edge of the echo produced by the lumen‐intima interface of the far wall. The external diameter was defined as the distance between the 2 media‐adventitia interfaces. CCSA was calculated by the following formula13: CCSA = π × ([external diameter/2]2 − [internal diameter/2]2). The left and right CIMTs and CCSAs were averaged for further analysis. Abnormal arterial damage was defined as CIMT > 900 μm or CCSA > 90th percentile.
2.4. Statistical analysis
Means and proportions were compared between men and women by Student's t‐test and Fisher's exact test, respectively. Continuous variables were presented as mean ± SD, and categorical variables as absolute numbers and percentage in parenthesis. Pearson's correlation analysis was applied for the association of CIMT and CCSA with systolic BPV. Multiple linear and logistic regression models were built to evaluate the association between systolic BPV and carotid damage. Receiver operator characteristics (ROC) analysis was applied to compare the discriminatory ability of brachial and aortic systolic BPVs in detecting carotid damage. Statistical analysis was performed using SAS software, version 9.3 and Sigmaplot version 12.5. Statistical significance was defined as P < .05.
3. RESULTS
3.1. Characteristics of participants
Clinical characteristics of participants between men and women were presented in Table 1. The 445 participants had mean age of 54.0 ± 13.0 years and included 57.1% male gender, 80.7% hypertension, 2.7% coronary heart disease, 5.8% diabetes mellitus, and 2.3% stroke. Among hypertensive participants, 206 (57.4%) were taking antihypertensive agents. Participants' BP parameters were presented in Table 2. Women consistently had significantly greater systolic BPV at both brachial and aortic levels than men. The correlation coefficients between brachial BPV and aortic BPV were 0.89 for wSD and 0.86 for ARV.
Table 1.
Participants' characteristics
| Variables | Total (445) | Men (254, 57.1%) | Women (191, 42.9%) | P‐values |
|---|---|---|---|---|
| Age, year | 54.0 ± 13.0 | 51.5 ± 12.4 | 57.4 ± 13.0 | <.001 |
| Smoker, n (%) | 268 (60.4) | 170 (67.2) | 98 (51.3) | .001 |
| Body mass index, kg/m2 | 27.8 ± 4.5 | 28.4 ± 3.9 | 27.1 ± 5.0 | .002 |
| Intima‐media thickness, μm | 709.2 ± 144.0 | 694.9 ± 144.6 | 728.2 ± 141.4 | .02 |
| Carotid cross‐sectional area, mm2 | 14.8 ± 4.1 | 14.8 ± 4.3 | 14.7 ± 3.8 | .78 |
| Diseases and therapies | ||||
| Hypertensiona, n (%) | 359 (80.7) | 211 (83.1) | 148 (77.5) | .14 |
| Anti‐hypertensive, n (%) | 206 (57.4) | 110 (52.1) | 96 (64.9) | .02 |
| Coronary heart disease, n (%) | 12 (2.7) | 10 (3.9) | 2 (1.1) | .12 |
| Diabetes mellitus, n (%) | 26 (5.8) | 18 (7.1) | 8 (4.2) | .20 |
| Renal diseases, n (%) | 7 (1.6) | 5 (2.0) | 2 (1.1) | .44 |
| Stroke, n (%) | 10 (2.3) | 8 (1.8) | 2 (0.5) | .14 |
| Biochemical parameters | ||||
| Glucose, mmol/L | 5.4 ± 0.9 | 5.5 ± 1.1 | 5.3 ± 0.7 | .02 |
| Total cholesterol, mmol/L | 5.4 ± 1.0 | 5.3 ± 1.0 | 5.6 ± 1.0 | .01 |
| LDL, cholesterol, mmol/L | 3.4 ± 0.9 | 3.4 ± 0.9 | 3.5 ± 1.0 | .23 |
| HDL, cholesterol, mmol/L | 1.4 ± 0.4 | 1.3 ± 0.3 | 1.5 ± 0.3 | <.001 |
| Triglyceride, mmol/L | 1.4 ± 0.6 | 1.5 ± 0.7 | 1.2 ± 0.5 | <.001 |
| Serum creatinine, μmol/L | 78.7 ± 17.2 | 84.8 ± 16.3 | 70.8 ± 15.1 | <.001 |
| GFR, mL/min/1.73 m2 | 87.9 ± 17.6 | 90.8 ± 16.9 | 84.0 ± 17.9 | <.001 |
GFR, glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Values are presented as mean ± standard deviation and numbers and percentages in parenthesis for quantitative and qualitative variables, respectively. Student t‐test and Fisher's exact test were applied to compare variables between men and women, and the P values were shown on the right column.
