Abstract
The time trends regarding the etiology of renal artery stenosis (RAS) are changing, but few investigations have focused on these issues. This study aimed to analyze the time trends regarding the etiology of RAS in a large patient sample from the China Center for Cardiovascular Disease. Consecutive inpatients with RAS from January 1999 to December 2016 were enrolled in this study. The etiologic diagnosis of RAS was based on established criteria. We retrospectively analyzed the time trends regarding the etiology of RAS during an 18‐year period. A total of 2905 patients with RAS were enrolled. There were 2393 (82.4%) patients with atherosclerosis (AS), 345 (11.9%) with Takayasu arteritis (TA), 126 (4.3%) with fibromuscular dysplasia (FMD), and 41 (1.4%) with other causes. Among all patients (n = 2905), patients aged ≤ 40 years (n = 450), patients aged >40 years (n = 2455), female patients (n = 1097), male patients (n = 1808), female patients aged >40 years (n = 808), and male patients aged >40 years (n = 1647), there were a gradual increase in the proportion of atherosclerotic RAS (P < 0.05), a gradual decrease in the proportion of RAS caused by TA (P < 0.05), and almost no change in the proportion of RAS caused by FMD during the 18‐year period (P > 0.05). The data show that the primary causes of RAS are AS, TA, and FMD. The proportion of RAS caused by AS and TA gradually increased and decreased, respectively, over time, and the proportion of RAS caused by FMD showed no significant change.
Keywords: distribution, etiology, renal artery stenosis, trend
1. INTRODUCTION
Renal artery stenosis (RAS) is a major cause of secondary hypertension and a frequent cause of renal failure/end‐stage renal disease in the elderly.1 It may be present in 5%‐10% of the elderly, with a higher prevalence in high‐risk patients.2 The incidence of cardiovascular events and cardiovascular mortality is higher in patients with renovascular hypertension than in patients with essential hypertension.3 Atherosclerosis (AS) is the main cause of RAS and strongly correlates with smoking, male sex, hypertension, chronic kidney disease, diabetes mellitus, and coronary artery disease.4 Other relatively rare causes of RAS are Takayasu arteritis (TA) and fibromuscular dysplasia (FMD), which represent the most common causes of RAS in young patients with hypertension.5, 6, 7 The time trends regarding the etiology of RAS are currently changing, but few investigations have focused on these issues. This study aimed to analyze the time trends regarding the etiology of RAS in a large patient sample from the China Center for Cardiovascular Disease.
2. METHODS
2.1. Patients
Consecutive inpatients with RAS at Beijing Fuwai Hospital from January 1999 to December 2016 were continuously enrolled in the study. Beijing Fuwai Hospital is the China Center for Cardiovascular Disease and consultation center for renal artery disease in China. Patients with (1) renal artery trunk and/or primary branch diameter stenosis ≥ 50% on multidetector row computed tomography (CT) angiography (CTA) or selective renal artery arteriography and (2) grade II‐III hypertension (systolic blood pressure ≥ 160 mm Hg or diastolic blood pressure ≥ 100 mm Hg without the use of antihypertensive drugs)8 were included. A total of 2905 patients were enrolled. Patients could be divided into primarily 2 groups: those admitted to screen for the cause of hypertension and first diagnosed with RAS at our hospital, and those referred to our hospital for further treatment owing to RAS. After the study was approved by the ethics committees of our hospital (approval number: 2014‐542), we used standardized processes to train 4 investigators to collect clinical and nonclinical data.
