Abstract
Thyroid hormones (THs) have profound effects on cardiovascular functions, suggesting that THs may contribute to the development of elevated blood pressure (BP). Few studies, however, have systematically assessed the relationship between THs and elevated BP. We therefore conducted a cross‐sectional study to examine how serum THs concentrations are related to the prevalence of elevated BP in a euthyroid population. This study (n = 12 487) was performed in Tianjin, China. Serum free triiodothyronine (FT3), free thyroxine (FT4), and thyroid‐stimulating hormone (TSH) levels were measured by chemiluminescence immunoassay. Elevated BP was defined according to the JNC 8 criteria. Analysis of covariance and multiple logistic regression models were used to assess the relationships between FT3, FT4, and TSH quartiles and elevated BP. The multivariable‐adjusted odds ratios (95% confidence interval) of elevated BP for gradual increase in the FT3, FT4, and TSH quartiles, when compared to the lowest quartiles were 1.08 (0.97, 1.21), 1.24 (1.12, 1.39), and 1.32 (1.18, 1.47); 1.18 (1.06, 1.32), 1.18 (1.06, 1.31), and 1.24 (1.11, 1.38); 1.06 (0.96, 1.19), 1.06 (0.95, 1.18), and 1.03 (0.93, 1.15), respectively. Our study demonstrated that FT3 and FT4 are positively related to the prevalence of elevated BP in euthyroid adults, but no significant relationship was found between TSH and elevated BP.
Keywords: elevated blood pressure, epidemiology, serum free thyroid hormones, thyroid function, thyroid‐stimulating hormone
1. INTRODUCTION
Elevated blood pressure (BP) was defined as a systolic blood pressure (SBP) of 120‐139 mmHg and/or a diastolic blood pressure (DBP) of 80‐89 mmHg by the eighth report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 8).1 The global prevalence of elevated BP is rapidly increasing due to an aging population, urbanization, and associated lifestyle changes. The National Health and Nutrition Examination Survey reported that an estimated 31% of adults were suffering from elevated BP in the United States in 1999‐2000.2 A large‐scale population survey about Chinese from 2007 to 2011 reported that the prevalence of elevated BP was 36.4% (41.1% in males and 33.2% in females).3 On average, every 4 years, 19% of people with elevated BP progresses to clinical hypertension4 which contributes to the burden of heart disease, stroke and kidney failure and premature mortality and disability.5 The identification of potential risk factors is an essential step in the prevention and control of elevated BP.
Various clinical and experimental studies have suggested that thyroid hormones (THs) may directly and indirectly influence BP as described below: Firstly, THs have profound effects on the cardiovascular system, such as systemic vascular resistance, resting heart rate, left ventricular contractility, and blood volume.6, 7 Secondly, in endothelial cells, THs can activate endothelial NO synthase through the PI3K/Akt pathway and affect endothelial function.8, 9 Meanwhile, in euthyroidism, hyperthyroidism, or hypothyroidism, researchers also found that THs were closely related to the endothelial function.10, 11, 12 Finally, THs were found to positively regulate cardiac myocyte β1‐adrenergic receptors in rat experiments13 and activate the renin‐angiotensin‐aldosterone system.14
To date, numerous clinic‐based studies were all mainly focused on the relationships between subclinical and overt hyperthyroidism/hypothyroidism and hypertension.15, 16, 17, 18 Moreover, several studies have analyzed the relationships between THs, thyroid‐stimulating hormone (TSH), and hypertension in euthyroid adults.19, 20, 21, 22, 23 However, to the best of our knowledge, only a small‐scale study (n = 2282) assessed the relationships between free thyroxine (FT4), TSH levels and elevated BP in euthyroid subjects.24 Therefore, it is still unclear how THs are related to elevated BP in euthyroid subjects. Accordingly, the aim of the present study is to evaluate whether serum free triiodothyronine (FT3), FT4 concentrations within the reference range as well as TSH levels are related to elevated BP among a large‐scale adult population with euthyroid status.
2. MATERIALS AND METHODS
2.1. Participants
A large prospective dynamic cohort study, which is called The Tianjin Chronic Low‐grade Systemic Inflammation and Health (TCLSIH or TCLSIHealth) Cohort Study, is carried out in a general adult population living in Tianjin, China. Participants, who had received health examinations, and had completed questionnaires regarding their smoking and drinking habits and disease history over the course of January 2007 to December 2016, were recruited. Moreover, a detailed lifestyle questionnaire was administered to randomly selected subjects from this population since May 2013.25
The present study used data from the TCLSIHealth, ranging from the year 2013 to 2016. The participant selection process was described in Figure 1. During the research period, there were 22 971 participants who had received at least one health examination including BP, THs, and TSH tests, agreed to participate, and provided written informed consent for their data to be analyzed. The first health examination data were included in the final analysis. We excluded those with a history of cardiovascular disease (CVD; n = 1478), or cancer (n = 345), or hypertension (n = 6230). Moreover, participants who having a level exceeding the standard reference range of THs and/or TSH (free T3 [FT3] <3.5 pmol/L [n = 46] or >6.5 pmol/L [n = 303], free T4 [FT4] <11.5 pmol/L [n = 158] or >22.7 pmol/L [n = 101], TSH <0.55 mIU/L [n = 299] or >4.78 mIU/L [n = 1187]) were excluded. We also excluded those participants who have a history of thyroid diseases and/or use of anti‐thyroid drugs (amiodarone, methimazole, etc) and other drugs which may affect thyroid function (steroids, hormone replacement therapy, anti‐epilepsy drugs, and non‐steroidal anti‐inflammatory drug, etc; n = 337). Participants with more than one exclusionary criterion were counted only once. Moreover, in this study, CVD was defined as a composite of coronary heart disease (coronary death, myocardial infarction, coronary insufficiency, and angina), cerebrovascular events (ischemic stroke, hemorrhagic stroke, and transient ischemic attack), peripheral artery disease (intermittent claudication), and heart failure.26 Participants who take anti‐diabetic drugs and lipid‐lowering drugs were not excluded from this study. Owing to these exclusions, the final cross‐sectional study population comprised 12 487 participants. The protocol of this study was approved by the Institutional Review Board of the Tianjin Medical University.