Hypertension was defined by 24‐h brachial mean SBP ≥ 130 mm Hg or DBP ≥ 80 mm Hg or taking anti‐hypertensive agents for BP control.
Table 2.
Brachial and aortic blood pressure components and their variabilities
| Total (445) | Men (254) | Women (191) | P | |
|---|---|---|---|---|
| Brachial blood pressures and their variabilities | ||||
| 24‐h mean SBP, mm Hg | 127.4 ± 12.2 | 128.0 ± 11.4 | 126.5 ± 13.1 | .21 |
| 24‐h mean DBP, mm Hg | 80.9 ± 9.9 | 83.3 ± 8.8 | 77.9 ± 10.4 | <.001 |
| 24‐h systolic SD, mm Hg | 14.6 ± 3.6 | 13.8 ± 3.2 | 15.6 ± 3.8 | <.001 |
| 24‐h systolic wSD, mm Hg | 12.8 ± 3.3 | 12.0 ± 2.9 | 13.9 ± 3.5 | <.001 |
| 24‐h systolic ARV, mm Hg | 10.8 ± 3.1 | 10.0 ± 2.5 | 11.9 ± 3.5 | <.001 |
| Aortic blood pressures and their variabilities | ||||
| 24‐h mean SBP, mm Hg | 118.1 ± 11.7 | 118.5 ± 10.7 | 117.5 ± 12.8 | .36 |
| 24‐h mean DBP, mm Hg | 82.5 ± 10.0 | 84.8 ± 9.0 | 79.4 ± 10.5 | <.001 |
| 24‐h systolic SD, mm Hg | 13.1 ± 3.4 | 12.4 ± 3.0 | 14.2 ± 3.2 | <.001 |
| 24‐h systolic wSD, mm Hg | 11.8 ± 3.1 | 11.0 ± 2.7 | 12.8 ± 3.2 | <.001 |
| 24‐h systolic ARV, mm Hg | 10.5 ± 3.1 | 9.7 ± 2.6 | 11.6 ± 3.4 | <.001 |
ARV, average real variability; DBP, diastolic blood pressure; SBP, systolic blood pressure; SD, standard deviation; wSD, time‐weighted standard deviation.
Values were presented as mean ± standard deviation. Student t‐test was applied to compare variables between men and women, and the P values were shown on the right column.
3.2. Correlation of brachial and aortic BPVs with carotid damage
Pearson correlation analysis was performed to investigate relationship between BPVs and parameters of carotid damage, and Z test was used to determine the difference between brachial and aortic BPVs. As shown in Table 3, in total population, either unadjusted or adjusted for age, gender and 24‐hour mean systolic BP, CIMT was significantly correlated to brachial and aortic ARVs (adjusted correlation coefficient [CC] = 0.127, P < .01; CC = 0.112, P < .05; respectively) and to brachial and aortic wSDs (CC = 0.126, P < .01; CC = 0.139, P < .01; respectively). CCSA was also significantly correlated to brachial and aortic ARVs (CC = 0.096, P < .05; CC = 0.097, P < .05; respectively) and to brachial and aortic wSDs (CC = 0.102, P < .05; CC = 0.133, P < .01; respectively). Compared with brachial BPV, central BPV showed slightly higher correlation with carotid parameters; however, the differences did not reach statistical significance (all P > .05). The subgroup analysis in hypertensive population obtained similar results.
Table 3.
