Abstract
Vascular damage is aggravated in animal models of hypertension with mineralocorticoid (MR) excess and in hypertensive patients with primary hyperaldosteronism. MR antagonism has shown to provide effective blood pressure (BP)‐control in patients with treatment resistant hypertension (TRH), but the concurrent effects on the vasculature have not been examined. In a randomized, double‐blinded, placebo‐controlled parallel‐group study, 51 patients with TRH received either eplerenone 50 mg or placebo for 6 months together with additional antihypertensives titrated to achieve a BP target of <140/90 mm Hg. Pulse wave velocity (PWV), augmentation index (AIx), augmentation pressure (AP), AP normalized to a heart rate of 75/min (AP@HR75), renal resistive index (RRI), intima‐media thickness (IMT) and urinary albumin excretion rate (UAER) were assessed before and after treatment. PWV was reduced only with eplerenone (from 11.3±3.6 to 9.8±2.6 m/s, P˂.001), but not with placebo (10.3±2.0 to 10.1±1.8 m/s, P=.60), despite similar reductions in BP (−35±20/−15±11 mm Hg vs −30±19/−13±7 mm Hg, n.s.). Further, reductions in AP and AP@HR75 were greater with eplerenone, while changes in AIx, RRI, IMT and UAER were similar. Our data show that eplerenone beneficially affects markers of arterial stiffness and wave reflection in patients with TRH, independently of BP lowering. These data add to the evidence that MR antagonism should be the preferred treatment option in TRH.
Keywords: aldosterone, aortic stiffness, hypertension, pulse wave velocity
1. Introduction
The stiffening of large arteries is, to some degree, a natural process related to the course of ageing. However, this process has been found to be accelerated in patients with cardiovascular risk factors, notably in patients with arterial hypertension, diabetes mellitus, or chronic kidney disease.1, 2, 3 Increased aortic stiffness as determined by the measurement of pulse wave velocity (PWV) has been identified as an independent predictor of poor cardiovascular outcomes in patients with essential hypertension.4, 5
Several in vitro investigations have indicated that aldosterone may directly induce vascular dysfunction, inflammation, and fibrosis in large arteries and, thus, lead to an increase in aortic stiffness.6, 7 This concept has also been supported in humans through a variety of findings: in normotensive patients, short‐term systemic administration of aldosterone results in endothelial dysfunction and vasoconstriction.8, 9 Furthermore, PWV is greater in patients with primary hyperaldosteronism compared with patients with essential hypertension.10 In patients with essential hypertension, increased plasma levels of aldosterone were found to be associated with reduced arterial compliance.11
Drugs that block the mineralocorticoid receptor (MR) are increasingly used to treat patients with treatment‐resistant hypertension (TRH). A post hoc analysis of the Anglo‐Scandinavian Cardiac Outcomes Trial (ASCOT)12 showed that spironolactone at a median dose of 25 mg lowers blood pressure (BP) effectively when used as a fourth‐line drug in TRH. Similarly, in the recently published Addition of Spironolactone in Patients With Resistant Arterial Hypertension (ASPIRANT) and PATHWAY‐2 trials, addition of spironolactone was significantly more effective in achieving BP control in patients with TRH compared with placebo or other BP drugs, respectively.13, 14 Due to limited specificity of spironolactone for the MR and concomitant blockade of androgen receptors, the development of gynecomastia is a common side effect that limits its usefulness. To overcome this issue, eplerenone has been developed with greater selectivity for the MR.15 We hypothesized that MR antagonism with eplerenone would have beneficial effects on vascular parameters beyond BP control in patients with TRH.
2. Material and methods
2.1. Study design
The study was a single‐center, prospective, randomized, double‐blind parallel group study comparing eplerenone 50 mg vs placebo administered on top of other BP‐lowering medications. Randomization was performed by a computer program. Double‐blindness was secured through identical packaging of the study medication by our local pharmacy. The treatment duration was 26 weeks and target office BP was <140/90 mm Hg in both groups. Hence, during the treatment period, addition of other antihypertensive agents was allowed, with the exception of MR antagonists.