Figure 1.
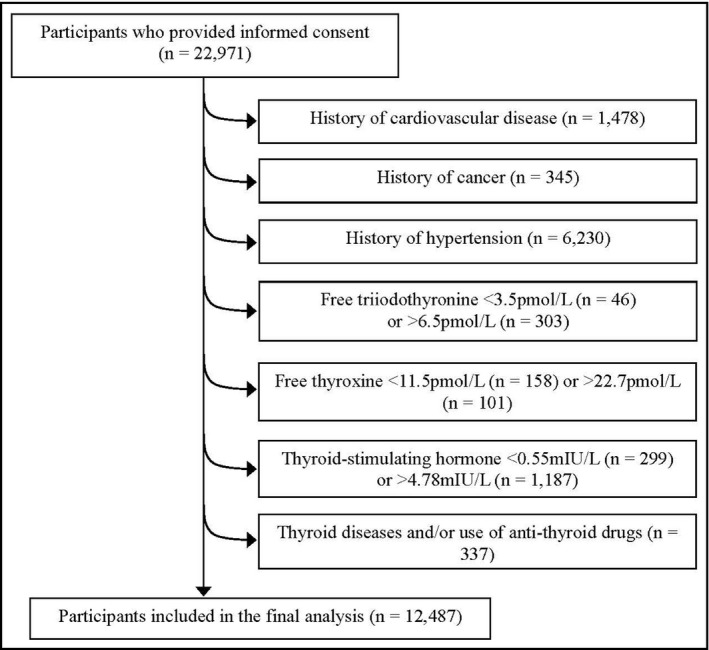
Flow diagram showing the selection of the study population
2.2. Assessment of BP
BP was measured twice from the upper right arm using an automatic device (KD598, Andon, Tianjin, China) after 5 minutes of rest in a seated position, and the mean of these two measurements was taken as the BP value.
2.3. Assessment of FT3, FT4 and TSH
Serum FT3 and FT4 were measured by chemiluminescence immunoassay using ADVIA Centaur FT3 analyzer and ADVIA Centaur FT4 analyzer (Siemens Healthcare Diagnostics, New York, NY), and expressed as pmol/L. The measuring range of FT3 and FT4 were 0.3‐30.8 pmol/L and 1.3‐155.0 pmol/L, respectively. Serum TSH was measured by chemiluminescence immunoassay using ADVIA Centaur TSH3‐Ultra analyzer (Siemens Healthcare Diagnostics), and expressed as mIU/L. The measuring range was 0.001‐150 mIU/L. The reference ranges of FT3, FT4, and TSH were 3.50 ~ 6.50 pmol/L, 11.50 ~ 22.70 pmol/L, and 0.55 ~ 4.78 mIU/L, respectively. Because previous studies have reported that sex‐specific difference was observed on the levels of THs, TSH,27 we divided participants into four categories (quartiles) according to FT3, FT4, and TSH concentrations by sex, and the first quartile for TSH, FT4, and FT3 was the reference.
2.4. Assessment of other variables
Levels of fasting blood glucose (FBG) were measured by glucose oxidase method. As for lipids, triglycerides (TG) and total cholesterol (TC) were measured by enzymatic methods. Low‐density lipoprotein (LDL) was measured by the polyvinyl sulfuric acid precipitation method, and high‐density lipoprotein (HDL) was measured by the chemical precipitation method using appropriate kits on a Cobas 8000 analyzer (Roche, Mannheim, Germany). Height and body weight were measured using a standard protocol, and body mass index (BMI) was calculated as weight/height2 (kg/m2). Waist circumference was measured at the umbilical level with subjects standing and breathing normally. Information on age, gender, smoking, and drinking status was obtained from a questionnaire survey. A detailed personal and family history of physical illness and current medications was noted from “yes” or “no” responses to relevant questions.
2.5. Definition of variables
Hypertension was finally assessed and diagnosed by physicians according to the criteria of the JNC 8: hypertension was defined as SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg or having history of hypertension or using antihypertensive drugs; elevated BP was defined as SBP ranging from 120 to 139 mmHg and/or DBP ranging from 80 to 89 mmHg.1 Diabetes was defined as FBG levels ≥7.0 mmol/L or having history of diabetes. Hyperlipidemia was defined as TC ≥5.17 mmol/L or TG ≥1.7 mmol/L or LDL ≥3.37 mmol/L or history of hyperlipidemia.