2.2. Study protocol
2.2.1. General information collection
The collected clinical data included demographic information, personal history (smoking and drinking status), past history (history of hypertension, diabetes mellitus, hyperlipidemia, chronic kidney disease, and other diseases), physical examination (height, weight, and blood pressure), and blood test results (fasting blood glucose, serum creatinine, total cholesterol, triglycerides, low‐density lipoprotein, and high‐density lipoprotein levels). Body mass index was calculated by dividing body weight in kilograms by height in meters squared. Renal function was evaluated using estimated glomerular filtration rate (eGFR), defined as follows: eGFR (mL/min/1.73 m2) = 186.3 × serum creatinine (mg/dL)−1.154 × age−0.203 × 0.742 (if female).9 Serum creatinine concentration was measured using the UniCel DxC 800 Synchron Clinical System (Beckman Coulter, Brea, CA) and was assessed using the Jaffe method. If eGFR was less than 60 mL/min/1.73 m2, the diagnosis of renal insufficiency was established.10 Related guidelines for the diagnosis of diabetes mellitus and hyperlipidemia were referred to.11, 12
2.2.2. Evaluation of RAS
Renal artery stenosis was diagnosed by renal artery angiography or CTA. The standard procedure was used for renal artery angiography, using a 6F or 5F catheter angiography for the abdominal aorta and selective renal artery angiography. Spiral CT was used to perform renal artery CTA examination, using 16‐row CT scanners (SOMATOM Sensation 16; Siemens, Munich, Germany) during the period of 2003‐2006 and 64‐row CT scanners (LightSpeed, GE Healthcare, Chicago, IL) during the period of 2007‐2016. After 4‐6 hours of fasting, an intravenous bolus injection of 100‐120 mL of contrast medium was administered through a median cubital vein at a rate of 3‐5 mL/s, and standard processes and technical parameters were used for renal artery reconstruction. CTA and angiographic images were analyzed by 2 experienced radiologists. A total of 2392 (82.3%) and 513 (17.7%) patients were diagnosed through renal artery angiography and CTA, respectively.
2.2.3. The etiologic diagnosis of RAS
The etiologic diagnosis of RAS was established based on clinical and radiographic features. AS was diagnosed based on the following criteria: (1) at least 1 risk factor for AS (diabetes, hyperlipidemia, age >40 years old, or long‐term smoking) and (2) at least 2 imaging findings for AS (tapered stenosis or occlusion of the renal artery, eccentric stenosis, irregular plaque, calcification, primary involvement of the proximal portion or ostium of the renal artery, AS present in other abdominal vasculature).13, 14 TA was diagnosed according to the 1990 criteria of the American College of Rheumatology15 or the criteria reported by Sharma and colleagues in 1996.16 The diagnosis of FMD involving the renal artery was based on the angiographic appearance, and AS, vasculitis, or other diseases needed to be excluded.17, 18 Other rare causes of RAS included Behcet's disease, polyarteritis nodosa, thrombosis, and dissection, the diagnoses of which were based on internationally agreed‐upon criteria.19, 20, 21
2.3. Statistical methods
Continuous variables are expressed as mean ± standard deviation, whereas categorical variables are expressed as frequency and percentage. The chi‐square test or Fisher's exact test was used to analyze categorical variables; continuous variables were compared among different groups using the variance test. We calculated the distribution of causes at each node (with 2 years considered a node during the past 18 years), and the time trends in the etiology of RAS were then analyzed using the Cochran‐Armitage test. The chi‐square test, Fisher's exact test, or variance test was performed using SPSS version 22.0 (IBM Corp., Armonk, NY) and the Cochran‐Armitage test was performed using SAS version 9.0 (SAS Institute Inc., Cary, NC). A P value <0.05 was considered statistically significant, and all reported P values were 2‐sided.
3. RESULTS
3.1. Demographic and clinical characteristics of patients with RAS of different etiologies
Between January 1999 and December 2016, a total of 2905 patients with RAS (mean age: 58.5 ± 16.5 years [range: 9‐89 years]) met the inclusion criteria; of these patients, 1097 patients (37.8%) were females. With respect to different etiologies, 2393 (82.4%) patients had AS, 345 (11.9%) patients had TA, 126 (4.3%) patients had FMD, and 41 (1.4%) patients had other causes (19 patients with thrombosis, 6 patients with dissection, 3 patients with polyarteritis nodosa, 1 patient with Behcet's disease, and 12 patients with undefined etiology). Patients with AS were older and had worse renal function, greater body mass index, and lower diastolic blood pressure than other patients (all P < 0.01). Detailed demographic and clinical characteristics of patients with RAS of different etiologies are shown in Table.
Table 1.
