Abstract
We investigated the association of endothelial glycocalyx damage with arterial stiffness, impairment of coronary microcirculatory function, and LV myocardial deformation in 320 untreated hypertensives and 160 controls. We measured perfused boundary region (PBR) of the sublingual microvessels, a marker inversely related with glycocalyx thickness, coronary flow reserve (CFR), and Global Longitudinal strain (GLS) by echocardiography, pulse wave velocity (PWV), and central systolic blood pressure (cSBP). Hypertensives had higher PBR, PWV cSBP, and lower CFR and GLS than controls (P < .05). In hypertensives, increased PBR was associated with increased cSBP, PWV, and decreased CFR and GLS after adjustment for age, sex, BMI, smoking LV mass, heart rate, hyperlipidemia, and office SBP (P < .05). PBR had an additive value to PWV, CFR, and office SBP for the prediction of abnormal GLS (x2 = 2.4‐3.8, P for change = .03). Endothelial glycocalyx is impaired in untreated hypertensives and is related to arterial stiffness, coronary, and myocardial dysfunction.
Keywords: arterial stiffness, endothelial glycocalyx, longitudinal strain, pulse wave velocity
1. INTRODUCTION
Endothelial glycocalyx consists of glycoproteins and proteoglycans and forms an endothelial surface layer that prevents the direct contact of blood cells to the endothelial surface in the vessels.1, 2, 3, 4, 5 The integrity of endothelial glycocalyx plays a vital role in vascular permeability, inflammation, and elasticity6, 7 and is also linked with the pathophysiological pathways of atherosclerosis.8, 9 Loss of endothelial glycocalyx intergrity has been shown to occur during exposure to atherogenic risk factors including hypeglycemia, hyperlidemia and smoking.10, 11, 12, 13, 14, 15 Studies have shown that endothelial glycocalyx serves as an effective buffer barrier for sodium.16 Damaged endothelial glycocalyx facilitates sodium entry into the endothelial cells and thus, endothelial glycocalyx damage has been proposed as the mechanism mediating the association between endothelial dysfunction and arterial hypertension observed in sodium abuse in experimental studies.16, 17 However, the association between endothelial glycocalyx damage and impaired arterial elastic properties in hypertensives have not been fully explored. Novel imaging techniques, using dedicated cameras, permit the non‐invasive assessment of the endothelial glycocalyx thickness of the sublingual arterial microvessels by measurement of the perfusion boundary region (PBR) of the luminal wall of the microvessels, with diameters ranging from 5 to 25 μm.14, 15
Two‐dimensional ultrasound speckle tracking imaging has permitted the accurate evaluation of left ventricular (LV) deformation.18, 19, 20 Left ventricular global longitudinal strain (GLS) is impaired in hypertensives and is associated with heart failure with preserved ejection fraction.21 Arterial stiffness, as assessed by pulse wave velocity (PWV), is one of the determinants for impaired LV longitudinal deformation. Moreover, coronary flow reserve (CFR), a marker of coronary microcirculatory function, is related to arterial stiffness and is impaired in hypertensive heart disease.22 The effect of endothelial glycocalyx damage on arterial wall properties, coronary flow reserve, and LV myocardial deformation has not been clarified.
We hypothesized that endothelial glycocalyx is impaired in newly diagnosed untreated hypertensives, leading to impaired arterial elasticity, coronary flow reserve, and consequently, abnormal myocardial deformation. Therefore, we investigated the association of endothelial glycocalyx damage with pulse wave velocity, coronary flow reserve, and LV myocardial deformation in untreated hypertensives.
2. MATERIALS AND METHODS
2.1. Study population
Out of 348 consecutive patients with new onset essential hypertension who attended our outpatient hypertension clinic, 320 patients (mean age: 51 ± 11 years, 67% males) with normal ejection fraction (>60%) had adequate 2‐dimensional echocardiography images for the analysis of speckle parameters (feasibility of the method 92%). The diagnosis of essential hypertension was defined as seated systolic blood pressure (SBP) > 140 mm Hg and/or diastolic pressure (DBP) > 90 mm Hg during at least 3 measurements and >125/80 mm Hg during 24 hour‐ambulatory BP monitoring.
One‐hundred and sixty normotensive individuals (age: 48 ± 13 years, 66% males) who visited our outpatient clinic for a routine check‐up were selected using a ratio of 1 control to 2 hypertensive patients as controls.
Exclusion criteria were history of diabetes, familiar hyperlipidemia, coronary artery disease (CAD), cardiomyopathy, and chronic pulmonary disease. All patients and controls had no history of CAD, normal resting electrocardiogram, and underwent a non‐invasive test (thallium scintigraphy after a treadmill exercise test or dobutamine stress echocardiography) to exclude myocardial ischemia. None of the female patients were on hormone replacement treatment. None of the patients or controls were on any kind of medication, including statins. All patients and controls had a glomerular filtration rate (GFR) > 60 mL/min/1.732 (MDRD formula) at enrolment.