2.6. Statistical analysis
All statistical analyses were performed using the Statistical Analysis System 9.3 edition for Windows (SAS Institute Inc, Cary, NC). Distributions of continuous variables were assessed for normality using the Kolmogorov‐Smirnov (n > 2000) or Shapiro‐Wilk (n ≤ 2000) test. Because the distributions of all the continuous variables were not normal in the present study, the natural logarithm was applied to normalize the data before statistical analysis. The continuous covariates after the log transformation approached normal distribution. Descriptive data are presented as the geometric mean (95% confidence interval, CI) for continuous variables and as percentages for categorical variables. For baseline characteristics analysis, the differences among no elevated BP and elevated BP were examined using analysis of covariance (ANCOVA) for continuous variables, and multiple logistic regression analysis for proportional variables after adjustment for age. The prevalence of elevated BP was used as dependent variables, and sex‐specific quartiles of FT3, FT4, and TSH concentrations were used as independent variables. The multiple logistic regression models were used to examine the relationships between quartiles of FT3, FT4, and TSH and the prevalence of elevated BP (0: no elevated BP, 1: elevated BP) with adjustment for the covariates: age (continuous variable), sex (males, females), BMI (continuous variable), smoking status (never, former, currently smoking), alcohol‐consumption status (everyday, sometime, former, never drinking), diabetes (yes or no), hyperlipidemia (yes or no), and family histories (yes or no) of CVD, hypertension, hyperlipidemia, and diabetes. We also performed a multiple linear regression analysis to assess the relationships between THs, TSH concentrations and BP after adjustment for the above covariates. For further analysis, the linearity assumption of the relationships between FT3, FT4, TSH and elevated BP were examined with generalized additive model. Furthermore, we performed a sensitivity analysis to assess the relationships between THs, TSH and the prevalence of stage 1 hypertension, which was defined as SBP ranging from 130 to 139 mmHg and/or DBP ranging from 80 to 89 mmHg according to the criteria of 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults.28 Odds ratios (ORs) with their corresponding 95% CI were calculated. Interactions between thyroid function (FT3, FT4, TSH concentrations) and all potential confounders were tested by addition of cross‐product terms to the regression analysis. All P values for linear trends were calculated using the median value (as continuous variable) of quartiles of FT3, FT4, and TSH. All tests were two‐tailed and P < 0.05 was defined as statistically significant.
3. RESULTS
A total of 12 487 participants without hypertension were included in our analysis. The prevalence of elevated BP was 50.7% (6332/12 487). Age‐adjusted participant characteristics in relation to elevated BP were presented in Table 1. Compared to participants without elevated BP, those with elevated BP tended to be older and to have higher BMI, waist circumference, TC, LDL, TG, SBP, DBP, FBG, FT3, FT4, and lower HDL (all P for trend <0.0001). A higher proportion of these participants were males, with a higher proportion of current smokers and alcohol consumers and a higher proportion of family history of hypertension (all P for trend <0.01). No significant differences were observed among TSH levels and the proportion of those with a family history of cardiovascular disease, hyperlipidemia, and diabetes (all P for trend >0.05).
Table 1.
Age‐adjusted participant characteristics by elevated BP status (n = 12 487)
| Elevated BP status | Pfor trenda | ||
|---|---|---|---|
| No | Yes | ||
| No. of subjects | 6155 | 6332 | ‐ |
| Age (y) | 41.4 (41.1, 41.7)b | 45.8 (45.5, 46.1) | <0.0001 |
| Sex (males, %) | 39.8 | 61.9 | <0.0001 |
| BMI (kg/m2) | 23.0 (23.0, 23.1) | 25.0 (25.0, 25.1) | <0.0001 |
| Waist circumference (cm) | 79.6 (79.3, 79.8) | 85.7 (85.4, 85.9) | <0.0001 |
| TC (mmol/L) | 4.66 (4.64, 4.68) | 4.83 (4.81, 4.86) | <0.0001 |
| LDL (mmol/L) | 2.64 (2.62, 2.66) | 2.79 (2.77, 2.81) | <0.0001 |
| HDL (mmol/L) | 1.40 (1.39, 1.41) | 1.28 (1.28, 1.29) | <0.0001 |
| TG (mmol/L) | 1.04 (1.02, 1.05) | 1.33 (1.31, 1.35) | <0.0001 |
| SBP (mmHg) | 106.8 (106.6, 107.0) | 122.5 (122.3, 122.8) | <0.0001 |
| DBP (mmHg) | 67.8 (67.7, 68.0) | 77.5 (77.3, 77.7) | <0.0001 |
| FBG (mmol/L) | 4.99 (4.97, 5.01) | 5.20 (5.18, 5.22) | <0.0001 |
| FT3 (pmol/L) | 5.10 (5.09, 5.12) | 5.28 (5.26, 5.29) | <0.0001 |
| FT4 (pmol/L) | 15.8 (15.8, 15.9) | 16.2 (16.2, 16.2) | <0.0001 |
| TSH (mIU/L) | 1.89 (1.87, 1.91) | 1.87 (1.85, 1.89) | 0.11 |
| Smoking status (%) | |||
| ≥15 cigarettes/d | 9.53 | 13.1 | <0.0001 |
| <15 cigarettes/d | 10.9 | 14.9 | <0.0001 |
| Ex‐smoker | 3.22 | 5.27 | <0.0001 |
| Non‐smoker | 76.4 | 66.8 | <0.0001 |
| Drinker (%) | |||
| Everyday | 2.29 | 4.73 | <0.0001 |
| Sometime | 52.8 | 58.9 | <0.0001 |
| Ex‐drinker | 6.36 | 4.84 | 0.02 |
| Non‐drinker | 38.5 | 31.6 | <0.0001 |
| Family history of diseases (%) | |||
| CVD | 28.1 | 30.1 | 0.36 |
| Hypertension | 41.4 | 46.0 | <0.0001 |
| Hyperlipidemia | 0.19 | 0.19 | 0.93 |
| Diabetes | 22.8 | 23.3 | 0.60 |
BMI, body mass index; CVD, cardiovascular disease; DBP, diastolic blood pressure; FBG, fasting blood glucose; FT3, free triiodothyronine; FT4, free thyroxine; HDL, high‐density lipoprotein cholesterol; LDL, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; TSH, thyroid‐stimulating hormone.
