Abstract
Background
Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) vaccines have been approved recently, and public concern regarding the risk of anaphylactic reactions arose after a few cases during the first days of mass vaccination. Polyethylene glycol (PEG) has been suggested as the most probable culprit agent for allergic reactions.
Objective
We describe the allergy work-up protocol implemented for the vaccination campaign in our Center, aiming to allow the greatest number of people to be vaccinated safely.
Methods
The protocol included the self-report of a history of suspected drug or vaccine allergies, and subsequent teleconsultation and allergometric tests for PEG and Polysorbate 80 (PS80). A desensitizing protocol of vaccine administration was applied to patients sensitized only to PS80, and to those with a suspect allergic reaction after the first vaccine dose.
Results
10.2% (414 out of 4042) of the entire vaccine population have been screened: only one patient resulted allergic to PEG and therefore excluded from the vaccination. Another patient was sensitized to PS80 only and safely vaccinated applying the desensitizing protocol. Seven subjects without a previous history of allergic disease experienced suspect hypersensitivity reactions to the first administered dose: one of them resulted allergic to PEG and was excluded from the second dose, while the others safely completed the vaccination with the desensitizing protocol.
Conclusion
A careful allergological risk-assessment protocol significantly reduces the number of patients who would have avoided SARS-CoV-2 vaccination for their allergies and to effectively identify and manage those rare patients with sensitization to PEGs and/or PS80.
Keywords: Allergy, Anaphylaxis, COVID-19, Polyethylen glycole, Vaccine
Introduction
From December 2019 to today, the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection causing Coronavirus-Disease-2019 (COVID-19) has affected over 100 million people worldwide causing over 2 million deaths,1 becoming the largest and most important pandemic of the last hundred years.
Vaccination, throughout history, has proven to be the best viable option for preventing the spread of infectious diseases. For COVID-19, vaccination represents an important hope and perhaps the only way to get out of the pandemic relatively quickly.2 For this reason, more than 200 potential vaccines are currently in development, few of which have been approved already by the regulatory authorities and recently put on the market: an unprecedented effort from the scientific community and the pharmacological industry.3 The first two SARS-CoV-2 vaccines used on a large scale were Pfizer-BionTech's BNT162B24 and Moderna's mRNA-1273,5 both mRNA vaccines.6
Public concern regarding the risk of an anaphylactic reaction to the SARS-CoV-2 vaccine spiked up in early December 2020 when, within the first days of mass vaccination with BNT162b2, two cases of anaphylaxis in the United Kingdom7 and six in the United States8 were reported after the inoculation of the vaccine. These cases have elicited a lot of clamor, prompting the necessity to determine the real prevalence of allergic reactions to the vaccines, especially because no cases of anaphylaxis were reported in randomized clinical trials with the vaccine.4,5
Early data from safety monitoring from the US Center for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) on the Pfizer-BioNTech vaccine have substantially reduced the risk of vaccine-related anaphylaxis, highlighting only 21 cases of systemic hypersensitivity reactions meeting the Brighton Collaboration case definition criteria for anaphylaxis9 out of 1893 360 vaccine doses administered during the 14–23 December 2020 time frame; of these cases, 86% of the patients had symptom onsets in the first 30 min from the administration of the vaccine, and 81% of these patients had a positive history for allergic reactions, particularly to drugs, foods, and insect stings.8 More recently, a similar report has been published about the Moderna vaccine: 108 cases of severe allergic reaction, including 10 cases of anaphylaxis, were identified among 4 041 396 administered doses as of January 10, 2021.10
Allergic reactions to vaccines, including anaphylaxis, are rare events and generally due to sensitization to excipients, adjuvants or other components, rather than the active ingredient (the antigen or, as in the case of the COVID-19 vaccine, the mRNA encoding a specific virus protein).11 A study carried out by McNeil et al from 2009 to 2011 reported 33 confirmed cases of influenza vaccine-triggered anaphylaxis in more than 25 million doses injected,12 confirming the extremely rare occurrence of vaccine-triggered anaphylaxis.