2.2. Study population
Fifty‐one patients were recruited by our clinical research competence center in Erlangen‐Nürnberg (www.crc-erlangen.de) for this study. All patients had TRH as defined by office BP ≥140/90 mm Hg despite treatment with at least three antihypertensive agents including one diuretic agent and an angiotensin‐converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB) in the highest tolerable dose. Main exclusion criteria were glomerular filtration rate <60 mL/min/1.73 m2, any type of secondary hypertension, diabetes mellitus, and dyslipidemia. Patients with manifest cardiovascular disease (including previous stroke/transitory ischemic attack, peripheral vascular disease, myocardial infarction, or any revascularization procedure) were also excluded from participation. Before enrollment in the study, written informed consent was obtained from each participant. The study protocol was approved by the institutional review board of the University of Erlangen‐Nürnberg. The study was performed in adherence to the principles of the Declaration of Helsinki and according to good clinical practice standards. The study was registered with ClinicalTrials.gov (NCT00138944).
2.3. BP measurement
A standardized sphygmomanometer was used for office BP measurements with the cuff size adjusted to the patient's arm circumference. Office BP was the mean of three consecutive measurements with the patient seated for 5 minutes, and 24‐hour ambulatory BP was performed by an automatic portable device (Spacelab No. 90207, Redmond, CA, USA). Measurement intervals were 15 minutes during the day (7 am to 10 pm) and 30 minutes during nighttime.
2.4. Pulse wave velocity
Carotid‐femoral PWV was determined using a SphygmoCor device (AtCor Medical, Sydney, NSW, Australia). Pulse waveforms of the common carotid artery and the femoral artery were obtained sequentially and PWV was calculated as the distance between the suprasternal notch and the femoral artery recording site, divided by the time interval between the feet of the flow waves. Previous studies have found PWV to be highly reproducible.16, 17
2.5. Pulse wave analysis
Radial artery waveforms were sampled by a noninvasive technique with the commercially available SphygmoCor System and calibrated to the brachial mean and diastolic BP of the same arm. Radial artery waveforms were recorded using high‐fidelity applanation tonometry (Millar Instruments, Houston, TX, USA). Corresponding central (aortic) waveforms were then automatically generated from the radial artery waveform by a validated transfer function. From the derived central waveforms, data on augmentation pressure (AP), augmentation pressure normalized to a heart rate of 75 beats per minute (AP@HR75), and on central augmentation index (AIx), defined as the pressure difference between these peaks, expressed as a percentage of central PP, are given. PWA has been found to be highly reproducible in previous studies.16, 17
2.6. Measurement of the IMT
Intima‐media thickness (IMT) measurements of the right and left common carotid artery were obtained from the far walls according to the Mannheim carotid intima‐media thickness consensus, using a Siemens G60S ultrasound machine with 10 MHz linear ultrasound transducer (Siemens Healthcare, Erlangen, Germany).18 IMT was found to be reproducible in previous studies.17, 19
2.7. Ultrasonographic determination of the RRI
The B‐mode measurements and the Doppler measurements of the renal resistive index (RRI) were performed using a Siemens G60S ultrasound machine with a 3.5‐MHZ sector transducer. Patients were placed in a supine position and both the right and the left kidneys were evaluated for morphologic criteria in order to exclude patients with any difference in size or morphology between kidneys. A lobular artery was located in the upper, middle, and lower third of the kidney using color flow imaging. Three measurements of maximum systolic blood flow velocity and minimum diastolic blood flow velocity were recorded on each kidney. The dimensionless RRI was calculated using the formula:
For analysis, RRI values from the six measurements were averaged. All ultrasonographic measurements were performed by the same examiners (TKS, BMWS). The repeatability of this method was found to be good in previous studies.17
2.8. Urinary albumin excretion rate
Urine was collected over 24 hours to measure urinary albumin excretion rate. Urine samples that contained <15 mg/kg body weight of creatinine over 24 hours were excluded because of assumed collecting error. Urinary albumin excretion was determined by applying the standard laboratory method of nephelometry.