Analysis of covariance or logistic regression analysis.
Geometric mean (95% confidence interval; all such values).
The adjusted relationships between FT3, FT4, TSH, and the prevalence of elevated BP were indicated in Figure 2 and Table 2. The adjusted ORs (95% CI) of elevated BP were related to the gradual increase in the FT3, FT4, and TSH concentrations as compared with participants who had the lowest concentrations were as follows: FT3, 1.08 (0.97, 1.21), 1.24 (1.12, 1.39), and 1.32 (1.18, 1.47; P for trend <0.0001); FT4, 1.18 (1.06, 1.32), 1.18 (1.06, 1.31), and 1.24 (1.11, 1.38; P for trend <0.001); TSH, 1.06 (0.96, 1.19), 1.06 (0.95, 1.18), and 1.03 (0.93, 1.15; P for trend = 0.58), respectively.
Figure 2.
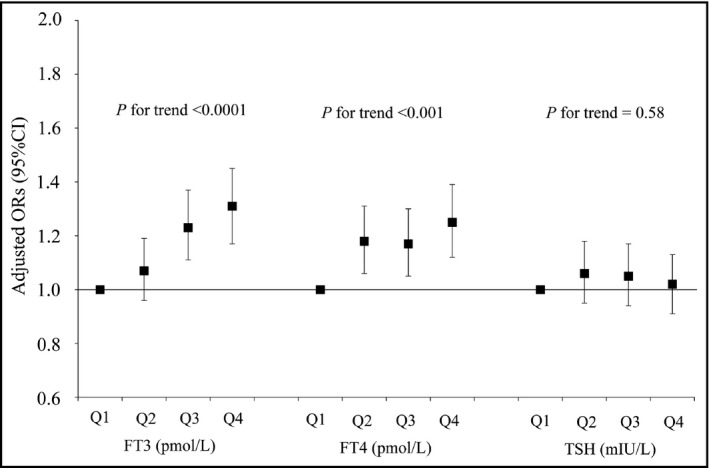
Adjusted relationships of quartiles of thyroid hormones concentrations to elevated blood pressure (n = 12 487). Adjusted for age, sex, body mass index, smoking status, alcohol‐consumption status, diabetes, hyperlipidemia, and family history of hypertension, cardiovascular disease, hyperlipidemia, and diabetes. CI, confidence interval; FT3, free triiodothyronine; FT4, free thyroxine; ORs, odds ratios; Q, quartiles; TSH, thyroid‐stimulating hormone
Table 2.
Adjusted relationships of gender‐specific quartiles of thyroid hormones concentrations to elevated BP (n = 12 487)
| Gender‐specific quartiles of FT3/FT4 (pmol/L, range) or TSH (mIU/L, range) | P for trenda | ||||
|---|---|---|---|---|---|
| Level 1 | Level 2 | Level 3 | Level 4 | ||
| FT3 concentration (pmol/L, range, males) | 3.50‐5.17 | 5.18‐5.46 | 5.47‐5.77 | 5.78‐6.50 | ‐ |
| FT3 concentration (pmol/L, range, females) | 3.50‐4.66 | 4.67‐4.94 | 4.95‐5.23 | 5.24‐6.50 | ‐ |
| No. of subjects | 3211 | 3072 | 3041 | 3163 | ‐ |
| No. of elevated BPb | 1551 | 1529 | 1591 | 1661 | ‐ |
| Crude | Reference | 1.06 (0.96, 1.17)c | 1.17 (1.06, 1.30) | 1.18 (1.07, 1.31) | <0.001 |
| Age‐ and BMI‐adjusted | Reference | 1.10 (0.99, 1.22) | 1.25 (1.12, 1.39) | 1.32 (1.18, 1.47) | <0.0001 |
| Multiple adjustedd | Reference | 1.08 (0.97, 1.21) | 1.24 (1.12, 1.39) | 1.32 (1.18, 1.47) | <0.0001 |
| FT4 concentration (pmol/L, range, males) | 11.50‐15.31 | 15.32‐16.60 | 16.61‐18.02 | 18.03‐22.69 | ‐ |
| FT4 concentration (pmol/L, range, females) | 11.50‐14.27 | 14.28‐15.46 | 15.47‐16.74 | 16.75‐22.57 | ‐ |
| No. of subjects | 3135 | 3130 | 3082 | 3140 | ‐ |
| No. of elevated BPb | 1556 | 1614 | 1554 | 1608 | ‐ |
| Crude | Reference | 1.08 (0.98, 1.19) | 1.03 (0.93, 1.14) | 1.07 (0.97, 1.18) | 0.37 |
| Age‐ and BMI‐adjusted | Reference | 1.19 (1.07, 1.33) | 1.18 (1.06, 1.31) | 1.27 (1.14, 1.41) | <0.0001 |
| Multiple adjustedd | Reference | 1.18 (1.06, 1.32) | 1.18 (1.06, 1.31) | 1.24 (1.11, 1.38) | <0.001 |
| TSH concentration (mIU/L, range, males) | 0.55‐1.29 | 1.30‐1.74 | 1.75‐2.35 | 2.36‐4.77 | ‐ |
| TSH concentration (mIU/L, range, females) | 0.55‐1.51 | 1.52‐2.10 | 2.11‐2.86 | 2.87‐4.78 | ‐ |
| No. of subjects | 3129 | 3117 | 3114 | 3127 | ‐ |
| No. of elevated BPb | 1530 | 1579 | 1607 | 1616 | ‐ |
| Crude | Reference | 1.07 (0.97, 1.19) | 1.11 (1.01, 1.23) | 1.12 (1.01, 1.23) | 0.02 |
| Age‐ and BMI‐adjusted | Reference | 1.07 (0.96, 1.19) | 1.08 (0.97, 1.20) | 1.07 (0.96, 1.19) | 0.25 |
| Multiple adjustedd | Reference | 1.06 (0.96, 1.19) | 1.06 (0.95, 1.18) | 1.03 (0.93, 1.15) | 0.58 |
BMI, body mass index; BP, blood pressure; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid‐stimulating hormone.