The two main SARS-CoV-2 vaccines that have been approved so far are novel mRNA vaccines encoding the “spike” protein of the virus inside a lipid nanoparticle. As most allergens tend to be proteins, and due to the fact that these vaccines lack proteins in their excipients, the most probable culprit of hypersensitivity reactions could be located inside the lipid particle, and in particular in its components Polyethylene glycol (PEG) 2000 dimyristoyl glycerol (DMG).
PEGs are hydrophilic polymers of different molecular weights that are found as excipients in drugs and everyday non-medicinal products such as cosmetics and food,13 which could explain how patients may undergo sensitization prior to the vaccination.14
Anaphylactic reactions to PEGs have been reported infrequently, however an increasing trend of allergic reactions to certain medications and personal hygiene products that contain PEGs has been observed in previous years,13,15, 16, 17, 18 as far as hypersensitivity reactions to the structurally similar molecule Polysorbate 80 (PS80) which has shown a certain degree of cross-reactivity with PEG.19 Interestingly, PS80 is one of the excipients of other two recently approved SARS-CoV-2 vaccines: the ChAdOx1 by AstraZeneca20 and Ad26.COV2.S by Janssen Vaccines & Prevention.21
As these excipients are present in the SARS-CoV-2 vaccines so far authorized, it is crucial to approach the vaccination with a thorough allergological assessment, especially in patients that have a history of allergic reactions to other vaccines or to some medications and cosmetics.13,22 In this article we describe the allergy work-up protocol implemented in the 2 locations of the IRCCS Humanitas Research Hospital, a large university hospital in Milan (Italy), for the vaccination campaign aimed at healthcare personnel. We have created this protocol with the aim of allowing the greatest number of people to be vaccinated safely, avoiding a reduced vaccination rate of potentially allergic risk subjects for fear of developing a serious allergic reaction.
Methods
Allergological work-up protocol
Together with the invitation to undergo anti-SARS-CoV-2 vaccination, a communication was sent to all the staff of the 2 hospitals involved, inviting them to notify via email to the Allergy Center any previous anaphylaxis, allergic reactions to drugs, and any clinical history of mastocytosis.
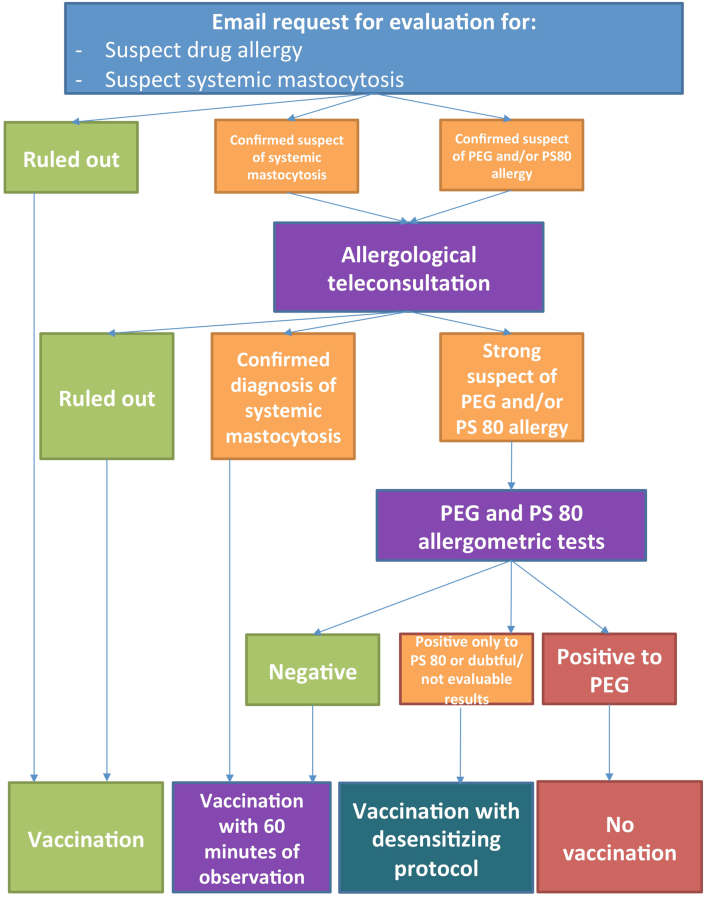
The reported cases were handled by a team of experienced allergists to identify those who had a history consistent with a previous hypersensitivity reaction to drugs containing PEG and/or PS80, or systemic mastocytosis: in these cases the patients were further investigated through a careful allergological history during a teleconsultation. If the outcome of the teleconsultation supported a reasonable suspicion of an allergic reaction to drugs containing PEG or PS80 or of systemic mastocytosis, patients were asked to perform skin allergy tests for the 2 excipients, as recently suggested by Banerji et al22 (see Table 1). Conversely, patients for whom the suspicion of previous allergic reactions to the excipients or the suspicion of mastocytosis was not confirmed, were asked to proceed with the vaccination.