Unadjusted and adjusted correlation coefficient between brachial and aortic blood pressure variability and CIMT and CCSA
| Variables | CIMT | CCSA | ||||||
|---|---|---|---|---|---|---|---|---|
| Correlation coefficienta | P † | Correlation coefficientb | P † | Correlation coefficienta | P † | Correlation coefficientb | P † | |
| Total population | ||||||||
| 24‐h systolic wSD | ||||||||
| Brachial | .329*** | .79 | .126** | .85 | .293*** | .76 | .102* | .65 |
| Aortic | .345*** | .139** | .312*** | .133** | ||||
| 24‐h systolic ARV | ||||||||
| Brachial | .283*** | .90 | .127** | .82 | .232*** | .79 | .096* | .99 |
| Aortic | .291*** | .112* | .249*** | .097* | ||||
| Hypertensives | ||||||||
| 24‐h systolic wSD | ||||||||
| Brachial | .237*** | .80 | .125* | .95 | .203*** | .75 | .097* | .76 |
| Aortic | .255*** | .130* | .226*** | .120* | ||||
| 24‐h systolic ARV | ||||||||
| Brachial | .250*** | .98 | .160** | .71 | .196*** | .79 | .119* | .90 |
| Aortic | .252*** | .132* | .215*** | .110* | ||||
ARV, average real variability; CCSA, carotid cross‐sectional area; CIMT, carotid intima‐media thickness; wSD, time‐weighted standard deviation.
Pearson's correlation analysis was performed to detect the unadjusted and adjusted correlations between BPVs and CIMT and CCSA. Corresponding correlation coefficients before (a) and after (b) adjustment for age, gender and 24‐h mean systolic BP were shown in the table. Z test was used to detect the difference between brachial and central correlation coefficient.
*P < .05, **P < .01, ***P < .001 for the correlation analysis. † P values for the differences between brachial and aortic BPV derived from Z‐test.
3.3. Multiple regression analysis on the association of brachial and aortic BPVs and carotid damage
Multiple linear and logistic regression models adjusted for age, gender, body mass index, smoking status, antihypertensive treatment, LDL, presence of diabetes, and brachial/aortic 24‐hour mean SBP were performed in total and hypertensive populations, respectively. As shown in Figure 1, either in brachial or in aortic models, 24‐hour mean systolic BP and BPV both were significantly associated with CIMT and CCSA. 1‐SD increase in brachial and aortic ARVs were significantly associated with 17.22 μm (P = .03) and 19.31 μm (P = .02) increases in CIMT, respectively, and 1‐SD increase in brachial and aortic wSDs were significantly associated with 20.80 μm (P = .02) and 19.68 μm (P = .02) increases in CIMT, respectively. As for CCSA, 1‐SD increase in aortic wSD and ARV were significantly associated with 0.50 mm2 (P = .04) and 0.47 mm2 (P = .04) increase in CCSA, respectively. Brachial wSD and ARV did not show statistical association with CCSA. Hypertensive subgroup analysis obtained similar results.
Figure 1.
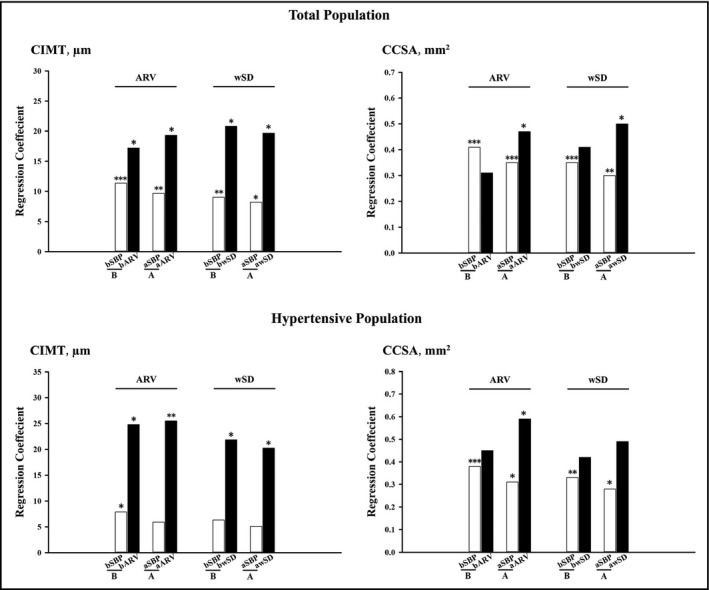