2.9. Statistical analysis
All statistical analyses were performed using SPSS software package (SPSS for Windows 22.0, SPSS Inc, Chicago, IL, USA). Chi‐square test was used to compare categorical variables. Student t test for paired samples was used for comparisons of normally distributed parameters before and after treatment. Student t test for unpaired samples was used for the comparison of treatment effects between the eplerenone and placebo groups. In addition, two‐way analysis of variance was used to compare the effects of group allocation (eplerenone vs placebo), time (before vs after therapy), and their interaction (group*time). The level of significance was set to P<.05 (two‐tailed) for the primary end point PWV. All other parameters were evaluated in an explorative way and therefore no correction for multiple testing was applied. All values are given as mean±standard deviation unless noted otherwise. Linear regression was performed to assess the effects of potential covariates on the reduction of PWV during the treatment phase. All explanatory variables were entered simultaneously into the model.
3. Results
3.1. Demographic data
Demographic data are shown in Table 1. The treatment groups were comparable with regard to sex distribution, body mass index (BMI), and duration of hypertension. There was a slight but significant difference in age, as patients randomized to eplerenone were slightly older than patients randomized to placebo. The number of antihypertensive drugs was similar between groups at baseline except for a greater number of patients taking α‐blockers in the group allocated to placebo. During the treatment phase, which aimed at a reduction of BP <140/90 mm Hg (and which allowed the addition of antihypertensive agents other than MR antagonists in both groups), the number of antihypertensive agents did not change in the patients allocated to eplerenone (P=.71 by paired t test), while there was an increase in the number of antihypertensive agents in the placebo group (P=.01 by paired t test). As a result, the number of antihypertensive agents was slightly greater in the placebo group at the end of the treatment phase, as was the number of α‐blockers and calcium channel blockers (Table 2, please note that the study medication, ie, eplerenone or placebo, was not counted towards the number of antihypertensive agents in this table).
Table 1.
Demographics of Study Participants
| Eplerenone | Placebo | P Value | |
|---|---|---|---|
| Age, y | 62.2±6.9 | 57.7±7.8 | .04 |
| Men/women | 19/6 | 22/4 | .44 |
| BMI, kg/m² | 29.3±4.6 | 28.1±3.2 | .28 |
| Duration of hypertension, mo | 214±146 | 166±158 | .27 |
| 24 h‐ambulatory BP, mm Hg | 143±13/82±12 | 143±12/86±8 | .99 |
| No. of antihypertensives | 3.8±0.6 | 3.9±0.8 | .89 |
| ACEIs, % | 48 | 54 | .68 |
| ARBs, % | 64 | 50 | .32 |
| Diuretics, % | 100 | 100 | 1.00 |
| Calcium antagonists, % | 56 | 65 | .50 |
| ß‐Blockers, % | 76 | 62 | .28 |
| Sympatholytics, % | 32 | 23 | .48 |
| α‐Blockers, % | 4 | 31 | .01 |
Abbreviations: ACEIs, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; BMI, body mass index; BP, blood pressure.
Table 2.
Antihypertensive Treatment at the End of the Study
| Eplerenone | Placebo | P Value | |
|---|---|---|---|
| No. of antihypertensives | 3.8±0.7 | 4.5±1.3 | .02 |
| ACEIs, % | 40 | 46 | .69 |
| ARBs, % | 68 | 71 | .89 |
| Diuretics, % | 100 | 100 | 1.00 |
| Calcium antagonists, % | 60 | 88 | .03 |
| ß‐Blockers, % | 76 | 63 | .32 |
| Sympatholytics, % | 28 | 50 | .21 |
| α‐Blockers, % | 04 | 25 | .04 |
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers.
