Abstract
Nonadherence to antihypertensive medication is considered as a reason of inadequate control of blood pressure. This meta‐analysis aimed to systemically evaluate the impact of fixed‐dose combination (FDC) therapy on hypertensive medication adherence compared with free‐equivalent combination therapies. Articles were retrieved from MEDLINE and Embase databases using a combination of terms “fixed‐dose combinations” and “adherence or compliance or persistence” and “hypertension or antihypertensive” from January 2000 to June 2017 without any language restriction. A meta‐analysis was performed to parallel compare the impact of FDC vs free‐equivalent combination on medicine adherence or persistence. Studies were independently reviewed by two investigators. Data from eligible studies were extracted and a meta‐analysis was performed using R version 3.1.0 software. A total of nine studies scored as six of nine to eight of nine for Newcastle‐Ottawa rating with 62 481 patients with hypertension were finally included for analysis. Results showed that the mean difference of medication adherence for FDC vs free‐equivalent combination therapies was 14.92% (95% confidence interval, 7.38%–22.46%). Patients in FDC group were more likely to persist with their antihypertensive treatment, with a risk ratio of 1.84 (95% confidence interval, 1.00–3.39). This meta‐analysis confirmed that FDC therapy, compared with free‐equivalent combinations, was associated with better medication adherence or persistence for patients with hypertension. It can be reasonable for physicians, pharmacists, and policy makers to facilitate the use of FDCs for patients who need to take two or more antihypertensive drugs.
Keywords: fixed‐dose combination, free‐equivalent combinations, hypertension, medication adherence, medication persistence
1. INTRODUCTION
Hypertension is the biggest risk factor for cardiovascular disease, with approximately one third of cardiovascular deaths attributed to uncontrolled hypertension.1 Globally, hypertension affected 31.1% of the global population, or 1.4 billion people, worldwide in 20102 and resulted in 9.4 million deaths annually.3 The control of high blood pressure (BP) by antihypertensive drugs is crucial for patients with hypertension, by reducing the risk of stroke and renal and cardiovascular disease.4 A standardized reduction of 10/5 mm Hg systolic BP/diastolic BP reduces the of stroke by 36%, heart failure by 43%, coronary events by 16%, cardiovascular death by 18%, and all‐cause mortality by 11%.5 However, a worldwide study showed that only 32.5% of patients receiving antihypertensive treatment have controlled BP.6
Nonadherence to antihypertensive medication is considered one of the major contributors to inadequate control of BP.7, 8 A recent review including 28 studies from 15 countries demonstrated that 45.2% of patients with hypertension were nonadherent to medications and 83.7% of medication nonadherence was found in patients with uncontrolled hypertension.9 It was reported that approximately two thirds of the patients with hypertension required two or more antihypertensive drugs to control BP.10 Compared with free‐equivalent combinations, use of fixed‐dose combination (FDC) therapies in patients who require combination medicines is believed to improve adherence to antihypertensive drugs as it reduces pill burden.11 However, studies reported that the use of FDCs in patients taking two or more guideline‐recommended antihypertensive medications was relatively low in clinical practices, from 10% to 50%.12, 13, 14 This meta‐analysis, which includes the latest studies, aimed to systemically evaluate the impact of FDCs on hypertensive medication adherence compared with free‐equivalent combination therapies according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) and Meta‐Analysis of Observational Studies in Epidemiology (MOOSE) guidelines.15, 16
2. METHODS
2.1. Search strategy
We performed a systematic literature search using MEDLINE and Embase databases using a combination of terms “fixed‐dose combinations” and “adherence or compliance or persistence” and “hypertension or antihypertensive” from January 2000 to June 2017 without any language restriction. We also searched the articles from the related publications of the retrieved studies and review articles for potential additional studies. The authors were contacted in case further information was needed in selected articles. A screening of titles or abstracts was performed, followed by a full‐text review.
2.2. Data abstraction
Two investigators independently assessed literature eligibility. Any discrepancies were resolved by consensus or a third investigator. We included studies of patients with hypertension, which directly parallel‐compared the impact of FDCs vs free‐equivalent combinations on medication adherence or persistence. No single‐arm studies were included. Relevant information from the selected studies was collected using a standard electronic form. We collected information about lead author, year of publication, patient characteristics, study design, drug therapy groups, measurements of adherence or persistence, and length of follow‐up, as well as the study outcomes.