Table 1.
Allergometric tests used for patients with suspect Polyethylen glycol (PEG) and/or Polysorbate 80 (PS80) hypersensitivity, modified from Banerji et al22 We used a cleansing preparation for colon endoscopy product as source of PEG 3350,100 mg/ml.17 Methyl-prednisolone Acetate (Depo-Medrol) 40 mg/ml and Triamcinolone acetonide (Kenacort) 40 mg/ml were used to intradermally test PEG 3350 and PS80 respectively
| Step | Tested drug | Dilution | Cumulative time (min) | |
|---|---|---|---|---|
| 1 | Positive control | Histamine | 1:1 | 0 |
| Negative control | Glycerin | 1:1 | ||
| Prick test | PEG 3350 | 1:100 | ||
| Prick test | Polysorbate 80 | 1:100 | ||
| 2 | Prick test | PEG 3350 | 1:10 | 30 |
| Prick test | Polysorbate 80 | 1:10 | ||
| 3 | Prick test | PEG 3350 | 1:1 | 60 |
| 4 | Intradermal | Methyl-prednisolone Acetate (Depo-Medrol) 40 mg/ml | 1:100 | 90 |
| Intradermal | Triamcinolone acetonide (Kenacort) 40 mg/ml | 1:100 | ||
| 5 | Intradermal | Methyl-prednisolone Acetate (Depo-Medrol) 40 mg/ml | 1:10 | 120 |
| Intradermal | Triamcinolone acetonide (Kenacort) 40 mg/ml | 1:10 | ||
| 6 | Observation | 180 | ||
Based on the outcome of the allergic tests, patients were invited: A) to be vaccinated with a prolonged post-administration observation period of 60 min (in case of allergy test negativity) following the guidelines provided by the main Italian allergological scientific societies (Italian Society of Allergology, Asthma and Clinical Immunology — SIAAIC; Italian Territorial and Hospital Allergologists and Immunologists Association — AAIITO),23 or B) to undergo a dosing protocol vaccine with fractionated doses for desensitizing purposes under the supervision of an allergist and an intensive care specialist (see Table 2) (in case of positive for PS80 or allergic tests doubtful/not evaluable), or C) to not proceed with vaccination (in case of positive allergometric tests for PEG).
Table 2.
Desensitization protocol used for the administration of BNT162B2 in patients resulted positive for Polysorbate 80 or with doubtful/not evaluable allergic tests, and in those who experienced a suspect hypersensitivity reaction to the first dose of vaccine
| Step | Dose (ml) | Cumulative dose (ml) | Cumulative time (min) |
|---|---|---|---|
| 1 | 0.03 | 0.03 | 0 |
| 2 | 0.07 | 0.10 | 30 |
| 3 | 0.10 | 0.20 | 60 |
| 4 | 0.10 | 0.30 | 90 |
| 6 | Observation | 120 |
Patients with confirmed diagnosis of systemic mastocytosis were advised to proceed to the vaccination with a premedication with H1 and H2 antihistamines, 1 h before, and montelukast 10 mg, the day before,24 and with a prolonged post-administration observation period of 60 min after vaccination.
