Abstract
Renal denervation (RDN) has been proposed as a novel interventional antihypertensive technique. However, existing evidence was mainly from patients with severe resistant hypertension. The authors aimed to evaluate the efficacy of RDN in patients with resistant hypertension with mildly elevated blood pressure (BP). Studies of RDN in patients with mild resistant hypertension (systolic office BP 140–160 mm Hg despite treatment with three antihypertensive drugs including one diuretic, or mean systolic BP by 24‐hour ambulatory BP measurement [ABPM] 135–150 mm Hg) were included. Two observational and one randomized cohort were identified (109 patients in the RDN group and 36 patients in the control group). Overall, the mean age of patients was 62±10 years, and 69.7% were male. Before‐after comparison showed that RDN significantly reduced ABPM as compared with the baseline systolic ABPM, from 146.3±13 mm Hg at baseline to 134.6±14.7 mm Hg at 6‐month follow‐up and diastolic ABPM from 80.8±9.4 mm Hg at baseline to 75.5±9.8 mm Hg at 6‐month follow up (both P<.001). This significant effect was not observed in the control group. Between‐group comparison showed a greater change in ABPM in the RDN group as compared with that in the control group (change in systolic ABPM: −11.7±9.9 mm Hg in RDN vs −3.5±9.6 mm Hg in controls [P<.001]; change in diastolic ABPM: −5.3±6.3 mm Hg in RDN vs −2.1±5.5 mm Hg in control [P=.007]). RDN was also associated with a significantly decreased office systolic/diastolic BP and reduced number of antihypertensive medications. No severe adverse events were found during follow‐up. RDN seems feasible to treat patients with mild resistant hypertension.
Keywords: blood pressure, prevention, renal denervation, resistant hypertension
1. Introduction
Hypertension remains the most common risk factor for major cardiovascular (CV) adverse events, affecting nearly half of the adult population globally and becoming more prevalent in the aging population.1 Despite a remarkable development of antihypertensive medications, there are still a portion of patients who fail to achieve target blood pressure (BP). Resistant hypertension (RH) is defined as a BP value >140/90 mm Hg despite receiving maximally tolerated doses of at least three antihypertensive drugs including a diuretic (based on guidelines of the European Society of Hypertension/European Society of Cardiology2 and the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure).3
The prevalence of RH is reported to range from 5% to 30% of the overall hypertensive population.2 In a recent large cohort analysis from the Spanish Ambulatory Blood Pressure Monitoring Registry, RH is present in 12% of the treated population.4 Patients with RH are associated with a significantly increased risk of cardiocerebralvascular complications such as atherosclerosis, stroke, arrhythmias, heart failure, and chronic kidney disease, requiring more intensive BP management to improve the prognosis of such a particular patient group.2, 3, 4, 5
Transcatheter renal denervation (RDN) has emerged as a novel technique to treat RH. Previous clinical trials have shown promising results of RDN in BP reduction; however, a majority of studies was conducted in patients with severe RH (defined as RH with office systolic BP [SBP] ≥160 mm Hg).2, 6, 7 Whereas in the clinical setting, a great number of patients with RH have office SBP between 140 and 160 mm Hg, ie mild RH.8 Whether RDN can reduce BP in patients with less severe forms of RH remains to be evaluated. We thus conducted a pooled‐data analysis to assess the effect of RDN in treating mild RH.
2. Methods
Electronic searches of the databases of PubMed and clinicaltrials.gov were performed until January 1, 2016. We used the search term “renal denervation,” restricted to clinical study of patients with “mild resistant hypertension.” Titles and abstracts were screened for eligibility. The references of relevant clinical studies or review articles were retrieved for manual searching.
2.1. Inclusion and exclusion criteria
Either observational or randomized trials were considered for inclusion if they evaluated the efficacy of RDN in mild RH and reported BP results of at least 6 months. Mild RH was defined as systolic office BP between 140 and 160 mm Hg or mean systolic BP on 24‐hour ambulatory BP monitoring (ABPM) between 135 and 150 mm Hg despite treatment with three antihypertensive drugs including one diuretic.3, 8, 9 Patients were excluded from study analysis if they had known secondary causes of hypertension, renal artery anatomy abnormalities, or other unstable comorbidities not suitable for an ablation procedure.
2.2. Data extraction
Data extraction included study design, location of center(s), inclusion criteria, sample size, follow‐up period, intervention and ablation procedure, and patient characteristics. Quality assessment was carried out based on the Cochrane guideline.