The investigation conforms to the principles outlined in the Declaration of Helsinki. The institute's ethics committee approved the study protocol, and all patients gave written informed consent to participate in the study.
Office systolic (SBP) and diastolic (DBP) blood pressure was obtained by mercury cuff sphygmomanometer. We calculated pulse pressure (PP) as PP = SBP‐DBP. Three measurements, 2 minutes apart, were averaged. Hyperlipidemia was defined as fasting total cholesterol >200 mg/dL or LDL cholesterol >130 mg/dL or fasting triglycerides >200 mg/dL. Normoglycaemic status was defined as a fasting glucose levels <110 mg/dL in the absence of usage of antidiabetic medications.
2.2. Measurements
2.2.1. Endothelial glycocalyx
We measured the perfused boundary region (PBR) of the sublingual arterial microvessels (ranged from 5 to 25 μm) using Sidestream Dark Feld imaging. This technique provides a direct, noninvasive, and fast method for the assessment of the endothelial glycocalyx. The software automatically (1) controls the quality of the video recordings with respect to tissue motion light intensity and image focus, resulting in an acceptable recording time of 1‐2 minutes; (2) identifies all available microvessels in the recording; and (3) defines small vascular segments every 10 μm along the length of the detected vasculature and calculates in them the perfused boundary region (PBR). The PBR is the cell‐poor layer that results from the phase separation between the flowing red blood cells (RBC) and plasma on the surface of the microvessel lumen. The PBR includes the most luminal part of glycocalyx that does allow cell penetration. Thus, an increased perfused boundary region is consistent with deeper penetration of erythrocytes into glycocalyx, indicating a loss of glycocalyx barrier properties and is a marker of reduced glycocalyx thickness.14, 23 The microvascular glycocalyx thickness, as assessed by PBR, correlated closely with systemic glycocalyx volume as assessed by an established tracer dilution technique as well as with cardiovascular risk factors in humans.14 The measurement of endothelial glycocalyx thickness using Sidestream Dark Field imaging is easy to perform (duration of 3 minutes) and is not biased by operator skills. Moreover, it has a standardized methodology, provides measurements of multiple sample sites (>3000 vascular segments of sublingual microvasculature), and the estimation of the glycocalyx integrity of microvessels with a diameter ranging from 5 to 25 μm has very good reproducibility.14, 23 Thus, the technique is proposed as a valid method to assess endothelial integrity by the European Society of Cardiology Working Group on Peripheral Circulation.15 The inter‐ and intra‐observer variabilities of PBR measurements were 5.2% and 4.3%, respectively. The feasibility of the examination was 97% in our laboratory.
2.3. Echocardiography
Studies were performed using a Vivid 7 ultrasound system. All studies were digitally stored in a computerized station and were analyzed by 2 observers, who had no access to clinical and laboratory data.
2.4. LV myocardial deformation
In all patients and controls, we measured longitudinal systolic strain (LS) from standard 2‐dimensional acquisitions (frame rate: 70‐80/s) with the use of a dedicated software. Global longitudinal strain (GLS) was calculated using the 17 LV segment model imaged from apical chamber views (4, 2, and 3 chamber view), as previously published.24 All variables represent the mean value of measurements taken in 3 consecutive cardiac cycles. Inter‐ and intraobserver variability of GLS was 8% and 5%, respectively.
2.5. Coronary flow reserve
Coronary flow velocity profiles in the left anterior descending artery were obtained using color‐guided pulse wave Doppler. We measured peak diastolic (Vd) coronary flow velocity of the diastolic coronary wave form at rest and after adenosine infusion (140 μg/kg min) for 3 minutes, according to previously published methodology.21, 22 In patients with weak resting Doppler signal of coronary flow, an IV bolus infusion of 0.3 mL of contrast agent was used at rest and a peak adenosine to enhance the signal.
Coronary flow reserve was calculated as the ratio of peak diastolic velocity after adenosine infusion to resting peak diastolic velocity. Measurements from 3 cardiac cycles were averaged. Inter‐ and intra‐observer variability were approximately 5% and 2% for Vd.
2.6. Arterial stiffness
PWV (m/s) was measured using tonometry by Complior, according to previously published methodology.25, 26, 27 Two non‐invasive pressure sensors were used to record the carotid and femoral waveforms and the distance between the 2 arterial sites was measured with a tape measure. PWV was calculated as the distance between carotid to femoral site divided by transit time between waves (m/s). Arteriograph was used in order to obtain central systolic blood pressure (cSBP‐mm Hg) and augmentation index (AI‐%) by means of an arm cuff using oscillometry, according to previously published methodology.25, 26 During systole, the blood volume having been ejected into the aorta generates pulse wave (early systolic peak, P1). This pulse wave runs down and reflects from the bifurcation of aorta, creating a second wave (late systolic peak, P2). Both early and late systolic peak were obtained and recorded on the computer as pulse waves. AI (%) was defined as (P2−P1/pulse pressure [PP]) × 100.