3.2. Laboratory assessments
Serum potassium values did not change in any of the two groups (eplerenone group: 4.2±0.4 mmol/L at baseline vs 4.2 mmol/L after treatment, P=.983; placebo group: 4.1±0.5 mmol/L at baseline vs 4.0±0.5 mmol/L after treatment, P=.337). Further, serum creatinine concentration did not change in any of the groups (eplerenone group: 0.9±0.2 mg/dL at baseline vs 0.9±0.2 mg/dL after treatment, P=.270; placebo group: 0.9±0.2 mg/dL at baseline vs 0.9±0.2 mg/dL after treatment, P=.493).
3.3. Blood pressure
Office BP at enrollment was 166±21/91±15 mm Hg in the eplerenone and 159±19/94±8 mm Hg in the placebo group (P=.23 for systolic and P=.47 for diastolic BP). 24‐hour ambulatory BP was also similar between the two groups: 143±13/82±12 mm Hg vs. 143±12/86±8 mm Hg (P=.99 and P=.21). Intensification of BP treatment in the group allocated to eplerenone resulted in a decrease in office BP of −35±20/−15±11 mm Hg. Intensification of BP treatment in patients allocated to placebo (but with discretionary adjustments of BP therapy other than MR antagonists) resulted in an office BP decrease of −30±19/−13±7 mm Hg. In both groups, the reductions in office BP were significant, but there was no interaction of the response with group allocation (P group*time=.3156 for office SBP [Figure 1A], P group*time=.4544 for office DBP [Figure 1B]). Target BP (<140/90 mm Hg) was achieved in 46% of patients randomized to eplerenone and in 54% in the placebo group (not significant, P=.21). Reductions in 24 hour BP were also significant, but, again, there was no difference between the two treatment groups (P group*time=.1330 for SBP, P group*time=.3318 for DBP).
Figure 1.
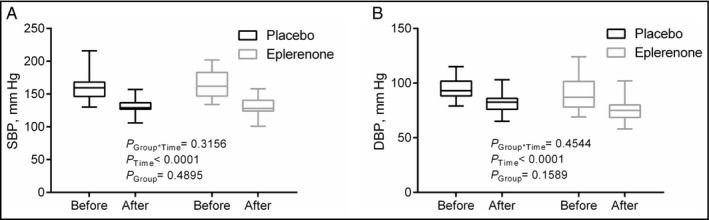
Office systolic blood pressure (SBP; A) and diastolic blood pressure (DBP; B) before and after therapy with placebo or eplerenone
3.4. Vascular markers
PWV at baseline was 11.3±3.6 m/s in the eplerenone group and 10.3±2.0 m/s in the placebo group (P=.81). In the eplerenone group, PWV decreased to 9.8±2.6 m/s (P<.001). In the placebo group, there was no significant change of PWV 10.1±1.8 m/s (P=.60). There was a significant interaction between group allocation (eplerenone vs placebo) and treatment effect over time (P group*time=.0270, Figure 2A). To study the relative role of BP reduction vs group allocation for the change in PWV during treatment, we performed linear regression analyses (Table 3). Only group allocation was identified as an independent explanatory variable of PWV change (P=.017).
Figure 2.
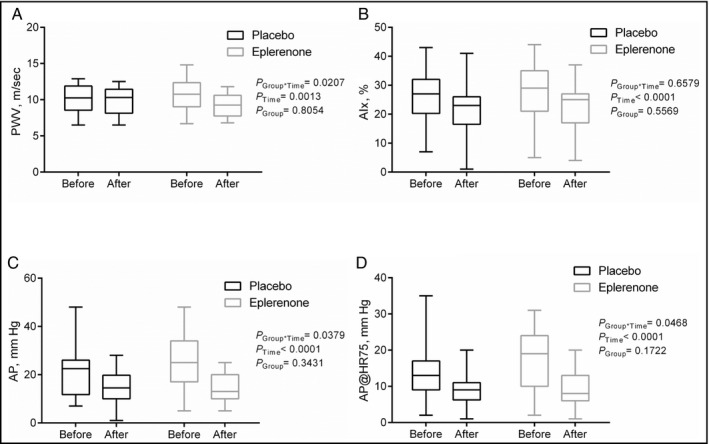
Pulse wave velocity (PWV; A), augmentation index (AIx; B), augmentation pressure (AP; C), and augmentation pressure normalized to a heart rate of 75 beats per minute (AP@HR75; D) before and after therapy with placebo or eplerenone
Table 3.