Medication adherence (also called compliance) was assessed by the medication possession ratio or proportion of days covered, which was measured by the sum of the days/medication supply for all fills of a given drug in a particular time period, divided by the number of days/medications in the time period. Medication persistence was defined as the percentage of patients who continuously refilled a prescription for either FDC or free‐equivalent combinations during the follow‐up period, and nonpersistence was assessed as the percentage of patients who, without authorization, stopped refilling prescribed medication without taking it up again.
The quality of the studies was assessed by the Newcastle‐Ottawa rating on a scale of one to nine, which is a risk of bias assessment tool for observational studies recommended by the Cochrane Collaboration.17 A study is judged on three broad perspectives by the Newcastle‐Ottawa rating: (1) the selection of the study groups; (2) the comparability of the groups; and (3) the ascertainment of either the exposure or outcome of interest for case‐control or cohort studies, respectively.
2.3. Statistical analysis
Meta‐analyses were performed using R version 3.1.0 software (R Foundation for Statistical Computing). Heterogeneity of the trial results was assessed by calculating the P value from the χ2 test. Statistically significant heterogeneity was defined as a P value <.1 based on the χ2 test or an I 2 statistic > 50%. In this case, a random effects model was used. Otherwise, a fixed‐effects model was chosen. The mean differences and risk ratio in adherence and persistence outcomes between the FDC and free‐equivalent combination groups (as well as 95% confidence intervals [CIs]) were calculated for each study where possible. The pooled effect for each grouping of trials was derived from the point estimate for each separate trial weighted by the inverse of the variance.
3. RESULTS
A total of 274 articles were yielded from the literature search, including 126 articles from Embase, 24 articles from MEDLINE, and 124 articles from both. After reviewing titles and abstracts and hand searching related citations, 27 potentially relevant articles were identified for full‐text review. Of those, after review of the full‐text articles, 19 studies were excluded for the following reasons: protocol papers (n = 2), review papers (n = 3), further medication adherence or persistence data not reported or obtainable from authors (n = 10), and not a parallel comparison of FDCs and free‐equivalent combinations (n = 4). Finally, we included eight articles with nine studies18, 19, 20, 21, 22, 23, 24, 25 in our analysis (Figure 1).
Figure 1.
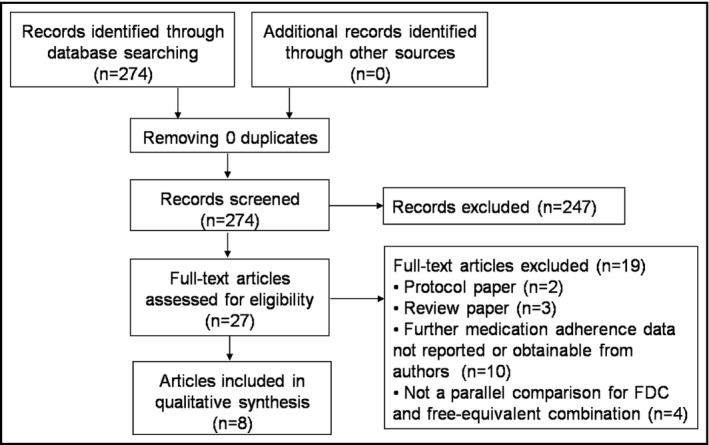
Flowchart describing the article selection process. FDC, fixed‐dose combination
In these nine studies, 62 481 patients with hypertension were included, with 30 103 patients taking FDCs and 32 378 patients taking free‐equivalent combinations. The characteristics of the studies are elaborated in the Table. All of studies were retrospective cohort trials except one prospective study. Seven studies investigated the impact of FDCs on medication adherence, while five studies measured medication persistence. All contents of the FDCs or corresponding free combinations were renin‐angiotensin system inhibitors with a diuretic or a calcium channel blocker. A study by Dezii and colleagues18 involved two subgroups: one using an FDC of lisinopril and hydrochlorothiazide and the other using an FDC of enalapril and hydrochlorothiazide. These studies were scored as six of nine to eight of nine for the Newcastle‐Ottawa rating.