The entire allergological work-up protocol is reported in Fig. 1.
Fig. 1.
Description of the allergological risk-assessment protocol for preventing and managing allergic reaction to SARS-CoV-2 vaccine
Subjects who reported an adverse event compatible with an allergic reaction after the first dose of the SARS-CoV-2 vaccine were also subjected to allergy testing for PEG and PS80 (see Table 1).
All the vaccinations have been performed with BNT162B2.
The entire allergological risk-assessment protocol here described was agreed with the Medical and Health Department and the Clinical Quality Department of the two hospitals, and approved by the internal review board. All the subjects evaluated were informed of the protocol and gave their informed consent.
Results
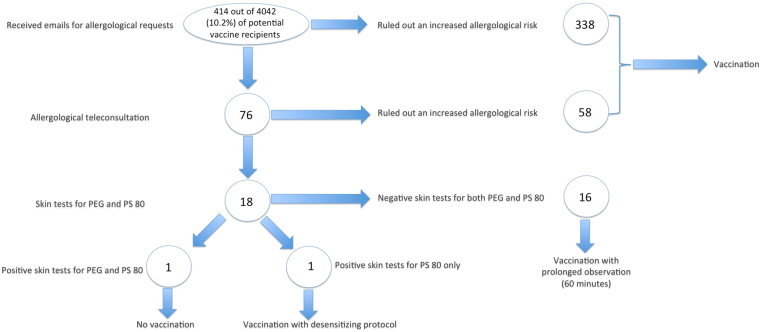
Four-hundred and fourteen emails were received with questions related to allergy risk assessment for SARS-CoV-2 vaccination, corresponding to 10.2% (414 out of 4042) of the entire vaccine population of the 2 hospitals to date. Of these, 338 were managed only via email because they related to allergic problems not related to a greater risk of hypersensitivity reactions to the vaccine, and therefore invited to be vaccinated. Other 76 were instead deemed worthy of further study and patients were invited to carry out an allergological teleconsultation. From the teleconsultations carried out, 18 patients with clinical history compatible with possible hypersensitivity to PEG and/or PS80 were identified and were subjected to allergometric tests; the remaining patients were asked to proceed with vaccination. One patient tested positive for both PEG and PS80 and, therefore, an allergological contraindication to vaccination was issued, while another patient tested positive for PS80 only and underwent a desensitizing vaccination protocol; the remaining 16 patients tested negative in allergic tests and, therefore, were vaccinated with the foresight of a prolonged observation period of 60 min post-administration. The patient treated with the desensitizing protocol tolerated vaccination without any clinical manifestation of hypersensitivity reactions, as all the other screened patients.
No patient with suspect systemic mastocytosis was screened.
The distribution of patients evaluated in the allergy work-up protocol is summarized in Fig. 2.
Fig. 2.
Distribution of patients evaluated in the allergological risk-assessment protocol for preventing and managing allergic reaction to SARS-CoV-2 vaccine
Seven people reported suspected hypersensitivity reactions to the first dose of vaccine (see Table 3); none of these had a previous clinical history suggesting an increased allergological risk for vaccination, and, therefore, were not previously screened in our risk-assessment protocol. Only 1 of them (the only one with a clear immediate reaction) tested positive for both PEG and PS80 allergy tests and, therefore, was excluded from the second administration; all the others received successfully the second dose of the vaccine with the desensitizing scheme (see Table 2) without showing hypersensitivity reactions.
Table 3.