Multiple linear regression analysis for the associations of CIMT and CCSA with brachial and aortic BPVs in total and hypertensive populations. Brachial (B) and aortic (A) multiple linear regression models with adjustment for age, gender, body mass index, smoking status, antihypertensive treatment, LDL, presence of diabetes, and bSBP/aSBP were conducted to investigate the associations of CIMT and CCSA with brachial and aortic BPVs, in total and hypertensive populations, respectively. Corresponding regression coefficients (SBP per 5 mm Hg and wSD/ARV per 1 SD) were shown in the figure. *P < .05; **P < .01; ***P < .001. bARV/aARV, average real variability of 24‐hour brachial/aortic SBP; bSBP/aSBP, 24‐hour brachial/aortic mean SBP; bwSD/awSD, time‐weighted standard deviation of 24‐hour brachial/aortic SBP; CCSA, carotid cross‐sectional area; CIMT, carotid intima‐media thickness
Results derived from multiple logistic regression analysis were shown in Figure 2. In brachial models, 24‐hour mean systolic BP was significantly associated with carotid damage, but BPV was not. In aortic models, contrarily, 24‐hour systolic BPV was significantly associated with carotid damage, but mean systolic BP was not. CIMT > 900 μm was significantly and independently associated with aortic ARV (Odds Ratio [OR] = 1.38; 95% confidence interval [CI]: 1.04‐1.84), aortic wSD (OR = 1.65; 95% CI: 1.19‐2.29), and brachial AVR (OR = 1.53; 95% CI: 1.07‐2.18), but not with brachial wSD. CCSA > 90th percentile was significantly and independently associated with aortic ARV (OR = 1.50; 95% CI: 1.07‐2.10) and aortic wSD (OR = 1.70; 95% CI: 1.12‐2.56), rather than with brachial ARV and wSD. Hypertensive subgroup analysis acquired consistent results.
Figure 2.
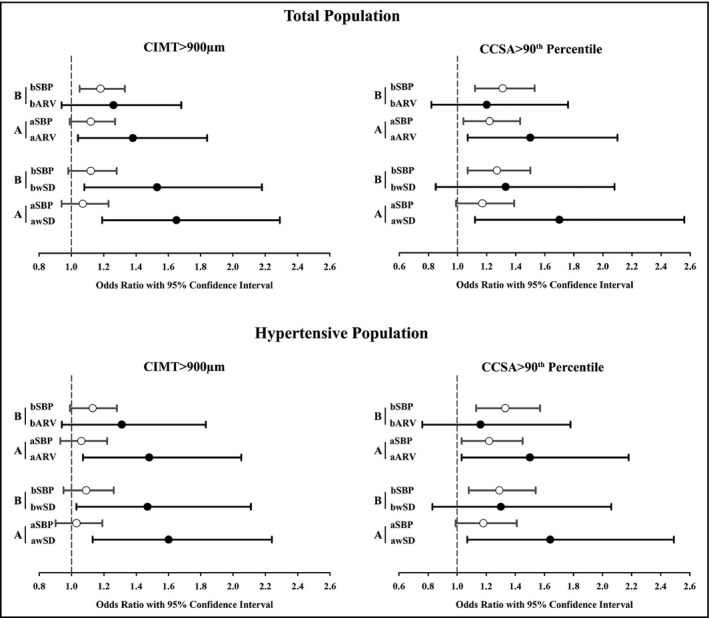
Multiple logistic regression analysis for the associations of CIMT > 900 μm and CCSA > 90th percentile with brachial and aortic BPVs in total and hypertensive populations. Brachial (B) and aortic (A) multiple logistic regression models with adjustment for age, gender, body mass index, smoking status, antihypertensive treatment, LDL, presence of diabetes, bSBP/aSBP, were conducted in total and hypertensive populations, respectively. Corresponding odds ratio (OR) with 95% Confidence Interval (SBP per 5 mm Hg and wSD/ARV per 1 SD) was shown in the figure. bARV/aARV, average real variability of 24‐hour brachial/aortic SBP; bSBP/aSBP, 24‐hour brachial/aortic mean SBP; bwSD/awSD, time‐weighted standard deviation of 24‐hour brachial/aortic SBP; CCSA, carotid cross‐sectional area; CIMT, carotid intima‐media thickness
3.4. ROC curve analysis on the ability of brachial and aortic BPVs in discriminating carotid damage
Receiver operator characteristics curve analysis and Z test in total and hypertensive populations were performed to investigate the ability of brachial and aortic BPVs in discriminating carotid damage, as shown in Figure 3. In total population, aortic ARV and wSD presented greater area under curve (AUC) than brachial ARV and wSD in identifying CIMT > 900 μm, but the differences between aortic and brachial BPVs did not reach statistical significance (AUC 0.67 vs 0.65, Z = 0.99, P = .32; AUC 0.72 vs 0.69, Z = 1.82, P = .06; respectively). In identifying CCSA > 90th percentile, aortic wSD is better than brachial wSD (AUC 0.73 vs 0.68, Z = 2.86, P < .01), however there is no difference between aortic and brachial ARV (AUC 0.69 vs 0.65, Z = 1.51, P = .13). Hypertensive subgroup analysis obtained consistent results.