Multiple logistic regression analysis.
Elevated BP is defined as SBP ranging from 120 to 139 mmHg and/or DBP ranging from 80 to 89 mmHg, according to the JNC 8 criteria.
Adjusted odds ratios (95% confidence interval; all such values).
Adjusted for age, body mass index, waist, smoking status, alcohol‐consumption status, diabetes, hyperlipidaemia and family history of cardiovascular disease, hypertension, hyperlipidemia, and diabetes.
The analysis with generalized additive model did not indicate non‐linear relationships between FT3, FT4, TSH and elevated BP (P = 0.15, 0.52, and 0.23, respectively). Furthermore, through multiple linear regressions, after multiple adjustment, standardized β coefficients (standard error of mean) of FT3, FT4, and TSH concentrations for BP levels were as follows: SBP, 0.089 (0.0092), 0.046 (0.0068), and 0.012 (0.0018), (all P values <0.0001, except TSH); DBP, 0.063 (0.0104), 0.050 (0.0078), and 0.001 (0.0021), (all P values <0.0001, except TSH), respectively.
We further performed a sensitivity analysis to assess the relationships between THs, TSH, and the prevalence of stage 1 hypertension. Compared with the reference groups, the adjusted ORs (95% CI) for elevated BP across the quartile of FT3, FT4, and TSH concentrations were as follows: FT3, 1.03 (0.93, 1.16), 1.14 (1.02, 1.28), and 1.21 (1.08, 1.35; P for trend <0.001); FT4, 1.20 (1.08, 1.34), 1.19 (1.07, 1.33), and 1.30 (1.16, 1.45; P for trend <0.0001); TSH, 1.03 (0.92, 1.15), 1.00 (0.90, 1.12), and 1.02 (0.91, 1.14; P for trend = 0.86), respectively.
4. DISCUSSION
In this large‐scale cross‐sectional study, we have examined the relationships between THs, TSH and elevated BP in an adult population. We found that FT3 and FT4 are positively related to the prevalence of elevated BP. However, no significant relationship was observed between TSH and elevated BP.
We adjusted for multiple potentially confounding factors in our analysis. This study suggests that numerous factors (age, sex, BMI, TC, TG, drinking, smoking status, family history of some diseases) are correlated with the prevalence of elevated BP. Since studies have shown that serum THs and TSH levels are related to age29 and BMI,30 we first adjusted for these two variables. Adjustment for age and BMI significantly affected the relationships between serum FT3, FT4 levels, and elevated BP, leading us to conclude that age and BMI are major confounding factors. We subsequently adjusted for waist circumference, smoking status, drinking status, diabetes, hyperlipidemia (influential factors on THs and TSH levels31, 32), and genetic factors, such as family history of CVD, hypertension, hyperlipidemia, and diabetes (influential factors on elevated BP34). However, after these adjustments, serum FT3, FT4 levels still had an obvious correlation with elevated BP.
Few studies have explored the relationships of FT3, FT4, TSH levels with the prevalence ofelevated BP. To the best of our knowledge, only a small‐scale cohort study (n = 2282) investigated whether FT4 and TSH within reference range were risk factors for elevated BP in Tehran population.24 Their findings indicated that a 1 ng/dL higher FT4 was related to 40% increased risk of elevated BP (OR [95%CI]: 1.40 [1.02‐1.90]),14 but no significant relationship was observed between TSH and elevated BP. Moreover, FT3 as the biologically active thyroid hormone was not measured in their study. Therefore, we firstly assessed the relationships between FT3, FT4, TSH, and the prevalence of elevated BP. Our results found that FT3 and FT4 were positively related to the prevalence of elevated BP in 12 487 euthyroid adults, but the relationship between TSH and elevated BP were not found. Further studies are required to explore the results in other population. Moreover, the size of the present study is large enough; therefore, the finding is reliable.
Multiple putative mechanisms could explain the relationship between THs and the increased BP values. Firstly, previous study has shown that hyperthyroidism (elevated T3 levels) increases SBP by decreasing systemic vascular resistance, increasing heart contractility and heart rate, and raising cardiac output.18 Secondly, the outliers of FT4 may alter BP salt sensitivity35 in euthyroid individuals, a factor considered physiologically relevant to the onset of hypertension. However, Itterman et al suggested that the effect of variation in THs levels on BP is only a direct, short‐term effect, since only associations with current and not incident hypertension were found in their study.21 Thirdly, Poplawska‐Kita A et al11 suggested that both subclinical and overt hyperthyroidism are related to endothelial dysfunction, which plays an important role in pathogenesis of high BP.36 Furthermore, a previous study also showed that thyroid function was intrinsically linked to variables of endothelial function in healthy euthyroid subjects.10 Fourthly, previous review has suggested that hyperthyroidism and states of adrenergic hyperactivity have many common clinical features and several components of the cardiac myocyte β‐adrenergic system are regulated by THs, such as the β1‐adrenergic receptor, guanine nucleotide regulatory proteins, and adenylate cyclase.7 The adrenergic system is the major regulator of cardiac and vascular function, and this is accomplished also through the activation of specific receptors located on endothelial surface by local and systemic release of catecholamines.37, 38 Thus, the effects of THs on elevated BP may be through the regulation of adrenergic system. However, there also has been experimental data indicating that the metabolic and cardiovascular effects of THs excess are largely independent of β‐adrenergic receptor.39 Therefore, further studies are needed to explore relevant mechanisms whether the effects of THs on elevated BP are through the regulation of adrenergic system. Finally, recent studies found that genetic variation in the hypothalamus‐pituitary‐thyroid axis has been linked to susceptibility to hypertension.40 The Thr92Ala polymorphism in the type 2 deiodinase, which locally converts the pro‐hormone T4 to the biological active T3, has been linked to BP.40 However, most of above trial and experiment studies were all focus on subclinical and overt hyperthyroidism, and few studies have aimed to assess the relationships between normal THs, TSH levels and elevated BP in euthyroid subjects.24 Therefore, further studies are needed to explore the relevant mechanisms under the relationships between normal THs, TSH levels and elevated BP in euthyroid subjects.