Demographic and clinical characteristics, and allergometric tests outcomes of patients with suspect hypersensitivity reaction after the administration of the first dose of vaccine.
| ID | Sex | Age (ys) | Hypersensitivity symptoms after the first vaccine dose | Time of onset of symptoms | Allergometric tests results for PEG and PS80 | Hypersensitivity symptoms after the second vaccine dose with desensitization protocol |
|---|---|---|---|---|---|---|
| 1 | Male | 27 | Unilateral left eyelid angioedema, spontaneously regressed within 24 h. | 20 h | Negative | None |
| 2 | Female | 31 | Urticaria (wheals localized mainly at lower limbs and face) spontaneously regressed within 48 h. | 11 h | Negative | None |
| 3 | Female | 24 | Bilateral eyelid angioedema and pruritus localized at neck and face; regressed within 48 h by taking Cetirizine 10 mg and Prednisone 25 mg. | 22 h | Negative | None |
| 4 | Female | 46 | Urticaria (wheals localized mainly upper limbs and face) spontaneously regressed within 72 h. | 8.5 h | Negative | None |
| 5 | Female | 38 | Hypotension (90/50 mmHg) not associated with skin rashes or other systemic symptoms. Treated with intravenous Metilprednisolone 40 mg, with regression of symptoms within 30 min. | 20 min | Positive to PEG 1:10 intradermal and PS 80 1:10 intradermal | Second dose not administered |
| 6 | Female | 54 | Unilateral right eyelid and labial angioedema, spontaneously regressed within 24 h. | 24 h | Negative | None |
| 7 | Female | 49 | Widespread itching with no evidence of skin rash, resolved within approximately 10 days with hydroxyzine 25 mg daily. | 20 h | Negative | None |
PEG: Polyethylen glycol; PS80: Polysorbate 80
Discussion
The main result of this study is that the application of an accurate protocol for the identification and management of subjects with potential risk of allergic reactions to the components of the SARS-CoV-2 vaccine can allow potentially at-risk subjects to be vaccinated safely, thus coping at least in part to the vaccine hesitancy of allergy sufferers. Furthermore, our protocol has allowed the identification of those rare patients (one out of 4042 people in our case) really allergic to PEG and for whom vaccination with Pfizer-BionTech and Moderna products would have represented a risk of hypersensitivity reaction, even potentially serious. Both of these results are perfectly in line with what is suggested by the main international scientific societies of allergology and clinical immunology,25,26 and confirm that the allergological risk from SARS-CoV-2 vaccination is extremely low in the face of a relatively widespread allergy-related vaccine hesitation.
As regards the suspected hypersensitivity reactions arising after the administration of the first dose of the vaccine, only 1 had the characteristics of an immediate reaction (although not severe) and exactly that patient tested positive in allergic tests for PEGs; the other subjects experienced mild and delayed symptoms, most likely attributable to non-hypersensitivity adverse events.
Another important result of the present work is the demonstration of the safety of a desensitization protocol specifically designed to be used with this vaccine. This aspect takes on particular importance considering that, to date, the only desensitization protocol for vaccines published relates to the vaccine for Diphteria, Pertussis, Tetannus (DPT)27 and is suggested as an example for any other vaccine;28,29 however, the desensitization protocol for the DPT vaccine involves the administration of a first dose previously diluted 1:10, which is difficult to apply to the SARS-CoV-2 vaccine because it would result in a waste of a portion of the drug which, given the current emergency and the need to carry out a mass vaccination, would be ethically impractical.