Figure 3.
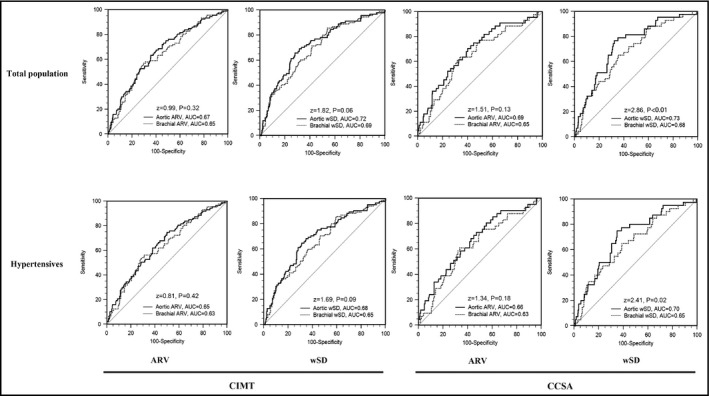
The comparisons between brachial and aortic BPVs on the ability of identifying CIMT > 900 μm and CCSA > 90th percentile in total population and hypertensives. Receiver operator characteristics curve analysis and z‐test were built to compare the difference between aortic and brachial BPVs in identifying CIMT > 900 μm and CCSA > 90th percentile in total population and hypertensives, respectively. Area under curve, P and z values were shown in the figure. ARV, average real variability of 24‐hour systolic BP; CCSA, carotid cross‐sectional area; CIMT, carotid intima‐media thickness; wSD, time‐weighted standard deviation of 24‐hour systolic BP
3.5. The associations of carotid damage with BPV when using the crude SD of 24‐hour BP as marker of BPV
The mean values of brachial and aortic crude SD of 24‐hour systolic BP were shown in Table 2. The correlation coefficient between brachial and aortic crude SDs was 0.88. In unadjusted correlation analysis, brachial and aortic crude SDs were significantly correlated with CIMT (CC: 0.220 vs 0.256, P for difference > .05) and CCSA (CC: 0.185 vs 0.220, P for difference > .05). In adjusted correlation analysis and multiple linear and logistic regression analysis, neither brachial nor aortic crude SD associated with carotid damage.
4. DISCUSSION
The present study indicated that both brachial and aortic 24‐hour systolic BPVs, as estimated by ARV and wSD were significantly and independently associated with CIMT and CCSA, markers of carotid damage, independently of 24‐hour mean systolic BP. Compared with brachial BPV, aortic BPV showed equal association with CIMT, but slightly stronger association with CCSA.
The relationships of brachial BPV with development and severity of cardiovascular organ damages, as well as with events and mortality, have been elaborated in previous investigations.8, 9, 11, 27, 28, 29 Through invasive measurements of 24‐hour BP in 108 hypertensives, Parati et al28 first reported that subjects with an increased 24‐hour brachial BPV presented greater prevalence and severity of TODs after adjustment for mean BP. Later, Mancia et al,8 in 1663 hypertensives from the ELSA study, offered further evidence that 24‐hour brachial systolic BPV was a determinant of CIMT. Sander et al,30 after 3.3 years of follow‐up for 268 patients with age >55 years, indicated that daytime brachial systolic BPV was a strong predictor of early atherosclerosis progression. Our results were in line with these findings and provided additional evidence that brachial 24‐hour systolic BPV is an independent risk factor for alteration of CIMT. Importantly, in the present study, we first reported the significant and independent association of aortic systolic BPV with carotid damage. Of note, in the present study, multiple linear regression analysis showed aortic 24‐hour mean systolic BP and BPV both were significantly associated with carotid parameters, and multiple logistic regression analysis showed that aortic 24‐hour systolic BPV rather than mean BP was significantly associated with carotid damage. This suggested that aortic 24‐hour systolic BPV might be a better BP‐related parameter than aortic mean BP level in association with carotid damage. More importantly, our study indicated that aortic systolic BPV showed a slightly stronger association with carotid damage (increased CCSA) than brachial BPV, in spite of mild evidence. This finding may add a new dimension on the investigation of BPV, but more studies with solid evidence are required to confirm this finding, especially the studies on the comparison between brachial and aortic BPV in terms of predicting cardiovascular morbidity and mortality.