2.7. Blood pressure monitoring
Clinical SBP and DBP blood pressure was obtained by mercury cuff sphygmomanometer on the left brachial artery after 5 minutes of rest. Three measurements 2 minutes apart were averaged.
Each patient rested in a supine position for 10 minutes in a quiet room at 23°C before the baseline hemodynamic measurements were recorded. Brachial blood pressure (BP) and heart rate (HR) were measured in the right arm with an automated digital oscillometric sphygmomanometer. Two sequential measurements separated by 2‐minutes interval were obtained and the mean was used for statistical analysis.28
2.8. Statistical analysis
All variables are expressed as mean ± SD. Statistical analysis was performed using SPSS 21.0 statistical software package. Categorical data were analyzed using the standard chi‐square test. Variables were tested by the Kolmogorov‐Smirnov test to assess the normality of distribution. Parameters without normal distribution were transformed into ranks for further analysis. Mean values of continuous variables were compared between groups using unpaired Student's t‐test or the Mann‐Whitney U‐test, where applicable.
ANOVA was used to compare vascular and echocardiographic parameters between groups after adjusting for age, sex, BMI, smoking, hyperlipidemia, heart rate, and office systolic blood pressure. Simple linear regression was used to investigate relations between variables. Multiple linear relations were checked by multiple linear regression analysis using forward or backward procedure in hypertensives. Clinic SBP, DBP, and PP were entered in the multivariable model separately to avoid collinearity. Associations are presented by means of standardized regression coefficient (b). All covariates included in the final models were tested for interactions. Tolerance values for each covariate was >0.5 in the multivariate models.
3. RESULTS
3.1. Study population
The demographic characteristics of the study cohort are presented in Table 1. Hypertensive patients and controls had similar age, sex, body mass index (BMI), and incidental atherosclerotic risk factors. Compared to controls, hypertensives had higher PBR, PWV, cSBP, SBP, DBP, and PP (P < .05, Table 1). Additionally, hypertensives had lower GLS and GLSr values compared to controls (P < .05 for all comparisons, Figure).
Table 1.
Clinical characteristics of the study population
| Variable | Hypertensives n = 320 | Healthy controls n = 160 | P |
|---|---|---|---|
| Age (y) | 51 ± 11 | 48 ± 13 | .7 |
| Sex (male) (%) | 214 (67) | 106 (66) | .99 |
| Smoking (%) | 99 (31) | 48 (31) | .8 |
| Hypelipidemia (%) | 64 (20) | 33 (21) | .7 |
| BMI (kg/m2) | 30.33 ± 5.362 | 29 ± 4.085 | .5 |
| Cholesterol (mg/dL) | 216.4 ± 34.5 | 205.6 ± 35.9 | .17 |
| Triglycerides (mg/dL) | 116.9 ± 52.9 | 124.2 ± 46.7 | .54 |
| HDL (mg/dL) | 53.6 ± 13.6 | 53.5 ± 10.6 | .97 |
| LDL (mg/dL) | 140.0 ± 33.7 | 128.7 ± 33.1 | .16 |
| SBP | 147 ± 17 | 120 ± 10 | <.001 |
| DBP | 89 ± 10 | 78 ± 9 | <.001 |
| PP (mm Hg) | 52.85 ± 11.93 | 43.21 ± 12.74 | .003 |
| PBR (5‐25 μm) | 2.049 ± 0.276 | 1.769 ± 0.306 | .037 |
| PBR (5‐9 μm) | 1.156 ± 0.104 | 1.051 ± 0.132 | .036 |
| PBR (10‐19 μm) | 2.185 ± 0.327 | 1.993 ± 0.365 | .049 |
| PBR (20‐25 μm) | 2.573 ± 0.444 | 2.269 ± 0.492 | .026 |
| cSBP (mm Hg) | 142.5 ± 20.76 | 121.4 ± 12.74 | .002 |
| AI % | 27.23 ± 17.23 | 23.58 ± 16.37 | .012 |
| PWV (m/s) | 11.73 ± 2.415 | 10.21 ± 5.770 | .007 |
| CFR | 2.583 ± 0.880 | 3.311 ± 0.518 | .002 |
| LV mass (gr) | 159 ± 39 | 130 ± 37 | <.001 |
| GLS (%) | −16.97 ± 3.639 | −21.91 ± 1.543 | .005 |
| GLSr (L/s) | −1.01 ± 0.17 | −1.21 ± 0.1 | .04 |
AI, augmentation index; CFR, coronary flow reserve; cSBP, central systolic blood pressure; GLS, global longitudinal strain; GLSr, global longitudinal strain rate; PBR, perfused boundary region; PP, pulse pressure; PWV, pulse wave velocity.
Parameters are expressed as mean values ± standard deviation (SD).
Figure 1.