Linear Regression Model for Explanation of the Change in PWV
| Parameter | R 2=.219, Corrected R 2=.164, P=.031 | |
|---|---|---|
| Standard β Value | P Value | |
| Office SBP reduction | .143 | .402 |
| Group (eplerenone vs placebo) | −.426 | .017 |
Abbreviations: PWV, pulse wave velocity; SBP, systolic blood pressure.
AIx was reduced in both groups after treatment, but there was no interaction between group allocation and treatment effect (P group*time=.6579 [Figure 2B]). AP and AP@HR75 were also reduced in both groups after treatment. Again, there was an interaction between group allocation and treatment effect on AP and AP@HR75 (P group*time=.0379 [Figure 2C] and P group*time=.0468 [Figure 2D], respectively). Other related parameters derived from PWA are presented in Table 4.
Table 4.
Central Aortic Parameters
| Eplerenone | Placebo | |||||
|---|---|---|---|---|---|---|
| Before | After | P Value | Before | After | P Value | |
| Heart rate, beats per min | 57±9 | 58±11 | .337 | 56±9 | 55±7 | .433 |
| pSBP, mm Hg | 167±26 | 131±12 | <.001 | 159±17 | 136±14 | <.001 |
| pDBP, mm Hg | 90±16 | 78±10 | <.001 | 93±9 | 85±9 | <.001 |
| pPP, mm Hg | 77±21 | 53±10 | <.001 | 66±15 | 52±13 | <.001 |
| pMP, mm Hg | 117±18 | 96±10 | <.001 | 117±11 | 103±9 | <.001 |
| cSBP, mm Hg | 159±26 | 123±12 | <.001 | 151±18 | 129±14 | <.001 |
| cDBP, mm Hg | 91±16 | 79±10 | <.001 | 94±9 | 85±9 | <.001 |
| cPP, mm Hg | 68±20 | 45±10 | <.001 | 57±16 | 44±12 | <.001 |
Abbreviations: cDBP, central diastolic blood pressure; cPP, central pulse pressure; cSBP, central systolic blood pressure; pDBP, peripheral diastolic blood pressure; pMP, peripheral mean pressure; pPP, peripheral pulse pressure; pSBP, peripheral systolic blood pressure.
In contrast, none of the other parameters of vascular damage were affected disparately between treatment groups (Figure 3A for IMT of the right common carotid artery, Figure 3B for IMT of the left common carotid artery, Figure 3C for RRI of the right kidney, and Figure 3D for RRI of the left kidney). Further, no change was noted in urine albumin‐to‐creatinine ratio in either of the two groups (eplerenone: 15±19 mg at baseline vs 10±15 mg after treatment, P=.349; placebo: 11±8 mg at baseline vs 11±15 mg after treatment, P=.983).
Figure 3.