Growing evidence indicated that aortic BP, measured by either invasive or noninvasive method, was a better marker for predicting hypertensive TODs and cardiovascular mortality than brachial cuff‐based BP.14, 15, 16, 17 Our study showed that aortic systolic BPV was slightly more strongly associated with carotid damage independently of systolic BP level and other covariates when compared with brachial systolic BPV. Taken together, these findings supported the superiority of central BP profiles. However, the mechanisms of the superiority of central BP profiles over brachial BP profiles are not well understood. The following 3 aspects may be involved. First, brachial and central arteries have different arterial stiffness and distensibility. Second, the timing and magnitude of reflective pressure waveforms vary from the aorta to peripheral artery. Third, peripheral and central arteries may respond differently to physiological regulations, pathophysiological stimulations, and pharmaceutical effects. These mechanisms result in variations of local BP magnitude and fluctuation along arterial tree. Thus, on the one hand, BP profiles measured at brachial arteries cannot accurately indicate the BP profiles measured at aortic arteries. On the other hand, aortic BP profiles, including BP level and fluctuation as the direct load that organs confront pathophysiologically, predict organ damage better than brachial BP. But, further investigations are needed to further explore the potential mechanisms of the discrepancy between peripheral and central BP profiles.
Office brachial BP has its limitations despite it has been referenced as the optimal BP parameter to diagnose hypertension and guide antihypertensive therapy for many decades. For example, in patients with white‐coat hypertension or masked hypertension,31 office brachial BP may not accurately reflect the patient's usual BP profile. Many new technologies and concepts then were developed to overcome these limitations, such as brachial 24‐hour ABPM and non‐invasive central BP measurement. Based on extensive investigation and validation, ABPM was indicated to be superior to brachial office BP in terms of its prognostic value and its current use in clinical practice.32 However, either measured once at office or monitored for 24 hours, brachial BP is a poor surrogate for central BP, which is supposed to provide more information for organ damage and cardiovascular risk. Thus, office non‐invasive assessment of central BP was developed decades ago and subsequently demonstrated more strongly associated with TODs and cardiovascular events, though no final consensus is reached so far. In recent years, cuff‐based aortic 24‐hour ABPM is also available for routine clinical practice,20, 33 but its clinical significance in hypertension diagnosis and treatment needs to be confirmed. Previous studies showed that 24‐hour aortic mean BP was better associated with left ventricular diastolic dysfunction18 and left ventricular hypertrophy19, 34 than brachial 24‐hour or office BP. In the present study, we indicated that 24‐hour aortic BPV showed a slightly stronger association with carotid damage than 24‐hour brachial BPV. Together, these findings suggested the potential value of aortic 24‐hour ABPM beyond brachial BP. However, it is far too early to make statements about the aortic 24‐hour ABPM in guiding the diagnosis and treatment of hypertension, more studies with solid evidences are required.
Our findings need to be interpreted within the context of its limitations. First, the same device measured both the brachial and central BP and its variation, so we cannot exclude the effect of noise of similar measurement in the comparison. Second, as the study is cross‐sectional, no causality data were available. Third, the sample size of the study was relatively small. Further studies with a larger sample size and covering a wider range of patient characteristics, including ethnicity, regional, and environmental factors, especially using 2 different methods for central and brachial BP measurement, would assist in validating our findings.