Table 1.
Characteristics of included studies
| Authors | Sample size, No. | Age, y | Men, % | Follow‐up, mo | Design | Contents of FDC | Definition of Adherence or Persistence | Difference in Persistence | Difference in Adherence (MPR or PDC) | Newcastle‐Ottawa Rating |
|---|---|---|---|---|---|---|---|---|---|---|
| Dezii, 200018 | 2268 | – | – | 12 | Retrospective cohort | Lisinopril/hydrochlorothiazide | Patients were regarded as persistent if they renewed their prescription within three times the number of days supplied by the previous prescription | 68.7% vs 57.8%, P < .05 | – | 6 of 9 |
| Dezii, 200018 | 1674 | – | – | 12 | Retrospective cohort | Enalapril/hydrochlorothiazide | Patients were regarded as persistent if they renewed their prescription within three times the number of days supplied by the previous prescription | 70.0% vs 57.5%, P < .05 | – | 6 of 9 |
| Taylor, 200319 | 5732 | 53 | 50 | 12 | Retrospective cohort | Amlodipine besylate/benazepril HCl | Adherence was measured by the MPR during the study period | ‐ | 80.8% vs 73.8%, P < .001 | 8 of 9 |
| Brixner, 200820 | 2189 | – | 47.1 | 12 | Retrospective cohort | Valsartan and hydrochlorothiazide |
Adherence was measured by calculating the MPRs for all patients with at least two prescription fills for dual therapy. MPR was defined as the total days supplied divided by the difference in days between the first fill and the last day of the last days supplied Patients were classified as persistent if they remained on dual therapy and did not discontinue therapy at 365 days. |
54% vs 19%, P < .001 | 62.1% vs 53.0%, P < .001 | 7 of 9 |
| Dickson, 200821 | 5704 | 76.0 ± 7.2 | 17.4 | 12 | Retrospective cohort | Amlodipine besylate/benazepril HCl | Compliance defined as the MPR, which was the total days’ supply of drug (excluding last prescription fill) divided by the length of follow‐up | – | 63.4% vs 49.0%, P < .0001 | 6 of 9 |
| Hess, 200822 | 14449 | 62.5 | 43.1 | 12 | Retrospective cohort | ARB/hydrochlorothiazide, ACEI/hydrochlorothiazide, ACEI/CCB |
Compliance, defined as MPR, was measured over 12 mo Persistence was measured as the percentage of patients who did not experience a lapse in therapy of more than 30 d since their last prescription refill |
58.3% vs 14.9%, P < .001 Regression‐adjusted differences: 42.5% (40.6%–44.5%), P < .001 |
76.9% vs 54.4%, P < .001 Regression‐adjusted differences: 22.1% (19.9%–24.1%), P < .001 |
7 of 9 |
| Hsu, 201523 | 7348 | 55.2 | 55.6 | 24 | Prospective cohort | ARB and thiazide diuretics |
Adherence was measured as the MPR, calculated as the number of days’ supply of medication dispensed during a specified follow‐up period divided by the number of days from the first dispensing to the end of the follow‐up period Persistence was measured as continuously refilling the prescription for either an FDC or free combination during the follow‐up period |
26.1% vs 19.5%, P < .001 | 42.06% vs 32.45%, P < .001 | 8 of 9 |
| Tung, 201524 | 16505 | 60.4 | 52.0 | 15.2 | Retrospective cohort | ARB/CCB | Adherence was measured as PDC | – | 80.35 ± 21.90% vs 72.57 ± 25.95%, P < .001 | 6 of 9 |
| Levi, 201625 | 6612 | 67.1 | 48.2 | 6 | Retrospective cohort | Olmesartan/amlodipine | Adherence was estimated by calculating the PDC | – | 55.1% vs 15.9%, P < .001 | 8 of 9 |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; FDC, fixed‐dose combination; HCl, hydrochloric acid; MPR, medication possession ratio; PDC, proportion of days covered.