Demographic and clinical characteristics of patients with different etiologies of RAS
| Variables | AS group, n = 2393 | TA group, n = 345 | FMD group, n = 126 | Other group, n = 41 | P value |
|---|---|---|---|---|---|
| Age (years) | 64.3 ± 10.1 | 31.2 ± 13.0 | 27.8 ± 9.6 | 40.2 ± 15.1 | <0.001 |
| Female | 738 (30.8) | 264 (76.5) | 82 (65.1) | 13 (31.7) | <0.001 |
| Smoking | 1307 (54.6) | 46 (13.3) | 23 (18.3) | 12 (29.3) | <0.001 |
| BMI (kg/m2) | 24.6 ± 3.1 | 22.9 ± 3.7 | 22.3 ± 3.6 | 22.8 ± 3.5 | <0.001 |
| Coronary heart disease | 1277 (53.4) | 24 (7.0) | 5 (4.0) | 8 (19.5) | <0.001 |
| Diabetes mellitus | 655 (27.4) | 10 (2.9) | 4 (3.2) | 2 (4.9) | <0.001 |
| Hyperlipidemia | 1222 (51.1) | 24 (7.0) | 8 (6.3) | 6 (14.6) | <0.001 |
| Stroke | 436 (18.2) | 10 (2.9) | 9 (7.1) | 2 (4.9) | <0.001 |
| SBP (mm Hg) | 155.1 ± 18.4 | 156.7 ± 20.1 | 155.3 ± 18.4 | 157.6 ± 22.7 | 0.433 |
| DBP (mm Hg) | 86.2 ± 12.6 | 89.7 ± 15.1 | 93.4 ± 14.7 | 97.0 ± 17.4 | <0.001 |
| Antihypertensive drugs (n) | 2.3 ± 0.9 | 2.2 ± 0.8 | 2.1 ± 1.0 | 2.0 ± 1.0 | 0.022 |
| Malignant hypertension | 35 (1.5) | 5 (1.4) | 2 (1.6) | 0 (0) | 0.879 |
| Serum creatinine (μmol/L) | 93.7 ± 30.1 | 77.8 ± 24.8 | 81.3 ± 28.3 | 76.0 ± 18.6 | <0.001 |
| eGFR (mL/min/1.73 m2) | 77.5 ± 27.2 | 95.1 ± 29.9 | 95.1 ± 35.4 | 103.1 ± 31.2 | <0.001 |
| Renal dysfunction | 669 (28.0) | 23 (6.7) | 7 (5.6) | 1 (2.4) | <0.001 |
| In need of dialysis | 16 (0.7) | 1 (0.3) | 0 (0) | 0 (0) | 0.618 |
Values are n (%) or mean values ± standard deviation.
AS, atherosclerosis; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FMD, fibromuscular dysplasia; SBP, systolic blood pressure; TA, Takayasu arteritis.
3.2. Time trends regarding the etiology of RAS among all patients
Figure 1 shows the time trends regarding the etiology of RAS among all patients. The proportion of atherosclerotic RAS (ARAS) obviously increased over time (Z = −7.84, P < 0.001) from approximately 50% in the early stage of the study to nearly 85% in recent years. The proportion of RAS caused by TA gradually decreased over time (Z = 7.03, P < 0.001) from 31% to 10%. The proportion of RAS caused by FMD did not essentially unchanged, fluctuating between 2.9% and 6.5% (Z = 0.11, P = 0.911).
Figure 1.