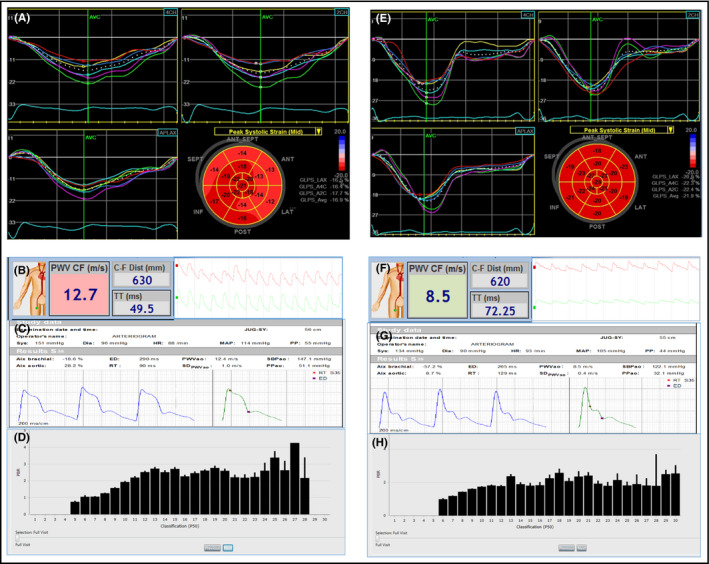
Representative examples of Global longitudinal strain (GLS), pulse wave velocity (PWV), aortic systolic blood pressure (SBPao) and perfused boundary region of sublingual microvessel (PBR) reflecting glycocalyx thickness in a hypertensive patient (A, B, C and D respectively) and a normal subject (E, F, G and H respectively). Echocardiography images show the 4‐chamber, 2‐chamber, and apical long‐axis views with time‐strain curves along with bull's eye plot of the Global Longitudinal Strain (GLS) (A and E). The measured PWV by Complior with the carotid and femoral pulse wave tracings (B and F), the central aortic systolic blood pressure (SBPao) by Arteriograph with the aortic pulse wave tracings (C and G) and the mean PBR values of microvessel ranging from 5 to 30 μm diameter as bars by Glycocheck (D and H) are shown. The hypertensive patient had a GLS of −16.9%, a PWV of 12.7 m/s, central SBP of 147.1 mm Hg, a PBR 5‐25 of 2.3 and PBR 20‐25 of 2.9 μm compared to a control subject with GLS of −21.9%, a PWV of 8.5 m/s, a central SBP of 122.1 mm Hg, a PBR 5‐25 of 1.89 and PBR 20‐25 of 2.09 μm
3.2. Association of endothelial glycocalyx with vascular function
Reduced endothelial glycocalyx thickness as assessed by increased PBR measured in the microvessels ranged from 5 to 25 micrometer (PBR5‐25) in hypertensives was related to increasing values of cSBP (b = .25, P = .001) and the association was more evident for PBR measured in the microvessels ranged from 20 to 25 micrometers (PBR20‐25; b = .30, P < .001). Increased PBR5‐25 was also related to increased PWV (b = .19, P = .02,) and in this correlation the association was more evident for PBR20‐25 (b = .22, P = .005). Furthermore, increasing values of PBR5‐25 were associated with decreasing CFR (b = −.18, P = .02). There was a borderline relation of PBR5‐25 with office SBP (b = .15, P = .06) and AI (b = .16, P = .05).
By multivariable regression analysis the association of PBR5‐25 with CFR, PWV and cSBP remained significant after adjustment for age, sex, BMI, smoking LV mass, heart rate, hyperlipidemia, and office SBP (Table 2, P < .05). Similar results were obtained after inclusion of office diastolic BP or mean BP instead of systolic BP in multivariable analysis (P < .05, data not shown).
Table 2.
Univariate and Multivariable analysis of the determinants in hypertensive patients
| CFR | Longitudinal strain | PWV | cSBP | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariablea | Univariate | Multivariablea | Univariate | Multivariablea | Univariate | Multivariablea | |||||||||
| Beta | P | Beta | P | Beta* | P | Beta | P | Beta | P | Beta | P | Beta | P | Beta | P | |
| Sex (female) | −.68 | .39 | −.02 | .59 | −.02 | .8 | −.03 | .7 | −.02 | .79 | −.03 | .71 | .03 | .69 | −.04 | .64 |
| Age | .06 | .45 | .05 | .55 | .22 | .005 | .18 | .04 | .22 | .005 | .18 | .04 | .40 | <.001 | .38 | <.001 |
| Heart rate | −.004 | .95 | .009 | .98 | −.04 | .66 | −.03 | .82 | −.04 | .66 | −.03 | .82 | .09 | .27 | .07 | .51 |
| BMI | .08 | .31 | .09 | .42 | .02 | .72 | .01 | .88 | .02 | .72 | .01 | .88 | .03 | .99 | .01 | .98 |
| LVM | .22 | .006 | .19 | .01 | .17 | .03 | .19 | .02 | .17 | .03 | .19 | .02 | .17 | .03 | .19 | .02 |
| Smoking | .22 | .005 | .18 | .02 | .01 | .84 | .06 | .44 | .01 | .84 | .06 | .44 | .19 | .028 | .07 | .38 |
| Hyperlipidemia | .13 | .11 | .10 | .1 | .15 | .06 | .10 | .1 | .15 | .06 | .10 | .1 | .13 | .11 | .09 | .25 |
| Office SBP | .17 | .03 | .07 | .81 | .20 | .011 | .17 | .035 | .20 | .011 | .17 | .035 | .30 | <.001 | .25 | <.001 |
| PBR5‐25 | −.19 | .02 | −.17 | .035 | .22 | .006 | .20 | .01 | .19 | .02 | .18 | .03 | .25 | .001 | .20 | .01 |
Beta, indicates standardised coefficient b; BMI, Body Mass Index; CFR, coronary flow reserve; cSBP, Central Systolic Blood Pressure; GLS, Longitudinal strain, global longitudinal of the left ventricle; LVM, left ventricular mass; PBR5‐25, perfused boundary region increased; PWV, pulse wave velocity; SBP, Systolic Blood Pressure.