Among seven studies reporting medication adherence, the results showed that the mean difference of medication adherence for FDC vs free‐equivalent combination therapies was 14.92% (95% CI, 7.38%–22.46%) with an I 2 estimate of 98% (Figure 2), indicating that the FDC significantly improved antihypertensive medication adherence. The largest differences were found in Levi's25 and Hess's22 studies, which were 39.20% and 22.10%, respectively.
Figure 2.
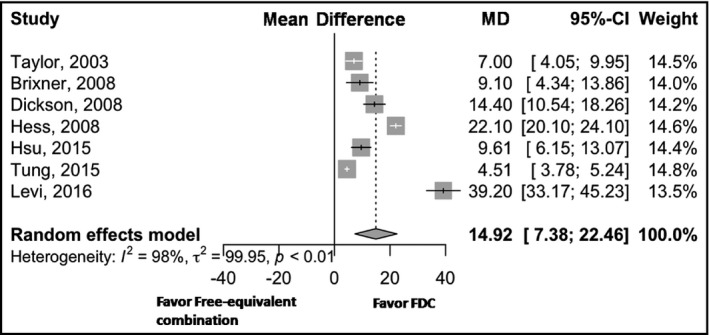
Forest plot for medication adherence. CI, confidence interval; FDC, fixed‐dose combination; MD, mean difference
Five studies measured medication persistence for FDC vs free‐equivalent combinations. The risk ratio was 1.84 (95% CI, 1.00–3.39) for the FDC with an I 2 estimate of 100% (Figure 3), which showed that FDCs improved medication persistence with a marginally statistical significance. The largest improvement was found in Hess's study,22 with a risk ratio of 3.91.
Figure 3.
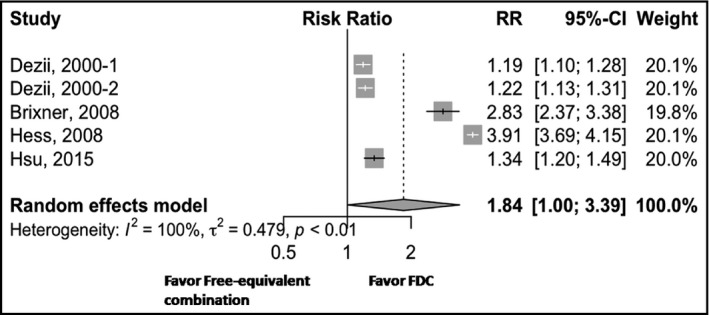
Forest plot for medication persistence. CI, confidence interval; FDC, fixed‐dose combination; RR, risk ratio
4. DISCUSSION
This study aimed to evaluate the impact of FDC vs corresponding free combination therapies on medication adherence or persistence of hypertensive medication use. Results showed that the use of an FDC was related to significantly better medication adherence or persistence. Compared with free‐equivalent combinations, FDCs were associated with an additional 14.92% (95% CI, 7.38%–22.46%) of prescribed medicine taking and nearly a doubled number of patients (1.84‐fold; 95% CI, 1.00–3.39) who continuously refilled the prescription during the follow‐up period.
Although several recent studies were included, the results of this meta‐analysis were consistent with previous similar reviews.26, 27 Gupta and colleagues26 conducted a meta‐analysis in 2009 to assess compliance and persistence associated with FDCs in comparison with their free‐equivalent components. They found that the use of FDCs was associated with significantly better compliance (odds ratio, 1.21; 95% CI, 1.03–1.43 [P = .02]) and a nonsignificant improvement in persistence (odds ratio, 1.54; 95% CI, 0.95–2.49 [P = .08]) compared with corresponding free combinations. Another meta‐analysis in 2011 by Sherrill and colleagues27 showed that FDCs were related to a mean medication possession ratio difference of 13.31% and a 2.13 risk ratio for medication persistence compared with free‐equivalent components, which were similar to our results. We found in recent studies that the two‐class combination of antihypertensives––an angiotensin receptor blocker with a calcium channel blocker––was used more frequently compared with mainly a renin‐angiotensin system inhibitor with diuretics in previous reviews. Despite having some combination class changes, the benefit of an FDC on medication compliance or persistence remained the same.