In addition, previous studies have indicated that hyperthyroidism can cause hypertension.7 Interestingly, many studies have also demonstrated that hypothyroidism is positively related to hypertension.15, 41 Increased peripheral vascular resistance and low cardiac output have been suggested to be the possible link between hypothyroidism and diastolic hypertension.41 Moreover, overt and subclinical hypothyroidism were also related to endothelial dysfunction,42, 43 which was considered as a cause of high BP.36 Alibaz Oner, et al12 found that L‐thyroxin therapy can improve endothelial functions in patients with subclinical hypothyroidism. Findings from above studies suggest a U‐shape relationship between THs within the whole range (subclinical and overt hyperthyroidism, euthyroidism, and subclinical and overt hypothyroidism) and BP levels. However, to the best of our knowledge, few studies have analyzed the relationships between THs, TSH levels and elevated BP in euthyroid subjects.24 In the present study, we found positive linear relationships between FT3, FT4 concentrations within the reference range and elevated BP. These results implied that when THs decline to certain concentration, it may gradually cause elevated BP for certain group of people. Therefore, further studies are needed to determine this cut‐off point for THs levels to detect those subjects with elevated BP.
It is important to acknowledge the relative merits and weaknesses of the present study. The present study appears to be the first to explore the relationships between the FT3, FT4, TSH levels within reference range and the prevalence ofelevated BP in a large‐scale adult population. Furthermore, we controlled for various potential confounders, such as age, sex, BMI, smoking status, alcohol‐consumption status, diabetes, hyperlipidemia and family history of CVD, hypertension, hyperlipidemia, and diabetes. However, the present study has several limitations. Firstly, this is a cross‐sectional study, which is impossible to infer causality. Further cohort studies and intervention trials should be undertaken to establish a causal relationship between THs and elevated BP. However, the present large‐scale cross‐sectional study supports the important hypothesis that THs levels even within the euthyroid range, may contribute to the development of elevated BP in the general population. Secondly, although our analyses made adjustment for a considerable number of confounders, there is the potential for confounding, such as many lifestyle factors (including dietary factors,44, 45 physical activity46) and cardiorespiratory fitness,47 which may affect the relationship between THs and elevated BP. Thus, a well‐designed randomized controlled trial is required to verify these results. Thirdly, genetic factor plays an important role in the pathogenesis of thyroid dysfunction48, 49 and hypertension.50, 51 In the present study, we took into consideration the role of genetic background in hypertension by adjusting for family history of hypertension. However, we did not adjust for the genetic factor of thyroid function because of lack of pertinent data. Therefore, future studies should explore genetically relationship between thyroid function and elevated BP. Finally, the study results only represent the present age group (mean age 44.9 years) and consisted of relatively young subjects. Therefore, it is possible that our results cannot be generalized to the general population. Further studies are needed to verify the results in other population.
5. CONCLUSION
The present findings demonstrated that FT3 and FT4 are positively related to the normal BP and the prevalence of elevated BP in euthyroid adults, but no significant relationship was found between TSH and elevated BP. These results imply that thyroid function may contribute to the regulation of BP in euthyroid individuals. Further cohort study and clinical trial are needed to verify our results.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
The authors gratefully acknowledge all the people that have made this study.
Gu Y, Zheng L, Zhang Q, et al. Relationship between thyroid function and elevated blood pressure in euthyroid adults. J Clin Hypertens. 2018;20:1541–1549. 10.1111/jch.13369
Funding information
This study was supported by grants from the National Natural Science Foundation of China (No. 81673166, 81372118, 81372467 and 81302422), the key technologies R&D programme of Tianjin (Key Project: No. 11ZCGYSY05700, 12ZCZDSY20400, 13ZCZDSY20200, and 15YFYZSY00020), the National Science and Technology Support Program (No. 2012BAI02B02), 2012 and 2016 Chinese Nutrition Society (CNS) Nutrition Research Foundation—DSM Research Fund (No. 2014‐071, 2016‐046 and 2016‐023), the Technologies development programme of Beichen District of Tianjin (No. bcws2013‐21, bcws2014‐05 and 2015‐SHGY‐02), the technologies project of Tianjin Binhai New Area (No. 2013‐02‐04 and 2013‐02‐06), the Science Foundation of Tianjin Medical University (No. 2010KY28 and 2013KYQ24), the Key Laboratory of Public Health Safety (Fudan University), Ministry of Education (No. GW2014‐5), and the National Training Programs of Innovation and Entrepreneurship for Undergraduates (No. 201510062013), China.