The effectiveness of a mass vaccination campaign like the one currently underway for SARS-CoV-2 is closely related to the speed with which as many people as possible are vaccinated. A major obstacle to this process is vaccine hesitation that may be at least in part due to previous reported allergic reactions.30 A careful protocol of identification and management of patients with previous allergic reactions, in particular to drugs, is therefore essential to contribute to the success of the vaccination campaign.25,26
There are objective difficulties in identifying patients with previous allergic reactions to PEGs or PS80, which include the anecdotalism of reactions reported in the scientific literature,13,15, 16, 17, 18, 19 the lack of extracts and standardized allergy test protocols,31 and the difficulty in finding exhaustive lists of drugs containing PEGs and/or PS80 as excipients. Another diagnostic problem is related to the lack of knowledge concerning the level of cross-reactivity between PEGs of different molecular weight and between PEGs and PS80.16,31, 32, 33 The diagnostic scheme we used and recently proposed by Banerji et al22 has the advantage of being practical and of including easily available diagnostic material. Obviously the ideal would be to be able to test the vaccine itself in vivo (and possibly also with serological or functional tests) but, as previously commented on the administration with desensitizing scheme, this would involve an unacceptable waste of part of the vaccine doses. However, despite all these limitations, our allergological work-up protocol also allowed the identification of a patient who was truly sensitized to PEG and PS80, avoiding a very likely allergic reaction for her, and another who was sensitized to PEG and PS80 and had the first hypersensitivity reaction ever after the administration of the first vaccine dose.
In conclusion, our study demonstrates that a careful allergological risk-assessment protocol is able to significantly reduce the number of patients who would have avoided SARS-CoV-2 vaccination and to effectively identify and manage those rare patients with sensitization to PEGs and/or PS80. This also clearly highlights one of the fundamental roles that the allergist has during a pandemic like the one we are experiencing, which is added to the careful management of allergic patients already previously described by our group.34
Abbreviations
AAIITO: Italian Territorial and Hospital Allergologists and Immunologists Association; CDC: Center for Diseases Control; COVID-19: Coronavirus-Disease-2019; DMG: Dimyristoyl glycerol; DPT: Diphteria Pertussis Tetanus; FDA: Food and Drug Administration; PEG: Polyethylene glycol; PS80: Polysorbate 80; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; SIAAIC: Italian Society of Allergology Asthma and Clinical Immunology.
Authors’ contributions
Enrico Heffler concepted the study, contributed in collecting and interpreting data, and contributed in writing the article. Giovanni Paoletti, Francesca Racca and Giorgio Walter Canonica contributed in the conception of the study, collecting and interpreting data, and contributed in writing the article. Francesca Racca, Alessandra Piona and Morena Merigo contributed in collecting and interpreting data. Giulio Melone contributed in interpreting data and writing the article. Francesca Puggioni, Sebastian Ferri, Donatella Lamacchia, Giuseppe Cataldo contributed in interpreting data. Elena Azzolini and Michele Lagioia contributed in the conception of the study and in interpreting data. Maurizio Cecconi contributed in critically interpreting data. All the Authors reviewed the manuscript, revised it critically before submission, and approved the final version of it.
Ethics statement
The protocol here described was agreed with the Medical and Health Department and the Clinical Quality Department of the two hospitals, and approved by the internal review board. All the subjects evaluated were informed of the protocol and gave their informed consent.
Editorial policy confirmation and agreement
All the Authors confirm that they consent to the publication of this article, and agree with the Editorial policy.
Availability of data and materials
Upon request.
Declaration of competing interest
-
-
Giovanni Paoletti does not have any conflict of interest to report.
-
-
Francesca Racca does not have any conflict of interest to report.
-
-
Alessandra Piona does not have any conflict of interest to report.
-
-
Giulio Melone does not have any conflict of interest to report.
-
-
Morena Merigo does not have any conflict of interest to report.
-
-
Francesca Puggioni reports personal fees from Astrazeneca, Chiesi, GSK, Guidotti, Menarini, Mundipharma, Novartis, Sanofi, Valeas, Allergy therapeutics, Almirall outside the submitted work.
-
-
Sebastian Ferri does not have any conflict of interest to report.
-
-
Elena Azzolini does not have any conflict of interest to report.
-
-
Michele Lagioia does not have any conflict of interest to report.
-
-
Donatella Lamacchia does not have any conflict of interest to report.
-
-
Giuseppe Cataldo does not have any conflict of interest to report.
-
-
Maurizio Cecconi does not have any conflict of interest to report.
-
-
Enrico Heffler reports participation to advisory boards and personal fees from AstraZeneca, Sanofi, GSK, Novartis, Circassia, Nestlè Purina, Boheringer Ingheleim, Valeas, Stallergenes Greer outside the submitted work.