PBR indicates reduced glycocalyx thickness.
b and P values are derived from the multivariable model including LV mass, systolic blood pressure, age, sex, heart rate, BMI, smoking, and hyperlipidemia.
3.3. Association of endothelial glycocalyx with longitudinal deformation
Increasing PBR5‐25 was also related with reduced GLS (b = .22, P = .006) and GLSr (b = .24, P = .003), and the association remained significant after adjustment for age, sex, BMI, smoking LV mass, heart rate, hyperlipidemia, and office SBP (b = .20, P = .01 and b = .22, P = .007, respectively; Table 2).
In a multivariable model including age, sex, BMI, smoking, hyperlipidemia, heart rate, LV mass index, and office systolic blood pressure, increasing PWV and decreasing CFR were related to impaired GLS (b = .21 and b = −.23, respectively, P < .01) and GLSr (b = .26 and b = −.30, respectively, P < .01) in hypertensives. LV mass index was also associated with longitudinal strain and systolic strain rate (b = .19 and b = .19, respectively, P < .05). The addition of PBR5‐25 to a multivariate model including PWV, CFR, age, sex, BMI, smoking, hyperlipidemia, heart rate, LV mass index, and office systolic blood pressure increased the model's predictive value for abnormal GLS (r = .375 and x2 = 2.4 of initial model to r = .42 and x2 = 3.8 after addition of PBR5‐25, P for change = .03). Similar results were obtained after inclusion of office diastolic BP or mean BP instead of systolic BP in multivariable analysis (P < .05, data not shown).
4. DISCUSSION
In our study we have shown that endothelial glycocalyx thickness, arterial elastic properties, coronary flow reserve, and LV myocardial deformation are impaired in untreated hypertensives compared with healthy controls. Reduced endothelial glycocalyx thickness, as indicated by increased PBR, was associated with increased arterial stiffness markers (PWV and cSPB) and impaired CFR in hypertensives. Furthermore impaired endothelial glycocalyx was an independent determinant of abnormal LV longitudinal strain and strain rate, and had an additive value to PWV and CFR for the prediction of an impaired LV myocardial deformation in hypertensive patients.
4.1. Glycocalyx and blood pressure
There is growing interest concerning the role of vascular endothelial glycocalyx in the pathophysiology of cardiovascular system. Arterial stiffness is a predictive marker of cardiovascular events26, 27 and its assessment is suggested by the guidelines of the European Society of Hypertension/European Society of Cardiology for the stratification of cardiovascular risk in hypertensives.28
Destruction of endothelial bed is associated with a pathophysiological sequelae, including capillary leak syndrome, edema formation, accelerated inflammation, platelet hyper‐aggregation, hypercoagulation, loss of vascular tone, atheroma formation, and hypertension.1, 5
Studies indicate that reduction glycocalyx thickness is associated with an increased permeability to various proteins and circulating blood cells compromising its protective effects on the subendothelial vascular layers, resulting in intimal damage.29 Additionally, intimal hyperplasia contributes to the genesis of atheroma.6 Thus, prevention of glycocalyx damage could be an effective means of protection in many pathological situations, including hypertension.5
Recent clinical studies have revealed the significant variation in shear stress in microcirculation and demonstrated the complexity of the flow field in the gap between endothelial cell and arterial wall. After precise measurement of the shear stress, its diversity was found less profound in arterioles with wider cell‐free layers.30 In agreement with these studies, in our study, we have shown that there is an association between impaired glycocalyx, as assessed by increased perfused boundary region of microvessel ranged from 5 to 25 μm and increased central systolic blood pressure and pulse wave velocity and a borderline association with office systolic blood pressure and augmentation index in untreated hypertensives. This unveils a link between endothelial glycocalyx thickness, peripheral blood pressure, and arterial stiffness. Moreover, this association was more evident for the damage of endothelial glycocalyx, observed in the larger microvessels ranged from 20 to 25 μm. Our findings suggest that in the newly diagnosed hypertensive patients the glycocalyx barrier appears to be more compromised in the larger size microvasculature, which usually consist the aortic vasa vasorum, thus affecting the elastic aortic wall properties.