2.3. Outcome measurements
The primary outcome was the change in 24‐hour ABPM (ΔABPM). The secondary outcomes consisted of change in office BP, medications, and procedure‐related serious adverse events.
2.4. Statistical analysis
Statistical analyses were conducted following the principles of the Cochrane guideline. Continuous variables were described as means±standard deviations (SDs) and categorical variables as frequencies and percentages. Means and SDs were combined using the following approximation formula recommended by the Cochrane Handbook (version 5.1, Higgins and Green's, The Cochrane Collaboration Handbook for Systemic Reviews of Interventions, Oxford, UK):
| Formulae for Combining Groups | Group 1 | Group 2 | Combined Groups | |
|---|---|---|---|---|
| Sample size | N1 | N2 | N1+N2 | |
| Mean | M1 | M2 |
|
|
| SD | SD1 | SD2 |
|
We presumed that the included patients were from one random statistical population. The between‐group and before‐after comparisons of BP were performed using Student t test (SPSS software version 17.0; SPSS Inc, Chicago, IL, USA). Changes in ABPM were synthesized and the difference between ΔABPM in the RDN group vs the control group was estimated using the Review Manager Soft Package (RevMan5.1, The Cochrane Collaboration, Oxford, UK). The effect was assessed by a statistical value of weighted mean difference. The significance level was set at a P value of .05.
3. Results
3.1. Studies inclusion
Forty‐five citations were retrieved after initial searching. After the screening of titles and abstracts and review of full‐text relevant articles, three clinical studies fulfilled the inclusion criteria and were thus identified.10, 11, 12 The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) studies selection flow diagram is presented in Figure S1.
3.2. Baseline characteristics
The features in study design, conducting centers, and study intervention are summarized in Table 1. There were two self‐controlled prospective studies and one randomized sham‐controlled trial, all performed in experienced RDN centers. A total of 145 patients were included (mean age 62±10 years, 69.7% male), of whom 109 patients with mild RH underwent RDN. The follow‐up period was 6 months. The RDN procedure was performed using a conventional ablation protocol.10, 11, 12 A summary of the patients’ baseline characteristics is presented in Table 2
Table 1.
Individual Trial Design
| Study/First Author | Design | Conducting Location | Inclusion Criteria | Sample Size, No. | Mean Age, y | Men, No. (%) | Follow‐up, mo | Intervention | Mean Ablation Points |
|---|---|---|---|---|---|---|---|---|---|
| Kaltenbach10 | Self‐controlled, single‐center, prospective | Frankfurt | Mild resistant hypertension, with SBP between 140 and 160 mm Hg | 20 | 61±10.8 | 11 (59) | 6 | Conventional RDN | Left 5.6; right 5.6 |
| Ott11 | Self‐controlled, multicenter, prospective | Erlangen, Homburg, Columbus, and New York | Mild resistant hypertension, with SBP between 140 and 160 mm Hg | 54 | 63.6±11 | 38 (70) | 6 | Conventional RDN | At least 4 each |
| Desch12 | Randomized sham‐controlled | Lübeck, Leipzig | Mild resistant hypertension, with a mean daytime SBP on ABPM between 135 and 149 mm Hg | 35/[36 as sham] | 64.5±7.6 [57.4±8.6] | 27 (77)/[25 (69)] | 6 | Conventional RDN | Left 5.5, right 5.7 |
| Pooled | Retrospective pooled analysis | Multicenter | Mild resistant hypertension | 145 | 62±10 | 101 (69.7) | 6 | Conventional RDN | ≥4 ablation each side |
Abbreviations: ABPM, ambulatory blood pressure monitoring; RDN, renal denervation; SBP, systolic blood pressure.
Table 2.
Clinical Characteristics of Included Patients
| Study/First Author | Ethnicity | BMI | Diabetes Mellitus, No. (%) | CHD, No. (%) | GFR | No. of Tolerated Antihypertensive Drugs at Baseline |
|---|---|---|---|---|---|---|
| Kaltenbach10 | White | — | 10 (50) | 10 (50) | 77.4±24.8 | 5.4±1.5 |
| Ott11 | White | 31.1±5.2 | 27 (50) | 14 (26) | 69.5±21 | 5.1±1.4 |
| Desch12 | White | 31.9±4.4 | 19 (54) | 21 (60) | 79±20 | 4.4±1.3 |
| Pooled | White | 31.4±4.9 | 56 (51) | 45 (41) | 74±22 | 4.9±1.4 |
Abbreviations: BMI, body mass index; CHD, coronary heart disease; GFR, glomerular filtration rate.