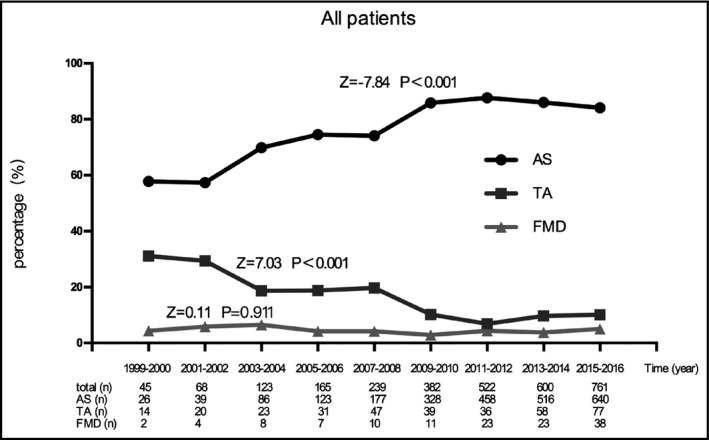
Time trends regarding the etiology of renal artery stenosis in all patients. AS, atherosclerosis; TA, Takayasu arteritis; FMD, fibromuscular dysplasia
3.3. Time trends regarding the etiology of RAS in different age groups
Figure 2 shows the time trends regarding the etiology of RAS in different age groups. In patients aged ≤ 40 years or patients aged >40 years, the proportion of ARAS gradually increased over time (all P < 0.001), whereas the proportion of RAS caused by TA gradually decreased over time (all P < 0.05). In the early stages of the study, we identified very few patients aged ≤ 40 years with ARAS, and the primary causes of RAS in this age group were TA and FMD. In recent years, ARAS accounted for approximately 10% of patients aged ≤ 40 years (Figure 2A). In patients aged >40 years, the proportion of ARAS increased from 70% in the early stage of the study to 97% in recent years, and the proportion of RAS caused by TA sharply decreased from 25% to 1% (Figure 2B).
Figure 2.

Time trends regarding the etiology of renal artery stenosis in patients aged ≤ 40 y (A) and >40 y (B). AS, Atherosclerosis; TA, Takayasu arteritis; FMD, Fibromuscular dysplasia
3.4. Time trends regarding the etiology of RAS in different sexes
Figure 3 displays the time trends regarding the etiology of RAS in different sexes. In female patients, the proportion of ARAS obviously increased over time, whereas the proportion of RAS caused by TA showed significantly decreased over time (all P < 0.001). The proportion of ARAS sharply increased from 30% to 75%, whereas the proportion of RAS caused by TA decreased from 54% to 18% (Figure 3A). AS has replaced TA as the most common etiology of RAS in female patients. In male patients, although the proportion of RAS caused by AS and TA gradually increased and decreased, respectively, over time, the degrees of change were small compared with those in female patients (Figure 3B).
Figure 3.

Time trends regarding the etiology of renal artery stenosis in female (A) and male (B) patients. AS, atherosclerosis; TA, Takayasu arteritis; FMD, fibromuscular dysplasia
3.5. Analysis of the time trends regarding the etiology of RAS based on sex and age
Figure 4 shows the time trends regarding the etiology of RAS based on two factors: sex and age. In female patients aged ≤ 40 years, ARAS accounted for a very small proportion of cases, and TA consistently remained the primary cause of RAS (Figure 4A). In male patients aged ≤ 40 years, the proportion of ARAS gradually increased over time (from 0% to 18.5%) (Z = −2.08, P = 0.037); there was no obvious change in the proportion of RAS caused by TA (Figure 4B). In female patients or male patients aged >40 years, the proportion of RAS caused by AS and TA gradually increased and decreased, respectively, over time (all P < 0.01; Figure 4C,D).
Figure 4.

Time trends regarding the etiology of renal artery stenosis in female patients aged ≤ 40 y (A), male patients aged ≤ 40 y (B), Female patients aged >40 y (C), and Male patients aged >40 y (D). AS, atherosclerosis; TA, Takayasu arteritis; FMD, fibromuscular dysplasia
4. DISCUSSION
In this study, we retrospectively analyzed the time trends regarding the etiology of RAS in 2905 patients at the China Center for Cardiovascular Disease during the past 18 years, which represents the largest sample size in the field. Our study showed that AS, TA, and FMD were sequentially the 3 primary causes of RAS. The proportion of RAS caused by AS and TA gradually increased and decreased, respectively, over time, and the proportion of RAS caused by FMD showed little change. The results of this study will help us to better understand the time trends regarding the different etiologies of RAS, especially in the Chinese patient population.