A possible explanation, concerning the protective features of glycocalyx, may be related to its mechanic properties. To be more specific, transmission of shear stress forces to endothelial cells, which are induced by blood flow, causes changes in the structure and architecture of glycocalyx, triggers the release of nitric oxide, and as a result contributes to the regulation of vasomotor tone. Hence, glycocalyx participates, through this rheological mechanism, in the maintenance of homeostasis of the peripheral tissues.31, 32 Indeed, denudation of the glycocalyx by heparinase III reduced NO production, hence the glycocalyx is deemed to play a significant role in the regulation of vasomotor tone.33 To this end in our study, we have found a close association between reduced endothelial glycocalyx thickness and impaired coronary flow reserve, which is at least partly NO dependent.
Evidence of degraded endothelial glycocalyx in hypertension was divulged, after studying the retinal and choroidal capillaries in rats. Endothelial glycocalyx in hypertensive rats was significantly impaired compared to controls.34 At the sites where glycocalyx was degraded, there was a remarkable adhesion of leucocytes and platelets on the luminal surface of endothelial cells.35
Salt‐overconsumption affects the cardiovascular system and has a direct impact on blood pressure. Endothelial glycocalyx was identified as being capable of selectively buffering sodium ions.36 Sodium excess damages the glycocalyx and renders vascular endothelium increasingly permeable for sodium. In the long term, sodium accumulates in the interstitium contributing to hypertension.36 Generally, there is an abundance of sodium in the blood, which is bound to negatively charged organic material and hence is osmotically inactive. The material, which is likely to store sodium, is the glycocalyx of blood vessels. It is constituted by membranous glycoproteins, proteoglycans, glycoaminoglycans, and associated plasma proteins. To be more specific, hyaluronic acid and the negatively charged heparan sulphateproteoglycans are its major elements. Glycocalyx lacking hyaluron lose its vasoprotective function, facilitating leukocyte adhesion, inflammation, and atherosclerosis.16, 35 Furthermore, enhanced sodium influx, triggered by small increases in plasma sodium concentration, directly affects vascular endothelial cells, leading to increased mechanical stiffness and reduced nitric oxide release. Initially, when sodium intake exceeds the excretory capacity of the kidney, sodium is retained. Repeated recurrence of this process is considered to be one of the early steps in the pathogenesis of hypertension and cardiovascular disease.17 In our study there was a close association between reduced glycocalyx thickness and increased central aortic blood pressure, and a borderline association with brachial systolic blood pressure, suggesting a role of perturbed glycocalyx to pathogenesis of hypertension.
Studies suggest that glycocalyx thickness correlates inversely with salt concentration in blood, introducing a positive feedback mechanism that leads to salt‐induced hypertension (provided that the thickness of glycocalyx declines).
Thus, the association of reduced endothelial glycocalyx thickness with increased central systolic blood pressure, pulse wave velocity, and its borderline association with augmentation index and brachial systolic blood pressure are in line with experimental data and suggest an important pathogenetic role of endothelial glycocalyx integrity in hypertension.
4.2. Glycocalyx and the heart
In our study, we observed that abnormal arterial stiffness, as assessed by increased PWV, was associated with impaired LV longitudinal deformation and impaired coronary microcirculation, as assessed by CFR, in hypertensive patients, as also shown in our previous studies.37, 38 However, in the current study we have expanded our previous findings by showing that reduced endothelial glycocalyx was associated with abnormal arterial elasticity and coronary flow reserve, as well as with impaired LV longitudinal deformation. Thus, this study indicated that impaired endothelial glycocalyx is a common pathogenetic link between abnormal vascular and myocardial function in untreated hypertensives.
Because of an increase in PWV in a stiff arterial tree, the reflected waves arriving in early systole boost the aortic systolic pressure further, whereas diastolic pressure falls sharply. This results in an elevated LV systolic afterload; therefore, increased oxygen demand along with a decrease in diastolic coronary perfusion pressure reduces oxygen delivery and leads to subendocardial ischemia and impaired subendocardial function.39 The subendocardial myocardial fibers determine the LV longitudinal deformation. Thus, subendocardial ischemia, resulting from increased arterial stiffness and abnormal wave reflection, as shown by the respective increase of PWV and AI in hypertensives compared to controls, directly affects LV longitudinal myocardial deformation. In addition, prolongation of ejection time and delayed onset of isovolumic myocardial relaxation, resulting from the augmented systolic aortic pressure, leads to elevated LV diastolic filling pressures, causing extravascular compression of the coronary microvessels contributing to reduced CFR. In the current study we have shown a direct association of abnormal endothelial glycocalyx with impaired myocardial and coronary microcirculatory function. However, it should be noted that increased arterial stiffness and coronary flow reserve also contributed to impaired LV myocardial deformation observed in untreated hypertensives.