In conclusion, both aortic and brachial 24‐hour systolic BPV were significantly and independently associated with CIMT and CCSA. Brachial and aortic BPV were roughly equal in their associations with CIMT, but aortic BPV is slightly stronger associated with CCSA than brachial BPV. Further studies are warranted to test our findings and investigate the clinical value of the novel aortic 24‐hour ABPM in terms of better cardiovascular risk stratification and management.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Yu S, Chi C, Protogerou AD, et al. 24‐hour aortic blood pressure variability showed a stronger association with carotid damage than 24‐hour brachial blood pressure variability: The SAFAR study. J Clin Hypertens. 2018;20:499–507. 10.1111/jch.13226
Contributor Information
Yi Zhang, Email: yizshcn@gmail.com.
Yawei Xu, Email: xuyaweicn@aliyun.com.
REFERENCES
- 1. Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the atherosclerosis risk in communities (aric) study, 1987‐1993. Am J Epidemiol. 1997;146:483‐494. [DOI] [PubMed] [Google Scholar]
- 2. Polak JF, Pencina MJ, O'Leary DH, et al. Common carotid artery intima‐media thickness progression as a predictor of stroke in multi‐ethnic study of atherosclerosis. Stroke. 2011;42:3017‐3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polak JF, Pencina MJ, Pencina KM, et al. Carotid‐wall intima‐media thickness and cardiovascular events. N Engl J Med. 2011;365:213‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bots ML, Hofman A, de Bruyn AM, et al. Isolated systolic hypertension and vessel wall thickness of the carotid artery. The rotterdam elderly study. Arterioscler Thromb. 1993;13:64‐69. [DOI] [PubMed] [Google Scholar]
- 5. Zanchetti A. Prevalence of carotid atherosclerosis in hypertension: preliminary baseline data from the european Lacidipine Study on Atheroscelerosis (ELSA). Blood Press Suppl. 1996;4:30‐35. [PubMed] [Google Scholar]
- 6. Zanchetti A, Bond MG, Hennig M, et al. Risk factors associated with alterations in carotid intima‐media thickness in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis. J Hypertens. 1998;16:949‐961. [DOI] [PubMed] [Google Scholar]
- 7. Nair SB, Malik R, Khattar RS. Carotid intima‐media thickness: ultrasound measurement, prognostic value and role in clinical practice. Postgrad Med J. 2012;88:694‐699. [DOI] [PubMed] [Google Scholar]
- 8. Mancia G, Parati G, Hennig M, et al. Relation between blood pressure variability and carotid artery damage in hypertension: baseline data from the European Lacidipine Study on Atherosclerosis (ELSA). J Hypertens. 2001;19:1981‐1989. [DOI] [PubMed] [Google Scholar]
- 9. Sega R, Corrao G, Bombelli M, et al. Blood pressure variability and organ damage in a general population: results from the pamela study (pressioni arteriose monitorate e loro associazioni). Hypertension. 2002;39:710‐714. [DOI] [PubMed] [Google Scholar]
- 10. Shintani Y, Kikuya M, Hara A, et al. Ambulatory blood pressure, blood pressure variability and the prevalence of carotid artery alteration: the ohasama study. J Hypertens. 2007;25:1704‐1710. [DOI] [PubMed] [Google Scholar]
- 11. Kawai T, Ohishi M, Kamide K, et al. Differences between daytime and nighttime blood pressure variability regarding systemic atherosclerotic change and renal function. Hypertens Res. 2013;36:232‐239. [DOI] [PubMed] [Google Scholar]
- 12. Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895‐905. [DOI] [PubMed] [Google Scholar]
- 13. Roman MJ, Pickering TG, Schwartz JE, et al. Relation of blood pressure variability to carotid atherosclerosis and carotid artery and left ventricular hypertrophy. Arterioscler Thromb Vasc Biol. 2001;21:1507‐1511. [DOI] [PubMed] [Google Scholar]
- 14. Roman MJ, Devereux RB, Kizer JR, et al. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the strong heart study. Hypertension. 2007;50:197‐203. [DOI] [PubMed] [Google Scholar]