In the 1980s, Chinese experts Zhao and colleagues22 reported that TA was the leading cause of RAS in 76 patients with renovascular hypertension, accounting for 61.9% of all patients. At the beginning of this century, Wang and colleagues23 studied data from 144 patients with RAS in a single center from 1979 to 2003 in China. They showed that TA was the primary cause of RAS before 1990, accounting for 71.7%. After 1990, with the improvement in examination techniques, more patients with RAS were diagnosed and AS became the most common cause of RAS, accounting for 55.3%. Peng and coauthors5 recently analyzed a relatively large study population with RAS in China and reported that the proportion of AS and TA was 81.5% and 12.7%, respectively. In summary, the proportion of ARAS obviously increased over time, and the proportion of RAS caused by TA gradually decreased over time in China. The results of our study are consistent with those previously reported. Therefore, it could be concluded that RAS caused by AS has become a common disease rather than a rare disease in China. The reason for this may be related to the changes in lifestyles, such as a trend from traditional to modern lifestyles. The trend may also be related to the aging of the Chinese population and the widespread use of CTA for RAS screening in clinical practice. In recent years, the proportion of ARAS tended to be stable, which may be associated with increasing efforts to effectively treat atherosclerotic cardiovascular disease by the Chinese government.
In Western countries, Maxwell and coauthors performed a retrospective study that included 880 patients with renovascular hypertension in 1972 and reported that AS accounted for 63% of all cases. In 1994, Anderson and colleagues24 reported that AS and FMD were the main causes of RAS, accounting for 84.5% and 15.5%, respectively. At the beginning of this century, studies showed that AS was the main cause of hypertension, accounting for approximately 90% of cases of renovascular hypertension.25, 26, 27 Hence, the proportion of ARAS gradually increased over time, similar to our findings.
Our study showed that the proportion of ARAS in patients aged ≤ 40 years increased over time, especially among males. Previous studies reported that TA was the primary cause of RAS in patients aged ≤ 40 years and that AS was rare in this age group.28 In our study, AS was rare in female patients aged ≤ 40 years, and there was no significant change during the past 18 years, which may be related to the protective effect of female sex before menopause.29 Therefore, although the proportion of ARAS gradually increased in these patients, TA or FMD should be first considered rather than AS. In recent years, the proportion of ARAS gradually increased in male patients aged ≤ 40 years, accounting for a large proportion of patients. When establishing etiological diagnosis for this patient group, we cannot ignore AS, especially in patients with a history of smoking, hypertension, hyperlipidemia, and other AS‐related risk factors.
Further, we showed that the proportion of RAS caused by TA gradually decreased over time in patients with RAS aged >40 years, especially among female patients. In the early stage of the study, the proportion of RAS caused by TA accounted for almost one‐half of all patients aged >40 years old, but in recent years, RAS caused by TA was not very common. The main reason for this result may be that many patients with TA were diagnosed late owing to poor medical screening in the early years of this study. In recent years, a growing number of patients with TA were promptly diagnosed with improvement in diagnostic capabilities.
In both male and female patients with RAS, the proportion of RAS caused by AS and TA gradually increased and decreased, respectively, over time. However, the proportion of RAS caused by TA in male patients was very small, and essentially remained at 10%, whereas the proportion of RAS caused by TA in female patients was remained at 20%. This is mainly related to the difference in the incidence of TA between males and females, which has been shown by other investigations.30, 31 In addition, our study showed that the prevalence of ARAS and FMD was always higher in men and women, respectively, over time, which is similar to the results of studies performed in Western countries.32, 33
With the continuous improvement in Chinese economic status and the increase in life expectancy, AS has become the primary cause of RAS. Considering that ARAS can lead to serious clinical consequences, we should focus on the prevention and treatment of RAS caused by AS.
This study has limitations. First, it was not a population‐based study; thus we could not ensure a random and unbiased selection. Second, our study was retrospective, and there may be information bias. In addition, the conditions for screening and diagnosing RAS have obviously been changed as the time span of data was large. Third, the indications for hospitalization, angiography, and percutaneous transluminal renal angioplasty might have changed over time, as the Angioplasty and Stenting for Renal Artery Lesions and Cardiovascular Outcomes in Renal Atherosclerotic Lesions trials were published during the study period, which was an additional potential confounding factor for our study. Fourth, the number of patients with RAS who were diagnosed at an early stage was small, which may have affected the results. Finally, we do not routinely perform ACE scan in all patients with RAS to assess the hemodynamic significance of the RAS lesion.34 In the future, we should use ultrasound as well as CTA and angiography to screen patients with RAS to further increase the detection rate.35
5. CONCLUSION
The data from the China Center for Cardiovascular Disease showed that AS, TA, and FMD were the 3 primary causes of RAS. The proportion of RAS caused by AS and TA gradually increased and decreased, respectively, over time, and the proportion of RAS caused by FMD did not significantly change during the 18‐year period.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
None.