-
-
Giorgio Walter Canonica reports grants as well as lecture or advisory board fees from: A. Menarini, Alk-Abello, Allergy Therapeutics, AstraZeneca, Boehringer-Ingelheim, Chiesi Farmaceutici, Genentech, Guidotti-Malesci, Glaxo Smith Kline, Hal Allergy, Mylan, Merck, Mundipharma, Novartis, Regeneron, Sanofi-Aventis, Sanofi-Genzyme, StallergenesGreer, UCB pharma, Uriach Pharma, Valeas, ViborPharma.
Acknowledgments
The Authors thank Ms. Melissa Sansonna for her unvaluable administrative help, and all the nurses of the vaccine service for their support.
Footnotes
Full list of author information is available at the end of the article.
References
- 1.WHO Coronavirus disease (COVID-19) dashboard. https://covid19.who.int
- 2.Cohen J. Shots of hope. Science. 2020;370(6523):1392–1394. doi: 10.1126/science.370.6523.1392. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Coira J., Sokolowska M. SARS-CoV-2 candidate vaccines - composition, mechanisms of action and stages of clinical development. Allergy. 2020 doi: 10.1111/all.14714. [published online ahead of print, 2020 Dec 19] [DOI] [PubMed] [Google Scholar]
- 4.Polack F.P., Thomas S.J., Kitchin N. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baden L.R., El Sahly H.M., Essink B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Confirmation of guidance to vaccination centres on managing allergic reactions following COVID-19 vaccination with the Pfizer/Biontech vaccine. https://www.gov.uk/government/news/confirmation-of-guidance-to-vaccination-centres-on-managing-allergic-reactions-following-covid-19-vaccination-with-the-pfizer-biontech-vaccine
- 8.Shimabukuro T., Nair N. Allergic reactions including anaphylaxis After receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. J Am Med Assoc. 2021 doi: 10.1001/jama.2021.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rüggeberg J.U., Gold M.S., Bayas J.M. Anaphylaxis: case definition and guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine. 2007;25(31):5675–5684. doi: 10.1016/j.vaccine.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 10.Center for Disease Control and Prevention (CDC) - Morbidity and Mortality Weekly Report (MMWR) Allergic reactions including anaphylaxis after receipt of the first dose of moderna COVID-19 vaccine — United States, December 21, 2020–January 10, 2021. 2021. https://www.cdc.gov/mmwr/volumes/70/wr/mm7004e1.htm [DOI] [PMC free article] [PubMed]
- 11.McNeil M.M., DeStefano F. Vaccine-associated hypersensitivity. J Allergy Clin Immunol. 2018;141(2):463–472. doi: 10.1016/j.jaci.2017.12.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNeil M.M., Weintraub E.S., Duffy J. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137(3):868–878. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabanillas B., Akdis C., Novak N. Allergic reactions to the first COVID-19 vaccine: a potential role of Polyethylene glycol? Allergy. 2020 doi: 10.1111/all.14711. [published online ahead of print, 2020 Dec 15. [DOI] [PubMed] [Google Scholar]
- 14.Kelso J.M. Anaphylactic reactions to novel mRNA SARS-CoV-2/COVID-19 vaccines. Vaccine. 2021;39(6):865–867. doi: 10.1016/j.vaccine.2020.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sellaturay P., Nasser S., Ewan P. Polyethylene glycol-induced systemic allergic reactions (anaphylaxis) J Allergy Clin Immunol Pract. 2021;9(2):670–675. doi: 10.1016/j.jaip.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 16.Badiu I., Guida G., Heffler E., Rolla G. Multiple drug allergy due to hypersensitivity to polyethylene glycols of various molecular weights. J Investig Allergol Clin Immunol. 2015;25(5):368–369. [PubMed] [Google Scholar]