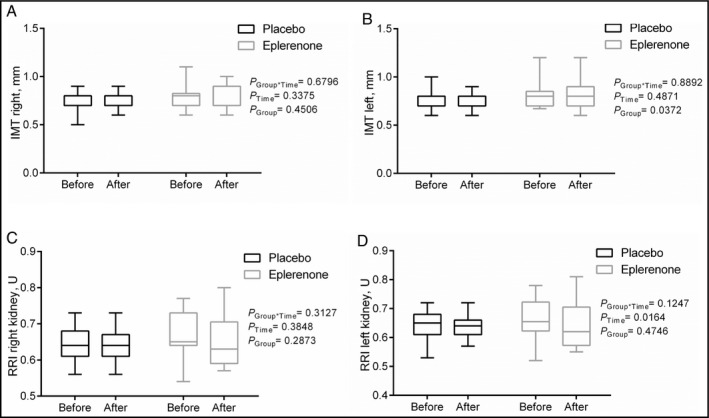
Intima‐media thickness (IMT) of the right (A) and left (B) common carotid artery and renal resistive index (RRI) of the right (A) and left (B) kidney
4. Discussion
In this double‐blind randomized clinical trial we demonstrated that several vascular markers were reduced in TRH by MR antagonism with low‐dose eplerenone. This was observed when eplerenone was added to established treatment with ACEIs or ARBs in patients, and this effect occurred independent of BP. This is of explicit clinical importance since increased arterial stiffness is associated with cardiovascular morbidity and mortality in hypertensive patients,4, 5 and improvement in arterial stiffness is associated with improved cardiovascular outcome.20
5. Study Limitations
Our findings are in line with several previous studies investigating the effects of spironolactone, such as the study by Davies and colleagues.21 In that randomized, double‐blinded crossover study, the investigators showed a significant reduction in PWV by application of spironolactone over 4 months in diabetic hypertensive patients.21 A major limitation of that study was the small sample size (10 patients). A similar benefit of MR antagonism was also observed in patients with chronic kidney disease.18 In patients with chronic kidney disease, the renin‐angiotensin‐aldosterone system plays a crucial role in the development of left ventricular hypertrophy and vascular stiffness.22 The Chronic Renal Impairment in Birmingham II (CRIB‐II) trial therefore investigated the effect of the addition of spironolactone to ACEIs or ARBs in 112 patients with early chronic kidney disease. The additional use of an aldosterone antagonist was effective in improving arterial stiffness in these patients.23 In our cohort, by chance, patients in the eplerenone group were slightly older than in the placebo group. This even more highlights the positive findings with eplerenone treatment, as age is an important risk factor for impaired PWV.24
There are also some studies on eplerenone. The study by Eguchi and colleagues was able to demonstrate that eplerenone was more effective than placebo in patients with TRH, especially regarding reduction of home and ambulatory awake BP.25 In addition to the BP reduction, this study also demonstrated an improvement in flow‐mediated vasodilation.25 Similarly, in a prospective, randomized study in 16 hypertensive patients, Savoia and colleagues26 found that eplerenone (compared with atenolol) was associated with reduced vascular stiffness and circulating inflammatory markers. Finally, White and colleagues27 studied the effects of eplerenone vs amlodipine on BP and PWV in patients with systolic hypertension over a treatment duration of 24 weeks. Eplerenone was as effective as amlodipine in the reduction of systolic BP, pulse pressure, and PWV. However, microalbuminuria was reduced to a greater extent with eplerenone than with amlodipine.
Our results are in contrast with some other reports.28, 29, 30 In those studies, the investigators did not find a beneficial effect on vascular stiffness with the administration of eplerenone. These studies, however, had several limitations including small sample size28, 29, 30 or a relatively short‐term treatment period of only 1 month.28, 29 It could be argued that it might take more than 1 month of treatment to detect the beneficial effects of MR blockade on vascular stiffness. Furthermore, patients in these studies had serious comorbidities and thus the results cannot be compared with our hypertensive study group without severe comorbidities.
How can we explain the BP‐lowering effects in the placebo group? In our study, all patients received ACEIs or ARBs and diuretics together with a third drug at baseline. To achieve target BP <140/90 mm Hg in both groups, forced titration of already prescribed agents as well as prescription of additional antihypertensive agents except for the use of MR antagonists was allowed, according to the discretion of the physician. At the end of study, the number of antihypertensive agents other than eplerenone or placebo was greater in the placebo group (Table 2).
Our other end points, IMT, RRI, and urinary albumin excretion rate have also been suggested as markers of target organ damage in TRH.31, 32, 33 However, we could not detect any beneficial effects of eplerenone on these other markers of vascular or renal injury. With regard to IMT, it is possible that even a treatment duration of 26 weeks is not long enough to observe a significant reduction in this parameter.
