Abstract
The effects of the concomitant infection by COVID-19 and Burkholderia cepacia (Bc) in CF are not known. We describe the case of a 34 years woman with CF, colonized by Bc and found SARS-CoV2 positive. In the first hospital week she suffered acute respiratory failure and chest imaging showed interstitial involvement and multiple thickenings. She was treated with antibiotics, dexamethasone, remdesivir and heparin, with gradual improvement and discharge at day 20th. The reciprocal role of SARS-CoV-2 and Bc, their potential interactions and the contribution of the individual therapies to the favourable outcome are unclear. It is debatable whether it was SARS-CoV2 that triggered a Bc pulmonary exacerbation or if the chronic Bc infection facilitated the development of a COVID-19 more aggressive than usually seen in CF. If the latter hypothesis were confirmed by similar cases, Bc colonization should be regarded as a risk factor for severe COVID-19 expression in CF.
Keywords: Cystic Fibrosis, Burkholderia cepacia, SARS-CoV-2 COVID-19, Pulmonary exacerbation
1. Introduction
Data so far collected by Cystic Fibrosis (CF) patient registries and Centers consortia suggest that in most patients with CF (pwCF) COVID-19 is less severe than initially predicted [1]. However, a subset of pwCF with well known negative CF prognostic factors, like diabetes and severely impaired lung function,have been shown to be prone to worse COVID-19 outcomes [2].
In the CF clinical setting, Burkholderia cepacia(Bc) infection can implicate an unfavorable evolution. Bc clinical manifestations are diverse, and include the cepacia syndrome, a rapidly progressing necrotizing pneumonia very frequently concluding with exitus. Factors contributing to a poor outcome are the multi-drug resistance of most strains and the severe systemic inflammatory response elicited by the infection. Combinations of IV antibiotics and, often, steroids, are used to tryand control the syndrome [3].
Bc in pwCF and SARS-CoV-2 in individuals with accompanying morbidities share the capability to trigger a hyper-inflammatory reaction and cause critical acute respiratory distress syndrome. The effects of the concomitant infection by these two pathogens in CF is not known. We describe the first case of COVID-19 in a pwCF chronically infected by Bc.
2. Case report
The case concerns a 34 years woman affected by CF, F508del homozygote, pancreatic insufficient, chronically infected by Bc genomovar III. Her ppFEV1 was around 70 and her daily treatment consisted of lumacaftor/ivacaftor, azithromycin, DNAse and PERT.
In October 2020 the patient contacted the Gaslini Institute CF Center reporting high temperature and an episode of unusually low oxygen saturation (SpO2 92%) in the previous two days. Contrary to her usual practice of avoiding social contacts since the beginning of the SARS-CoV-2 epidemic, in the preceding week she had had close contacts with some friends. Because of the COVID-19 Institute policy, she was not seen at the CF Center and was referred to the Emergency Room.
On admission, the patient presented raised temperature (38°C), good oxygen saturation levels (SpO2 99-100%), right anterior basal rales at chest auscultation and her chest X-ray was analogous to previous ones. SARS-CoV2 antigenic and molecular swab tests resulted positive. The patient was moved to a COVID ward and, after consultation with the CF Center physicians, started IV meropenem (1500 mg qds) and oral sulfamethoxazole-trimetoprim (160 mg+800 mg tds).
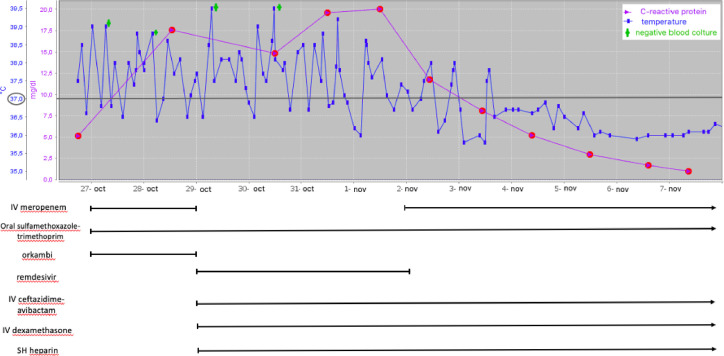
In the following two days clinical, biochemical and radiological parameters deteriorated: temperature raised, with spikes reaching 39.5°C and poor response to IV paracetamol and metamizole; chest auscultation revealed disseminated crackles; C-Reactive Protein level increased from 5.12 to 17mg/dl (N <0.46 mg/dl); transcutaneous oxygen saturation decreased to 90-92%; diarrhea, epigastric pain and intense musculoskeletal ache appeared. A chest CT showed bilateral areas of ground-glass opacities, dilated segmental and subsegmental vessels with a peripheral distribution and consolidations surrounded by ground-glass opacities (halo sign). (supplementary material- figure 1 ).
Fig. 1.
CRP and temperature trend during the hospital stay
She was moved to a sub-intensive care Unit, meropenem was switched to IV ceftazidime/avibactam (2000mg/500mg tds) and IV dexamethasone (5mg qd), IV remdesivir (induction dose of 200 mg followed by 100 mg qd for 4days) and SQ heparin (8000 IU qd) were started. Lumacaftor/ivacaftor was discontinued because of potential interactions between remdesivir and CYP3A4.