Xiong H‐l, Peng M, Jiang X‐j, et al. Time trends regarding the etiology of renal artery stenosis: 18 years’ experience from the China Center for Cardiovascular Disease. J Clin Hypertens. 2018;20:1302‐1309. 10.1111/jch.13356
Hong‐liang Xiong and Meng Peng equally contributed to the study as first authors.
REFERENCES
- 1. Textor SC, Wilcox CS. Renal artery stenosis: a common, treatable cause of renal failure? Annu Rev Med. 2001;52:421‐442. [DOI] [PubMed] [Google Scholar]
- 2. Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter‐Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463‐e654. [DOI] [PubMed] [Google Scholar]
- 3. Sauguet A, Honton B. Atherosclerotic renal artery stenosis. Ann Cardiol Angeiol (Paris). 2014;63:437‐441. [DOI] [PubMed] [Google Scholar]
- 4. Kwon SH, Lerman LO. Atherosclerotic renal artery stenosis: current status. Adv Chronic Kidney Dis. 2015;22:224‐231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peng M, Jiang XJ, Dong H, et al. Etiology of renal artery stenosis in 2047 patients: a single‐center retrospective analysis during a 15‐year period in China. J Hum Hypertens. 2016;30:124‐128. [DOI] [PubMed] [Google Scholar]
- 6. Persu A, Giavarini A, Touze E, et al. European consensus on the diagnosis and management of fibromuscular dysplasia. J Hypertens. 2014;32:1367‐1378. [DOI] [PubMed] [Google Scholar]
- 7. Sanidas EA, Seferou M, Papadopoulos DP, et al. Renal fibromuscular dysplasia: a not so common entity of secondary hypertension. J Clin Hypertens (Greenwich). 2016;18:240‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159‐2219. [DOI] [PubMed] [Google Scholar]
- 9. Peng M, Jiang XJ, Dong H, et al. A comparison of nephrotoxicity of contrast medium in elderly patients who underwent renal or peripheral arterial vascular intervention. Intern Med. 2016;55:9‐14. [DOI] [PubMed] [Google Scholar]
- 10. Thomas C, Thomas L. Renal failure–measuring the glomerular filtration rate. Dtsch Arztebl Int. 2009;106:849‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. American Diabetes Association . Standards of medical care in diabetes–2014. Diabetes Care. 2014;37(suppl 1):S14‐S80. [DOI] [PubMed] [Google Scholar]
- 12. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2889‐2934. [DOI] [PubMed] [Google Scholar]
- 13. Chrysochou C, Kalra PA. Epidemiology and natural history of atherosclerotic renovascular disease. Prog Cardiovasc Dis. 2009;52:184‐195. [DOI] [PubMed] [Google Scholar]
- 14. Tanemoto M, Saitoh H, Satoh F, et al. Predictors of undiagnosed renal artery stenosis among Japanese patients with risk factors of atherosclerosis. Hypertens Res. 2005;28:237‐242. [DOI] [PubMed] [Google Scholar]
- 15. Arend WP, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33:1129‐1134. [DOI] [PubMed] [Google Scholar]
- 16. Sharma BK, Jain S, Suri S, et al. Diagnostic criteria for Takayasu arteritis. Int J Cardiol. 1996;54(suppl):S141‐S147. [DOI] [PubMed] [Google Scholar]
- 17. Persu A, Touze E, Mousseaux E, et al. Diagnosis and management of fibromuscular dysplasia: an expert consensus. Eur J Clin Invest. 2012;42:338‐347. [DOI] [PubMed] [Google Scholar]
- 18. Yang YK, Zhang Y, Meng X, et al. Clinical characteristics and treatment of renal artery fibromuscular dysplasia with percutaneous transluminal angioplasty: a long‐term follow‐up study. Clin Res Cardiol. 2016;105:930‐937. [DOI] [PubMed] [Google Scholar]