6. Conclusions
In patients with resistant hypertension, intensification of BP treatment including use of eplerenone resulted in an improvement in markers of arterial stiffness and arterial wave reflection that appeared unrelated to the BP reduction. Since these markers have been related to worse cardiovascular outcomes,5, 34 our data add to the evidence that supports the preferential use of MR antagonists in TRH.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG; KFO 106, TP5) to PD Dr Schmidt and Professor Dr Schmieder. All authors disclose any significant financial arrangement with commercial companies that produce or sell products that are the subject of studies reported in the manuscript, or with competitors of such companies.
Kalizki T, Schmidt BMW, Raff U, et al. Low dose‐eplerenone treatment decreases aortic stiffness in patients with resistant hypertension. J Clin Hypertens. 2017; 19:669–676 10.1111/jch.12986
The present work was performed in fulfillment of the requirements for obtaining the degree “Dr. med.” for Tatjana Kalizki.
References
- 1. Salomaa V, Riley W, Kark JD, Nardo C, Folsom AR. Non‐insulin‐dependent diabetes mellitus and fasting glucose and insulin concentrations are associated with arterial stiffness indexes. The ARIC Study. Atherosclerosis Risk in Communities Study. Circulation. 1995;91:1432–1443. [DOI] [PubMed] [Google Scholar]
- 2. London GM, Marchais SJ, Guerin AP. Arterial stiffness and function in end‐stage renal disease. Adv Chronic Kidney Dis. 2004;11:202–209. [DOI] [PubMed] [Google Scholar]
- 3. Kaess BM, Rong J, Larson MG, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–1117. [DOI] [PubMed] [Google Scholar]
- 5. Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all‐cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. [DOI] [PubMed] [Google Scholar]
- 6. Rocha R, Rudolph AE, Frierdich GE, et al. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2002;283:H1802–H1810. [DOI] [PubMed] [Google Scholar]
- 7. Rocha R, Chander PN, Khanna K, Zuckerman A, Stier CT Jr. Mineralocorticoid blockade reduces vascular injury in stroke‐prone hypertensive rats. Hypertension. 1998;31:451–458. [DOI] [PubMed] [Google Scholar]
- 8. Farquharson CA, Struthers AD. Aldosterone induces acute endothelial dysfunction in vivo in humans: evidence for an aldosterone‐induced vasculopathy. Clin Sci. 2002;103:425–431. [DOI] [PubMed] [Google Scholar]
- 9. Schmidt BM, Oehmer S, Delles C, et al. Rapid nongenomic effects of aldosterone on human forearm vasculature. Hypertension. 2003;42:156–160. [DOI] [PubMed] [Google Scholar]
- 10. Bernini G, Galetta F, Franzoni F, et al. Arterial stiffness, intima‐media thickness and carotid artery fibrosis in patients with primary aldosteronism. J Hypertens. 2008;26:2399–2405. [DOI] [PubMed] [Google Scholar]
- 11. Blacher J, Amah G, Girerd X, et al. Association between increased plasma levels of aldosterone and decreased systemic arterial compliance in subjects with essential hypertension. Am J Hypertens. 1997;10:1326–1334. [DOI] [PubMed] [Google Scholar]
- 12. Chapman N, Dobson J, Wilson S, et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007;49:839–845. [DOI] [PubMed] [Google Scholar]
- 13. Vaclavik J, Sedlak R, Plachy M, et al. Addition of spironolactone in patients with resistant arterial hypertension (ASPIRANT): a randomized, double‐blind, placebo‐controlled trial. Hypertension. 2011;57:1069–1075. [DOI] [PubMed] [Google Scholar]
- 14. Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug‐resistant hypertension (PATHWAY‐2): a randomised, double‐blind, crossover trial. Lancet. 2015;386:2059–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Colussi G, Catena C, Sechi LA. Spironolactone, eplerenone and the new aldosterone blockers in endocrine and primary hypertension. J Hypertens. 2013;31:3–15. [DOI] [PubMed] [Google Scholar]