In our multivariable analysis, higher PBR values increased the predictive value of multivariable model, including PWV and CFR for the presence of reduced GLS, suggesting an independent and additive value of impaired endothelial glycocalyx for predicting myocardial dysfunction in hypertension.
5. CONCLUSIONS
Endothelial glycocalyx is damaged in newly diagnosed untreated hypertensives. This damage is related to abnormal aortic elastic properties and to impaired coronary microcirculatory function and contributes to impaired LV longitudinal deformation. Data from our study along with data from previous studies underline the importance of the protection and restoration of endothelial glycocalyx integrity not only as a therapeutic goal concerning prevention of hypertension but also protection vascular health and myocardial function.
5.1. Study limitations
In our study we used Sideview Dark Field imaging. This method allows a non‐invasive assessment of endothelial glycocalyx based on the erythrocyte column distribution. Thus, the limitation this imaging technique is the indirect estimation of endothelial glycocalyx thickness. On the other hand, the noninvasive nature of technique, the semi‐automated analyses, and the good reproducibility stand as important advantages. The cross sectional design of the study does not allow exploration into the causative role of the endothelial glycocalyx damage on abnormal arterial, coronary, and myocardial function observed in hypertensives.
CONFLICTS OF INTEREST
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
Ikonomidis I, Voumvourakis A, Makavos G, et al. Association of impaired endothelial glycocalyx with arterial stiffness, coronary microcirculatory dysfunction, and abnormal myocardial deformation in untreated hypertensives. J Clin Hypertens. 2018;20:672–679. 10.1111/jch.13236
REFERENCES
- 1. Becker BF, Jacob M, Leipert S, Salmon AH, Chappell D. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br J Clin Pharmacol. 2015;80:389‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fu BM, Tarbell JM. Mechano‐sensing and transduction by endothelial surface glycocalyx: composition, structure, and function. Wiley Interdiscip Rev Syst Biol Med. 2013;5:381‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tarbell JM, Cancel LM. The glycocalyx and its significance in human medicine. J Intern Med. 2016;280:97‐113. [DOI] [PubMed] [Google Scholar]
- 4. Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. 2010;87:300‐310. [DOI] [PubMed] [Google Scholar]
- 5. Becker BF, Chappell D, Jacob M. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res Cardiol. 2010;105:687‐701. [DOI] [PubMed] [Google Scholar]
- 6. Noble MI, Drake‐Holland AJ, Vink H. Hypothesis: arterial glycocalyx dysfunction is the first step in the atherothrombotic process. QJM. 2008;101:513‐518. [DOI] [PubMed] [Google Scholar]
- 7. Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121‐167. [DOI] [PubMed] [Google Scholar]
- 8. Mulivor AW, Lipowsky HH. Inflammation‐ and ischemia‐induced shedding of venular glycocalyx. Am J Physiol Heart Circ Physiol. 2004;286:1672‐1680. [DOI] [PubMed] [Google Scholar]
- 9. Speziale S, Sivaloganathan S. Poroelastic theory of transcapillary flow: effects of endothelial glycocalyx deterioration. Microvasc Res. 2009;78:432‐441. [DOI] [PubMed] [Google Scholar]
- 10. Nieuwdorp M, van Haeften TW, Gouverneur MC, et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes. 2006;55:480‐486. [DOI] [PubMed] [Google Scholar]
- 11. Ikonomidis I, Pavlidis G, Lambadiari V, et al. Early detection of left ventricular dysfunction in first‐degree relatives of diabetic patients by myocardial deformation imaging: the role of endothelial glycocalyx damage. Int J Cardiol. 2017;233:105‐112. [DOI] [PubMed] [Google Scholar]
- 12. Van Teeffelen JW, Brands J, Stroes ES, Vink H. Endothelial glycocalyx: sweet shield of blood vessels. Trends Cardiovasc Med. 2007;17:101‐105. [DOI] [PubMed] [Google Scholar]
- 13. Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT, Vink H. Damage of the endothelial glycocalyx in dialysis patients. J Am Soc Nephrol. 2012;23:1900‐1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nieuwdorp M, Meuwese MC, Mooij HL, et al. Measuring endothelial glycocalyx dimensions in humans: a potential novel tool to monitor vascular vulnerability. J Appl Physiol. 2008;104:845‐852. [DOI] [PubMed] [Google Scholar]
- 15. Lekakis J, Abraham P, Balbarini A, et al. Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur J Cardiovasc Prev Rehabil. 2011;18:775‐789. [DOI] [PubMed] [Google Scholar]
- 16. Oberleithner H, Peters W, Kusche‐Vihrog K, et al. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflugers Arch. 2011;462:519‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oberleithner H, Wilhelmi M. Vascular glycocalyx sodium store – determinant of salt sensitivity? Blood Purif. 2015;39:7‐10. [DOI] [PubMed] [Google Scholar]