REFERENCES
- 1. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507‐520. [DOI] [PubMed] [Google Scholar]
- 2. Wang Y, Wang QJ. The prevalence of prehypertension and hypertension among US adults according to the new joint national committee guidelines: new challenges of the old problem. Arch Intern Med. 2004;164(19):2126‐2134. [DOI] [PubMed] [Google Scholar]
- 3. Xu T, Liu J, Zhu G, et al. Prevalence of prehypertension and associated risk factors among Chinese adults from a large‐scale multi‐ethnic population survey. BMC Public Health. 2016;16(1):775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vasan RS, Larson MG, Leip EP, et al. Assessment of frequency of progression to hypertension in non‐hypertensive participants in the Framingham Heart Study: a cohort study. Lancet. 2001;358(9294):1682‐1686. [DOI] [PubMed] [Google Scholar]
- 5. Sharma KK. A nursing care study: congestive heart failure. Nurs J India. 1982;73(11):289‐290. [PubMed] [Google Scholar]
- 6. Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116(15):1725‐1735. [DOI] [PubMed] [Google Scholar]
- 7. Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344(7):501‐509. [DOI] [PubMed] [Google Scholar]
- 8. Cai Y, Manio MM, Leung GP, et al. Thyroid hormone affects both endothelial and vascular smooth muscle cells in rat arteries. Eur J Pharmacol. 2015;747:18‐28. [DOI] [PubMed] [Google Scholar]
- 9. Hiroi Y, Kim HH, Ying H, et al. Rapid nongenomic actions of thyroid hormone. Proc Natl Acad Sci USA. 2006;103(38):14104‐14109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernandez‐Real JM, Lopez‐Bermejo A, Castro A, et al. Thyroid function is intrinsically linked to insulin sensitivity and endothelium‐dependent vasodilation in healthy euthyroid subjects. J Clin Endocrinol Metab. 2006;91(9):3337‐3343. [DOI] [PubMed] [Google Scholar]
- 11. Poplawska‐Kita A, Siewko K, Telejko B, et al. The changes in the endothelial function and haemostatic and inflammatory parameters in subclinical and overt hyperthyroidism. Int J Endocrinol. 2013;2013:981638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alibaz Oner F, Yurdakul S, Oner E, et al. Evaluation of the effect of L‐thyroxin therapy on endothelial functions in patients with subclinical hypothyroidism. Endocrine. 2011;40(2):280‐284. [DOI] [PubMed] [Google Scholar]
- 13. Bahouth SW, Cui X, Beauchamp MJ, et al. Thyroid hormone induces beta1‐adrenergic receptor gene transcription through a direct repeat separated by five nucleotides. J Mol Cell Cardiol. 1997;29(12):3223‐3237. [DOI] [PubMed] [Google Scholar]
- 14. Danzi S, Klein I. Thyroid hormone and blood pressure regulation. Curr Hypertens Rep. 2003;5(6):513‐520. [DOI] [PubMed] [Google Scholar]
- 15. Kotsis V, Alevizaki M, Stabouli S, et al. Hypertension and hypothyroidism: results from an ambulatory blood pressure monitoring study. J Hypertens. 2007;25(5):993‐999. [DOI] [PubMed] [Google Scholar]
- 16. Volzke H, Ittermann T, Schmidt CO, et al. Subclinical hyperthyroidism and blood pressure in a population‐based prospective cohort study. Eur J Endocrinol. 2009;161(4):615‐621. [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez Gil L, de la Sierra A. Prevalence of hypertension and other cardiovascular risk factors in subjects with subclinical hypothyroidism. Med Clin. 2017;148(8):351‐353. [DOI] [PubMed] [Google Scholar]
- 18. Prisant LM, Gujral JS, Mulloy AL. Hyperthyroidism: a secondary cause of isolated systolic hypertension. J Clin Hypertens. 2006;8(8):596‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asvold BO, Bjoro T, Nilsen TI, et al. Association between blood pressure and serum thyroid‐stimulating hormone concentration within the reference range: a population‐based study. J Clin Endocrinol Metab. 2007;92(3):841‐845. [DOI] [PubMed] [Google Scholar]
- 20. Boekholdt SM, Titan SM, Wiersinga WM, et al. Initial thyroid status and cardiovascular risk factors: the EPIC‐Norfolk prospective population study. Clin Endocrinol. 2010;72(3):404‐410. [DOI] [PubMed] [Google Scholar]
- 21. Ittermann T, Tiller D, Meisinger C, et al. High serum thyrotropin levels are associated with current but not with incident hypertension. Thyroid. 2013;23(8):955‐963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Langen VL, Niiranen TJ, Puukka P, et al. Association between thyroid‐stimulating hormone and blood pressure in adults: an 11‐year longitudinal study. Clin Endocrinol. 2016;84(5):741‐747. [DOI] [PubMed] [Google Scholar]
- 23. Asvold BO, Bjoro T, Vatten LJ. Associations of TSH levels within the reference range with future blood pressure and lipid concentrations: 11‐year follow‐up of the HUNT study. Eur J Endocrinol. 2013;169(1):73‐82. [DOI] [PubMed] [Google Scholar]
- 24. Abdi H, Gharibzadeh S, Tasdighi E, et al. Associations Between Thyroid and Blood Pressure in Euthyroid Adults: A 9‐Year Longitudinal Study. Horm Metab Res. 2018;50(3):236‐241. [DOI] [PubMed] [Google Scholar]
- 25. Gu Y, Li H, Bao X, et al. The relationship between thyroid function and the prevalence of type 2 diabetes mellitus in euthyroid subjects. J Clin Endocrinol Metab. 2016;jc20162965. [DOI] [PubMed] [Google Scholar]