Evidence shows that BP control could be improved by better treatment compliance, but, in clinical practice, improving medication adherence remains a challenge.4 Pill burden was significantly associated with decreased adherence to antihypertensive therapies in real‐practice settings.27 The number of prescribed pills was significantly related to adherence, with 55.3%, 40.4%, and 32.6% of patients having proportion of days covered ≥ 80% in the single‐, double‐, and triple‐pill cohorts, respectively.28 This is was why we found that FDCs always improved the adherence to antihypertensive drugs. The use of an FDC has been rapidly increasing in the past decade with evidence that the simpler the therapeutic regimen the better the patient's adherence and outcomes of disease.25 It was reported in a German health insurance data analysis that in patients who started new antihypertensive therapy in 2007 or 2008 (n = 8,032), 10.8% of them started with an FDC of two drugs and only 8.2% started with a free combination of two drugs.29 Single‐pill therapy was associated with high medication adherence, and therapeutic simplification by the use of an FDC should be a nonignorable strategy to improve treatment adherence for hypertension.
5. STUDY LIMITATIONS
There are some limitations in our study. First, nearly all of the data sources were retrospective studies. Although in most studies known confounding factors were adjusted for benefit of FDC, the patient characteristics of patients between the FDC group and the free‐equivalent combination group may not be well balanced. Also, as in all retrospective studies, there may be recall bias for adherence assessment. Second, the definition and measurement of medication adherence is a key for our results, but we had a wide variety of measurement methods and definitions in our selected studies (Table). However, the results from different methods are consistent. Third, there was considerable heterogeneity among the included studies. Therefore, we used the random effects method to analyze the pooled data.
6. CONCLUSIONS
This meta‐analysis confirms that FDCs, compared with free‐equivalent combinations, are associated with better medication adherence or persistence in patients with hypertension. It is reasonable for physicians, pharmacists, and policy makers to facilitate the use of FDCs for patients who need to take two or more antihypertensive drugs.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
ACKNOWLEDGMENTS
The authors sincerely thank Quan Fang, MD, Professor and Director, Department of Cardiology, Peking Union Medical College Hospital, Beijing, China, for providing valuable literature and suggestions during the study.
Du L‐P, Cheng Z‐W, Zhang Y‐X, Li Y, Mei D. The impact of fixed‐dose combination versus free‐equivalent combination therapies on adherence for hypertension: a meta‐analysis. J Clin Hypertens. 2018;20:902–907. 10.1111/jch.13272
REFERENCES
- 1. Lewington S, Lacey B, Clarke R, et al. The burden of hypertension and associated risk for cardiovascular mortality in China. JAMA Intern Med. 2016;176:524‐532. [DOI] [PubMed] [Google Scholar]
- 2. Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population‐based studies from 90 countries. Circulation. 2016;134:441‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224‐2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peacock E, Krousel‐Wood M. Adherence to antihypertensive therapy. Med Clin North Am. 2017;101:229‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension. 1. Overview, meta‐analyses, and meta‐regression analyses of randomized trials. J Hypertens. 2014;32:2285‐2295. [DOI] [PubMed] [Google Scholar]
- 6. Chow CK, Teo KK, Rangarajan S, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high‐, middle‐, and low‐income countries. JAMA. 2013;310:959‐968. [DOI] [PubMed] [Google Scholar]
- 7. Elliott WJ. What factors contribute to the inadequate control of elevated blood pressure? J Clin Hypertens (Greenwich). 2008;10:20‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Kleef ME, Spiering W. Hypertension: overly important but under‐controlled. Eur J Prev Cardiol. 2017;24:36‐43. [DOI] [PubMed] [Google Scholar]