- 16. Wilkinson IB, Fuchs SA, Jansen IM, et al. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16:2079–2084. [DOI] [PubMed] [Google Scholar]
- 17. Liang YL, Teede H, Kotsopoulos D, et al. Non‐invasive measurements of arterial structure and function: repeatability, interrelationships and trial sample size. Clin Sci. 1998;95:669–679. [DOI] [PubMed] [Google Scholar]
- 18. Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima‐media thickness consensus (2004‐2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23:75–80. [DOI] [PubMed] [Google Scholar]
- 19. Lau KH, Fung YK, Cheung YT, Tsang WK, Ying M. Repeatability and reproducibility of ultrasonographic measurement of carotid intima thickness. J Clin Ultrasound. 2012;40:79–84. [DOI] [PubMed] [Google Scholar]
- 20. London GM, Pannier B, Guerin AP, et al. Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow‐up of an interventional study. J Am Soc Nephrol. 2001;12:2759–2767. [DOI] [PubMed] [Google Scholar]
- 21. Davies J, Gavin A, Band M, Morris A, Struthers A. Spironolactone reduces brachial pulse wave velocity and PIIINP levels in hypertensive diabetic patients. Br J Clin Pharmacol. 2005;59:520–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin‐angiotensin‐aldosterone system. Circulation. 1991;83:1849–1865. [DOI] [PubMed] [Google Scholar]
- 23. Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early‐stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009;54:505–512. [DOI] [PubMed] [Google Scholar]
- 24. Mitchell GF, Parise H, Benjamin EJ, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. [DOI] [PubMed] [Google Scholar]
- 25. Eguchi K, Kabutoya T, Hoshide S, Ishikawa S, Kario K. Add‐on use of eplerenone is effective for lowering home and ambulatory blood pressure in drug‐resistant hypertension. J Clin Hypertens (Greenwich). 2016;18:1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Savoia C, Touyz RM, Amiri F, Schiffrin EL. Selective mineralocorticoid receptor blocker eplerenone reduces resistance artery stiffness in hypertensive patients. Hypertension. 2008;51:432–439. [DOI] [PubMed] [Google Scholar]
- 27. White WB, Duprez D, St Hillaire R, et al. Effects of the selective aldosterone blocker eplerenone versus the calcium antagonist amlodipine in systolic hypertension. Hypertension. 2003;41:1021–1026. [DOI] [PubMed] [Google Scholar]
- 28. Hwang MH, Yoo JK, Luttrell M, et al. Role of mineralocorticoid receptors in arterial stiffness in human aging. Exp Gerontol. 2013;48:701–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hwang MH, Yoo JK, Luttrell M, et al. Mineralocorticoid receptors modulate vascular endothelial function in human obesity. Clin Sci. 2013;125:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boesby L, Elung‐Jensen T, Strandgaard S, Kamper AL. Eplerenone attenuates pulse wave reflection in chronic kidney disease stage 3‐4–a randomized controlled study. PLoS One. 2013;8:e64549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hering D, Lambert EA, Marusic P, et al. Renal nerve ablation reduces augmentation index in patients with resistant hypertension. J Hypertens. 2013;31:1893–1900. [DOI] [PubMed] [Google Scholar]
- 32. Lotufo PA, Pereira AC, Vasconcellos PS, Santos IS, Mill JG, Bensenor IM. Resistant hypertension: risk factors, subclinical atherosclerosis, and comorbidities among adults‐the Brazilian Longitudinal Study of Adult Health (ELSA‐Brasil). J Clin Hypertens (Greenwich). 2015;17:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raff U, Schmidt BM, Schwab J, et al. Renal resistive index in addition to low‐grade albuminuria complements screening for target organ damage in therapy‐resistant hypertension. J Hypertens. 2010;28:608–614. [DOI] [PubMed] [Google Scholar]
- 34. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness: a systematic review and meta‐analysis. J Am Coll Cardiol. 2010;55:1318–1327. [DOI] [PubMed] [Google Scholar]