- 26. D'Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743‐753. [DOI] [PubMed] [Google Scholar]
- 27. Porcu E, Medici M, Pistis G, et al. A meta‐analysis of thyroid‐related traits reveals novel loci and gender‐specific differences in the regulation of thyroid function. PLoS Genet. 2013;9(2):e1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. [DOI] [PubMed] [Google Scholar]
- 29. Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291(2):228‐238. [DOI] [PubMed] [Google Scholar]
- 30. Knudsen N, Laurberg P, Rasmussen LB, et al. Small differences in thyroid function may be important for body mass index and the occurrence of obesity in the population. J Clin Endocrinol Metab. 2005;90(7):4019‐4024. [DOI] [PubMed] [Google Scholar]
- 31. Altinova AE, Toruner FB, Akturk M, et al. Adiponectin levels and cardiovascular risk factors in hypothyroidism and hyperthyroidism. Clin Endocrinol. 2006;65(4):530‐535. [DOI] [PubMed] [Google Scholar]
- 32. Asvold BO, Bjoro T, Nilsen TI, et al. Tobacco smoking and thyroid function: a population‐based study. Arch Intern Med. 2007;167(13):1428‐1432. [DOI] [PubMed] [Google Scholar]
- 33. Balhara YP, Deb KS. Impact of alcohol use on thyroid function. Indian J Endocrinol Metab. 2013;17(4):580‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bergvall N, Cnattingius S. Familial (shared environmental and genetic) factors and the foetal origins of cardiovascular diseases and type 2 diabetes: a review of the literature. J Intern Med. 2008;264(3):205‐223. [DOI] [PubMed] [Google Scholar]
- 35. Gumieniak O, Perlstein TS, Hopkins PN, et al. Thyroid function and blood pressure homeostasis in euthyroid subjects. J Clin Endocrinol Metab. 2004;89(7):3455‐3461. [DOI] [PubMed] [Google Scholar]
- 36. Konukoglu D, Uzun H. Endothelial dysfunction and hypertension. Adv Exp Med Biol. 2017;956:511‐540. [DOI] [PubMed] [Google Scholar]
- 37. Izzo R, Cipolletta E, Ciccarelli M, et al. Enhanced GRK2 expression and desensitization of betaAR vasodilatation in hypertensive patients. Clin Transl Sci. 2008;1(3):215‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ciccarelli M, Sorriento D, Coscioni E, Iaccarino G, Santulli G. Endocrinology of the Heart in Health and Disease (Chapter 11: Adrenergic Receptors). Cambridge, MA: Academic Press; 2017:285‐315. [Google Scholar]
- 39. Bachman ES, Hampton TG, Dhillon H, et al. The metabolic and cardiovascular effects of hyperthyroidism are largely independent of beta‐adrenergic stimulation. Endocrinology. 2004;145(6):2767‐2774. [DOI] [PubMed] [Google Scholar]
- 40. Gumieniak O, Perlstein TS, Williams JS, et al. Ala92 type 2 deiodinase allele increases risk for the development of hypertension. Hypertension. 2007;49(3):461‐466. [DOI] [PubMed] [Google Scholar]
- 41. Stabouli S, Papakatsika S, Kotsis V. Hypothyroidism and hypertension. Expert Rev Cardiovasc Ther. 2010;8(11):1559‐1565. [DOI] [PubMed] [Google Scholar]
- 42. Clausen P, Mersebach H, Nielsen B, et al. Hypothyroidism is associated with signs of endothelial dysfunction despite 1‐year replacement therapy with levothyroxine. Clin Endocrinol. 2009;70(6):932‐937. [DOI] [PubMed] [Google Scholar]
- 43. Cikim AS, Oflaz H, Ozbey N, et al. Evaluation of endothelial function in subclinical hypothyroidism and subclinical hyperthyroidism. Thyroid. 2004;14(8):605‐609. [DOI] [PubMed] [Google Scholar]
- 44. Padwal R, Hackam D, Khan N, et al. Primary prevention of CVD: modification of diet in people with hypertension. BMJClin Evid. 2016;2016:214. [PMC free article] [PubMed] [Google Scholar]
- 45. Matana A, Torlak V, Brdar D, et al. Dietary factors associated with plasma thyroid peroxidase and thyroglobulin antibodies. Nutrients. 2017;9(11):1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Santulli G, Ciccarelli M, Trimarco B, et al. Physical activity ameliorates cardiovascular health in elderly subjects: the functional role of the beta adrenergic system. Front Physiol. 2013;4:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jae SY, Babu AS, Yoon ES, et al. Impact of cardiorespiratory fitness and risk of systemic hypertension in nonobese versus obese men who are metabolically healthy or unhealthy. Am J Cardiol. 2017;120(5):765‐768. [DOI] [PubMed] [Google Scholar]
- 48. Lombardi A, Menconi F, Greenberg D, et al. Dissecting the genetic susceptibility to Graves' disease in a cohort of patients of Italian origin. Front Endocrinol. 2016;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brown RS, Lombardi A, Hasham A, et al. Genetic analysis in young‐age‐of‐onset Graves' disease reveals new susceptibility loci. J Clin Endocrinol Metab. 2014;99(7):E1387–E1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Santulli G, Cipolletta E, Sorriento D, et al. CaMK4 Gene deletion induces hypertension. J Am Heart Assoc. 2012;1(4):e001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wei LK, Au A, Teh LK, et al. Recent advances in the genetics of hypertension. Adv Exp Med Biol. 2017;956:561‐581. [DOI] [PubMed] [Google Scholar]