- 18. Ikonomidis I, Tzortzis S, Andreadou I, et al. Increased benefit of interleukin‐1 inhibition on vascular function, myocardial deformation, and twisting in patients with coronary artery disease and coexisting rheumatoid arthritis. Circ Cardiovasc Imaging. 2014;7:619‐628. [DOI] [PubMed] [Google Scholar]
- 19. Yoshihiro S, Tomko I, Kazutaka A. Current status of 3‐dimensional speckle tracking echocardiography: a review from our experiences. J Cardiovasc Ultrasound. 2014;22:49‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Notomi Y, Lysyansky P, Setser RM, et al. Measurement of ventricular torsion by two‐dimensional ultrasound speckle tracking imaging. J Am Coll Cardiol. 2005;45:2034‐2041. [DOI] [PubMed] [Google Scholar]
- 21. Ikonomidis I, Tzortzis S, Triantafyllidi H, et al. Association of impaired left ventricular twisting‐untwisting with vascular dysfunction, neurohumoral activation and impaired exercise capacity in hypertensive heart disease. Eur J Heart Fail. 2015;17:1240‐1251. [DOI] [PubMed] [Google Scholar]
- 22. Ikonomidis I, Lekakis J, Papadopoulos C, et al. Incremental value of pulse wave velocity in the determination of coronary microcirculatory dysfunction in never‐treated patients with essential hypertension. Am J Hypertens. 2008;21:806‐813. [DOI] [PubMed] [Google Scholar]
- 23. Reitsma S, Slaaf DW, Vink H, van Zandvoort M, Egbrink MGA. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr. 2004;17:630‐633. [DOI] [PubMed] [Google Scholar]
- 25. Ikonomidis I, Ntai K, Kadoglou NP, et al. The evaluation of pulse wave velocity using arteriograph and complior apparatus across multiple cohorts of cardiovascular‐related diseases. Int J Cardiol. 2013;168:4890‐4892. [DOI] [PubMed] [Google Scholar]
- 26. Ikonomidis I, Makavos G, Lekakis J. Arterial stiffness and coronary artery disease. Curr Opin Cardiol. 2015;30:422‐431. [DOI] [PubMed] [Google Scholar]
- 27. Tritakis V, Tzortzis S, Ikonomidis I, et al. Association of arterial stiffness with coronary flow reserve in revascularized coronary artery disease patients. World J Cardiol. 2016;8:231‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension. Eur Heart J. 2013;34:2159‐2221. [DOI] [PubMed] [Google Scholar]
- 29. Gouverneur M, Berg B, Nieuwdorp M, Stroes E, Vink H. Vasculoprotective properties of the endothelial glycocalyx: effects of fluid shear stress. J Intern Med. 2006;259:393‐400. [DOI] [PubMed] [Google Scholar]
- 30. Yin X, Zhang J. Cell‐free layer and wall shear stress variation in microvessels. Biorheology. 2012;49:261‐270. [DOI] [PubMed] [Google Scholar]
- 31. Ushiyama A, Kataoka H, Iijima T. Glycocalyx and its involvement in clinical pathophysiologies. J Intensive Care. 2016;4:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yen W, Cai B, Yang J, et al. Endothelial surface glycocalyx can regulate flow‐induced nitric oxide production in microvessels in vivo. PLoS ONE. 2015;10:e0117133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Van den Berg BM, Nieuwdorp M, Stroes ES, Vink H. Glycocalyx and endothelial (dys) function: from mice to men. Pharmacol Rep. 2006;58:75‐80. [PubMed] [Google Scholar]
- 34. Kumase F, Morizane Y, Mohri S, Takasu I, Ohtsuka A, Ohtsuki H. Glycocalyx degradation in retinal and choroidal capillary endothelium in rats with diabetes and hypertension. Acta Med Okayama. 2010;64:277‐283. [DOI] [PubMed] [Google Scholar]
- 35. Lipowsky H. Protease activity and the role of the endothelial glycocalyx in inflammation. Drug Discov Today Dis Models. 2011;8:57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oberleithner H. Vascular endothelium: a vulnerable transit zone for merciless sodium. Nephrol Dial Transplant. 2014;29:240‐246. [DOI] [PubMed] [Google Scholar]
- 37. Ikonomidis I, Tzortzis S, Paraskevaidis I, et al. Association of abnormal coronary microcirculatory function with impaired response of longitudinal left ventricular function during adenosine stress echocardiography in untreated hypertensive patients. Eur Heart J Cardiovasc Imaging. 2012;13:1030‐1040. [DOI] [PubMed] [Google Scholar]
- 38. Ikonomidis I, Tzortzis S, Tsantes A, et al. The interplay between renin‐angiotensin system activation, abnormal myocardial deformation and neurohumoral activation in hypertensive heart disease: a speckle tracking echocardiography study. Int J Cardiovasc Imaging. 2017;33:323‐329. [DOI] [PubMed] [Google Scholar]
- 39. Vinereanu D, Nicolaides E, Boden L, Payne N, Jones CJ, Fraser AG. Conduit arterial stiffness is associated with impaired left ventricular subendocardial function. Heart. 2003;89:449‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]