- 17.Bommarito L., Mietta S., Nebiolo F., Geuna M., Rolla G. Macrogol hypersensitivity in multiple drug allergy. Ann Allergy Asthma Immunol. 2011;107(6):542–543. doi: 10.1016/j.anai.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Pizzimenti S., Heffler E., Gentilcore E. Macrogol hypersensitivity reactions during cleansing preparation for colon endoscopy. J Allergy Clin Immunol Pract. 2014;2(3):353–354. doi: 10.1016/j.jaip.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Badiu I., Geuna M., Heffler E., Rolla G. Hypersensitivity reaction to human papillomavirus vaccine due to polysorbate 80. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr.02.2012.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramasamy M.N., Minassian A.M., Ewer K.J. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadoff J., Le Gars M., Shukarev G. Interim results of a phase 1-2a trial of Ad26.COV2.S covid-19 vaccine. N Engl J Med. 2021 doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banerji A., Wickner P.G., Saff R. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2020;S2213–2198(20):31411–31412. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.– Italian Society of Allergology, Asthma and Clinical Immunology - SIAAIC; Italian Territorial and Hospital Allergologists and Immunologists Association – AAIITO Guidelines for management of patient at risk of allergic reaction to COVID-19 vaccines. http://www.siaaic.org/?p=5569
- 24.Rama T.A., Moreira A., Castells M. mRNA COVID-19 vaccine is well tolerated in patients with cutaneous and systemic mastocytosis with mast cell activation symptoms and anaphylaxis. J Allergy Clin Immunol. 2021;147(3):877–878. doi: 10.1016/j.jaci.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klimek L., Jutel M., Akdis C.A. ARIA-EAACI statement on severe allergic reactions to COVID-19 vaccines - an EAACI-ARIA position paper. Allergy. 2020 doi: 10.1111/all.14726. [DOI] [PubMed] [Google Scholar]
- 26.Turner P.J., Ansotegui I.J., Campbell D.E. COVID-19 vaccine-associated anaphylaxis: a statement of the world allergy organization anaphylaxis committee. World Allergy Organ J. 2021:100517. doi: 10.1016/j.waojou.2021.100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carey A.B., Meltzer E.O. Diagnosis and "desensitization" in tetanus vaccine hypersensitivity. Ann Allergy. 1992;69(4):336–338. [PubMed] [Google Scholar]
- 28.Kelso J.M., Greenhawt M.J., Li J.T. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130(1):25–43. doi: 10.1016/j.jaci.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson L., Brockow K., Alm J. Vaccination and allergy: EAACI position paper, practical aspects. Pediatr Allergy Immunol. 2017;28(7):628–640. doi: 10.1111/pai.12762. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen K.H., Srivastav A., Razzaghi H. COVID-19 vaccination intent, Perceptions, and reasons for not vaccinating among groups Prioritized for early vaccination — United States, september and december 2020. MMWR Morb Mortal Wkly Rep. 2021;70(6):217–222. doi: 10.15585/mmwr.mm7006e3. ePub: 9 February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wenande E., Garvey L.H. Immediate-type hypersensitivity to polyethylene glycols: a review. Clin Exp Allergy. 2016;46(7):907–922. doi: 10.1111/cea.12760. [DOI] [PubMed] [Google Scholar]
- 32.Yamasuji Y., Higashi Y., Sakanoue M. A case of anaphylaxis caused by polyethylene glycol analogues. Contact Dermatitis. 2013;69(3):183–185. doi: 10.1111/cod.12084. [DOI] [PubMed] [Google Scholar]
- 33.Wenande E., Kroigaard M., Mosbech H., Garvey L.H. Polyethylene glycols (PEG) and related structures: overlooked allergens in the perioperative setting. A A Case Rep. 2015;4(5):61–64. doi: 10.1213/XAA.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 34.Malipiero G., Heffler E., Pelaia C. Allergy clinics in times of the SARS-CoV-2 pandemic: an integrated model. Clin Transl Allergy. 2020;10:23. doi: 10.1186/s13601-020-00333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon request.