Two days later .32 FiO2 support was necessary during temperature spikes, CRP had increased further (21mg/dl) and a chest X-ray showed bilateral interstitial hypodiaphania in mid-basal areas, more represented in the right lung (supplementary-figure 2). Blood cultures had resulted repeatedly negative.
In doubt as to whether the deteriorating clinical conditions were connected with a Bc exacerbation or with COVID-19, meropenem was reintroduced and dexamethasone increased (6mg qd). In the following days the patient improved, with defervescence, better oxygen saturation and progressive reduction of inflammation markers. According to the local COVID-19 protocol, remdesivir was stopped at day 8th from admission and dexamethasone tapered.
Twelve days after the positive SARS-CoV2 swab and 3 days after symptoms disappearance the molecular swab tested negative. Thirteen days after admission SARS-CoV2 serology showed high levels of IgG (13.600AU/ml) and IgM (5.252 AU/ml) antibodies and chest CT had significantly improved (supplementary materials -figure 3).
Discharge occurred at day 20th in good clinical conditions, free of oxygen and with minimal loss of respiratory function compared to the value at the latest outpatient clinic visit (ppFEV1 65 versus 68). The patient was directed to a post-COVID follow-up program.
3. Discussion
We report the favourable outcome of a severe SARS-CoV2 infection in a pwCF colonized by Bc. The concomitance of COVID-19 and Bc chronic infection raises some issues about the interaction of these two pathogens in the complex CF lung environment.
None of the six confirmed cases of SARS-CoV2 in pwCF that have so far come to our attention required hospital admission, except for the one reported here. This patient suffered a severe course of COVID-19, with acute respiratory failure, and signs of interstitial involvement at chest x-ray. She also showed multiple parenchimal consolidation at chest CT, a manifestation suggestive of initial cepacia syndrome, but blood cultures sampled during temperature spikes resulted repeatedly negative.
We found it problematic to determine the interaction between SARS-CoV-2 and Bc in this uncommon presentation. A therapy aimed at SARS-CoV2 and Bc was used, but the interpretation of response to treatment is ambiguous. Steroids are used both in severe COVID-19 and in cepacia syndrome, and it seems reasonable arguing that IV dexamethasone had a significant role. The respective impacts of the multiple IV antibiotics and of the antiviral are more difficult to assess, and it might be speculated that both to some degree contributed to the positive outcome of this case.
CF pulmonary exacerbations may be triggered by respiratory viral infections [4]. Although this correlation has not been proved for SARS-CoV2, COVID-19 might have disrupted the balance between immune response and chronic Bc infection and the initial prevalent viral dominance could have been later outweighed by bacterial infection.
A complementary interpretation is that the chronic Bc infection facilitated the development of a SARS-CoV2 disease more aggressive than what is usually seen in pwCFs. High levels of neutrophils and pro-inflammatory cytokines are distinctive traits of CF lung disease since the first months of life [5]. A cepacia syndrome, even in its early stage, might have shifted such chronic inflammation background to an uncontrolled inflammatory response and expedited the onset of a clinical picture resembling the “cytokine storm” that characterizes severe COVID-19. If this hypothesis were confirmed by further similar cases, Bc colonization should be regarded as a risk factor for severe COVID-19 expression in CF, similarly to diabetes or poor lung function.
Finally, we would like to note that this case can also be seen from a completely different angle, that is the challenges posed by the care of a complex pwCF whose admission to a CF Center is prevented by SARS-CoV2. The integration between the temporary COVID-19 therapeutic needs and the demands of CF care, like support in physiotherapy and nutrition, and the interaction of different health professionals were challenging in the strict segregation context of COVID-19 care. Although the critical clinical decisions were always made in agreement by the CF and COVID teams, this hospital stay was quite different from an admission for a standard CF pulmonary exacerbation, and the patient suffered the lack of her usual points of reference.
Declaration of Competing Interest
None.
Acknowledgements
We have no specific funding to report for this case report.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcf.2021.03.024.
Appendix. Supplementary materials
References
- 1.Cosgriff R., et al. A multinational report to characterise SARS-CoV-2 infection in people withcystic fibrosis. J. Cyst.Fibros. 2020;19:355–358. doi: 10.1016/j.jcf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClenaghan E, Cosgriff R, Brownlee K, Ahern S, Burgel P-R, Byrnes C, et al. The global impact of SARS-CoV-2 in 181 people with cystic fibrosis. J Cyst Fibros. 2020 doi: 10.1016/j.jcf.2020.10.003. S1569-1993(20)30877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilchrist FJ, Webb AK, Bright-Thomas RJ, Jones AM. Successful treatment of cepacia syndrome with a combination of intravenous cyclosporin, antibiotics and oral corticosteroids. J Cyst Fibros. 2012;11:458–460. doi: 10.1016/j.jcf.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Wark PA, Tooze M, Cheese L, Whitehead B, Gibson PG, Wark KF, et al. Viral infections trigger exacerbations of cystic fibrosis in adults and children. European Respiratory Journal. 2012;40:510–512. doi: 10.1183/09031936.00202311. [DOI] [PubMed] [Google Scholar]
- 5.Khan T, Wagener JS, Bost T, Martinez TJ, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.