- 19. Criteria for diagnosis of Behcet's disease. International Study Group for Behcet's Disease. Lancet. 1990;335:1078‐1080. [PubMed] [Google Scholar]
- 20. Lightfoot RW Jr, Michel BA, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of polyarteritis nodosa. Arthritis Rheum. 1990;33:1088‐1093. [DOI] [PubMed] [Google Scholar]
- 21. Chaillou P, Moussu P, Noel SF, et al. Spontaneous dissection of the celiac artery. Ann Vasc Surg. 1997;11:413‐415. [DOI] [PubMed] [Google Scholar]
- 22. Zhao PZ, Meng JX, Chai GP, et al. Pathological typing of 76 patients with renovascular hypertension. Chin Circulation J. 1986;1:70‐72. [Google Scholar]
- 23. Wang F, Wang M, Liu YC, et al. The changing etiology and therapeutic situation of atherosclerotic renal artery stenosis. Zhonghua Yi Xue Za Zhi. 2005;85:2762‐2766. [PubMed] [Google Scholar]
- 24. Anderson GH Jr, Blakeman N, Streeten DH. The effect of age on prevalence of secondary forms of hypertension in 4429 consecutively referred patients. J Hypertens. 1994;12:609‐615. [DOI] [PubMed] [Google Scholar]
- 25. Textor SC. Ischemic nephropathy: where are we now? J Am Soc Nephrol. 2004;15:1974‐1982. [DOI] [PubMed] [Google Scholar]
- 26. Prisant LM, Szerlip HM, Mulloy LL. Fibromuscular dysplasia: an uncommon cause of secondary hypertension. J Clin Hypertens (Greenwich). 2006;8:894‐898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bokhari MR. Renal Artery Stenosis. Treasure Island, FL: StatPearls Publishing; 2018. [PubMed] [Google Scholar]
- 28. Bicakcigil M, Aksu K, Kamali S, et al. Takayasu's arteritis in Turkey ‐ clinical and angiographic features of 248 patients. Clin Exp Rheumatol. 2009;27:S59‐S64. [PubMed] [Google Scholar]
- 29. Nabulsi AA, Folsom AR, White A, et al. Association of hormone‐replacement therapy with various cardiovascular risk factors in postmenopausal women. The Atherosclerosis Risk in Communities Study Investigators. N Engl J Med. 1993;328:1069‐1075. [DOI] [PubMed] [Google Scholar]
- 30. Chaudhry MA, Latif F. Takayasu's arteritis and its role in causing renal artery stenosis. Am J Med Sci. 2013;346:314‐318. [DOI] [PubMed] [Google Scholar]
- 31. Yang L, Zhang H, Jiang X, et al. Clinical manifestations and longterm outcome for patients with Takayasu arteritis in China. J Rheumatol. 2014;41:2439‐2446. [DOI] [PubMed] [Google Scholar]
- 32. Olin JW, Froehlich J, Gu X, et al. The United States Registry for Fibromuscular Dysplasia: results in the first 447 patients. Circulation. 2012;125:3182‐3190. [DOI] [PubMed] [Google Scholar]
- 33. Chrysant SG, Chrysant GS. Treatment of hypertension in patients with renal artery stenosis due to fibromuscular dysplasia of the renal arteries. Cardiovasc Diagn Ther. 2014;4:36‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stratigis S, Stylianou K, Kyriazis PP, et al. Renal artery stenting for atherosclerotic renal artery stenosis identified in patients with coronary artery disease: does captopril renal scintigraphy predict outcomes? J Clin Hypertens (Greenwich). 2018;20:373‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grupp C, Koziolek MJ, Wallbach M, et al. Difference between renal and splenic resistive index as a novel criterion in Doppler evaluation of renal artery stenosis. J Clin Hypertens (Greenwich). 2018;20:582‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]