- 9. Abegaz TM, Shehab A, Gebreyohannes EA, et al. Nonadherence to antihypertensive drugs: a systematic review and meta‐analysis. Medicine (Baltimore). 2017;96:e5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tung YC, Huang YC, Wu LS, et al. Medication compliance and clinical outcomes of fixed‐dose combinations vs. combinations of an angiotensin II receptor blocker and a calcium channel blocker in hypertension treatment. J Clin Hypertens (Greenwich). 2017;19:983‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Erdine S. Compliance with the treatment of hypertension: the potential of combination therapy. J Clin Hypertens (Greenwich). 2010;12:40‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong MC, Tam WW, Cheung CS, et al. Antihypertensive prescriptions over a 10‐year period in a large Chinese population. Am J Hypertens. 2013;26:931‐938. [DOI] [PubMed] [Google Scholar]
- 13. Fontil V, Bibbins‐Domingo K, Nguyen OK, et al. Management of hypertension in primary care safety‐net clinics in the United States: a comparison of community health centers and private physicians’ offices. Health Serv Res. 2017;52:807‐825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fontil V, Pletcher MJ, Khanna R, et al. Physician underutilization of effective medications for resistant hypertension at office visits in the United States: NAMCS 2006‐2010. J Gen Intern Med. 2014;29:468‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008‐2012. [DOI] [PubMed] [Google Scholar]
- 16. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lo CK, Mertz D, Loeb M. Newcastle‐Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dezii CM. A retrospective study of persistence with single‐pill combination therapy vs. concurrent two‐pill therapy in patients with hypertension. Manag Care. 2000;9:2‐6. [PubMed] [Google Scholar]
- 19. Taylor AA, Shoheiber O. Adherence to antihypertensive therapy with fixed‐dose amlodipine besylate/benazepril HCl versus comparable component‐based therapy. Congest Heart Fail. 2003;9:324‐332. [DOI] [PubMed] [Google Scholar]
- 20. Brixner DI, Jackson KC 2nd, Sheng X, et al. Assessment of adherence, persistence, and costs among valsartan and hydrochlorothiazide retrospective cohorts in free‐and fixed‐dose combinations. Curr Med Res Opin. 2008;24:2597‐2607. [DOI] [PubMed] [Google Scholar]
- 21. Dickson M, Plauschinat CA. Compliance with antihypertensive therapy in the elderly: a comparison of fixed‐dose combination amlodipine/benazepril versus component‐based free‐combination therapy. Am J Cardiovasc Drugs. 2008;8:45‐50. [DOI] [PubMed] [Google Scholar]
- 22. Hess G, Hill J, Lau H, et al. Medication utilization patterns and hypertension‐related expenditures among patients who were switched from fixed‐dose to free‐combination antihypertensive therapy. P T. 2008;33:652‐666. [PMC free article] [PubMed] [Google Scholar]
- 23. Hsu CI, Hsiao FY, Wu FL, et al. Adherence and medication utilisation patterns of fixed‐dose and free combination of angiotensin receptor blocker/thiazide diuretics among newly diagnosed hypertensive patients: a population‐based cohort study. Int J Clin Pract. 2015;69:729‐737. [DOI] [PubMed] [Google Scholar]
- 24. Tung YC, Lin YS, Wu LS, et al. Clinical outcomes and healthcare costs in hypertensive patients treated with a fixed‐dose combination of amlodipine/valsartan. J Clin Hypertens (Greenwich). 2015;17:51‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Levi M, Pasqua A, Cricelli I, et al. Patient adherence to olmesartan/amlodipine combinations: fixed versus extemporaneous combinations. J Manag Care Spec Pharm. 2016;22:255‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed‐dose combinations of antihypertensive agents: a meta‐analysis. Hypertension. 2010;55:399‐407. [DOI] [PubMed] [Google Scholar]
- 27. Sherrill B, Halpern M, Khan S, et al. Single‐pill vs free‐equivalent combination therapies for hypertension: a meta‐analysis of health care costs and adherence. J Clin Hypertens (Greenwich). 2011;13:898‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xie L, Frech‐Tamas F, Marrett E, et al. A medication adherence and persistence comparison of hypertensive patients treated with single‐, double‐ and triple‐pill combination therapy. Curr Med Res Opin. 2014;30:2415‐2422. [DOI] [PubMed] [Google Scholar]
- 29. Grimmsmann T, Himmel W. Comparison of therapy persistence for fixed versus free combination antihypertensives: a retrospective cohort study. BMJ Open. 2016;6:e011650. [DOI] [PMC free article] [PubMed] [Google Scholar]