- 15. Wang KL, Cheng HM, Chuang SY, et al. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens. 2009;27:461‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McEniery CM, Cockcroft JR, Roman MJ, et al. Central blood pressure: current evidence and clinical importance. Eur Heart J. 2014;35:1719‐1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kollias A, Lagou S, Zeniodi ME, et al. Association of central versus brachial blood pressure with target‐organ damage: systematic review and meta‐analysis. Hypertension. 2016;67:183‐190. [DOI] [PubMed] [Google Scholar]
- 18. Zhang Y, Kollias G, Argyris AA, et al. Association of left ventricular diastolic dysfunction with 24‐h aortic ambulatory blood pressure: the safar study. J Hum Hypertens. 2015;29:442‐448. [DOI] [PubMed] [Google Scholar]
- 19. Protogerou AD, Argyris AA, Papaioannou TG, et al. Left‐ventricular hypertrophy is associated better with 24‐h aortic pressure than 24‐h brachial pressure in hypertensive patients: the safar study. J Hypertens. 2014;32:1805‐1814. [DOI] [PubMed] [Google Scholar]
- 20. Protogerou AD, Argyris A, Nasothimiou E, et al. Feasibility and reproducibility of noninvasive 24‐h ambulatory aortic blood pressure monitoring with a brachial cuff‐based oscillometric device. Am J Hypertens. 2012;25:876‐882. [DOI] [PubMed] [Google Scholar]
- 21. Weber T, Wassertheurer S, Rammer M, et al. Validation of a brachial cuff‐based method for estimating central systolic blood pressure. Hypertension. 2011;58:825‐832. [DOI] [PubMed] [Google Scholar]
- 22. Wassertheurer S, Kropf J, Weber T, et al. A new oscillometric method for pulse wave analysis: comparison with a common tonometric method. J Hum Hypertens. 2010;24:498‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones CR, Taylor K, Chowienczyk P, et al. A validation of the mobil o graph (version 12) ambulatory blood pressure monitor. Blood Press Monit. 2000;5:233‐238. [DOI] [PubMed] [Google Scholar]
- 24. Mena L, Pintos S, Queipo NV, et al. A reliable index for the prognostic significance of blood pressure variability. J Hypertens. 2005;23:505‐511. [DOI] [PubMed] [Google Scholar]
- 25. Bilo G, Giglio A, Styczkiewicz K, et al. A new method for assessing 24‐h blood pressure variability after excluding the contribution of nocturnal blood pressure fall. J Hypertens. 2007;25:2058‐2066. [DOI] [PubMed] [Google Scholar]
- 26. Parati G, Ochoa JE, Lombardi C, et al. Blood pressure variability: assessment, predictive value, and potential as a therapeutic target. Curr Hypertens Rep. 2015;17:537. [DOI] [PubMed] [Google Scholar]
- 27. Tatasciore A, Renda G, Zimarino M, et al. Awake systolic blood pressure variability correlates with target‐organ damage in hypertensive subjects. Hypertension. 2007;50:325‐332. [DOI] [PubMed] [Google Scholar]
- 28. Parati G, Pomidossi G, Albini F, et al. Relationship of 24‐hour blood pressure mean and variability to severity of target‐organ damage in hypertension. J Hypertens. 1987;5:93‐98. [DOI] [PubMed] [Google Scholar]
- 29. Hansen TW, Thijs L, Li Y, et al. Prognostic value of reading‐to‐reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55:1049‐1057. [DOI] [PubMed] [Google Scholar]
- 30. Sander D, Kukla C, Klingelhofer J, et al. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: a 3‐year follow‐up study. Circulation. 2000;102:1536‐1541. [DOI] [PubMed] [Google Scholar]
- 31. Krakoff LR. Blood pressure out of the office: its time has finally come. Am J Hypertens. 2016;29:289‐295. [DOI] [PubMed] [Google Scholar]
- 32. Quinn RR, Hemmelgarn BR, Padwal RS, et al. The 2010 canadian hypertension education program recommendations for the management of hypertension: part I – blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol. 2010;26:241‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luzardo L, Lujambio I, Sottolano M, et al. 24‐h ambulatory recording of aortic pulse wave velocity and central systolic augmentation: a feasibility study. Hypertens Res. 2012;35:980‐987. [DOI] [PubMed] [Google Scholar]
- 34. Weber T, Wassertheurer S, Schmidt‐Trucksass A, et al. Relationship between 24‐hour ambulatory central systolic blood pressure and left ventricular mass: a prospective multicenter study. Hypertension. 2017;70:1157‐1164. [DOI] [PubMed] [Google Scholar]