3.3. Primary outcome
3.3.1. Ambulatory BP monitoring
All included studies had ABPM follow‐up data. As shown in Table 3, for patients receiving RDN, ABPM was significantly decreased at 6‐month follow‐up as compared with that at baseline (ABPM SBP from 146.3±13 to 134.6±14.7 mm Hg [P<.001] and ABPM DBP from 80.8±9.4 to 75.5±9.8 mm Hg [P<.001]). For patients in the sham‐controlled group, ABPM at 6 months was not significantly different from that at baseline (ABPM SBP from 140.4±5.6 to 136.9±13.5 mm Hg [P=.155] and ABPM DBP from 80.6±7.1 to 78.5±9.2 mm Hg [P=.282].
Table 3.
Changes in ABPM During Follow‐Up
| Study/First Author | No. (Group) | Baseline ABPM SBP | 6‐mo Follow‐up ABMP SBP | ΔABPM SBP | Baseline ABPM DBP | 6‐mo Follow‐up ABPM DBP | ΔABPM DBP |
|---|---|---|---|---|---|---|---|
| Kaltenbach10 | 20 (RDN) | 147.0±9.8 | 135.7±14.1 | −11.3±8.6 | 79.5±10.1 | 75.4±12.2 | −4.1±7.3 |
| Ott11 | 54 (RDN) | 150±16 | 136±16 | −14±10.1 | 83±10 | 76±10 | −7±6.3 |
| Desch12 | 35 (RDN) | 140.2±4.6 (RDN) | 131.9±12.9 (RDN) | −8.3±9.6 (RDN) | 78.2±7.4 (RDN) | 74.9±8.2 (RDN) | −3.3±5 (RDN) |
| 36 (sham) | 140.4±5.6 (sham) | 136.9±13.5 (sham) | −3.5±9.6 (sham) | 80.6±7.1 (sham) | 78.5±9.2 (sham) | −2.1±5.5 (sham) | |
| Pooled | 109 (RDN) | 146.3±13 | 134.6±14.7 | −11.7±9.9 | 80.8±9.4 | 75.5±9.8 | −5.3±6.3 |
| 36 (sham) | 140.4±5.6 | 136.9±13.5 | −3.5±9.6 | 80.6±7.1 | 78.5±9.2 | −2.1±5.5 |
Abbreviations: ABPM, ambulatory blood pressure monitoring; DBP, diastolic blood pressure; RDN, renal denervation; SBP, systolic blood pressure; Δ, change.
As for the comparison of ΔABPM during follow‐up, pooled analysis (shown in Figures 1 and 2) showed a greater reduction in ABPM in the RDN group as compared with that in the control group (ΔABPM SBP: −11.7±9.9 mm Hg in the RDN group vs −3.5±9.6 mm Hg in the control group [P<.001]; ΔABPM DBP: −5.3±6.3 mm Hg in the RDN group vs −2.1±5.5 mm Hg in the control group [P=.004]).
Figure 1.
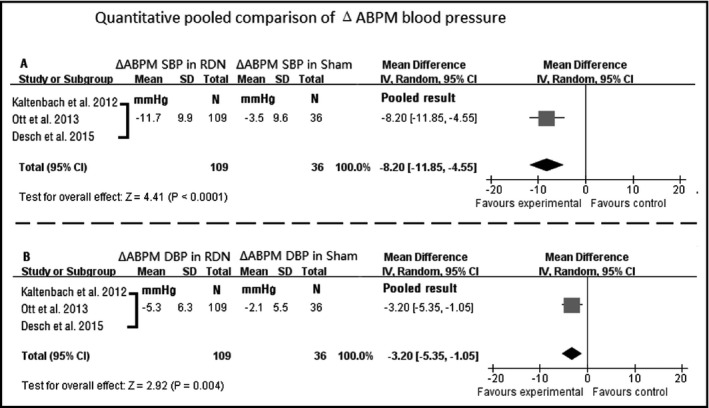
Quantitative pooled comparison of changes in ambulatory blood pressure monitoring (ABPM). CI indicates confidence interval; DBP, diastolic blood pressure; RDN, renal denervation; SBP, systolic blood pressure; SD, standard deviation; Δ, change
Figure 2.
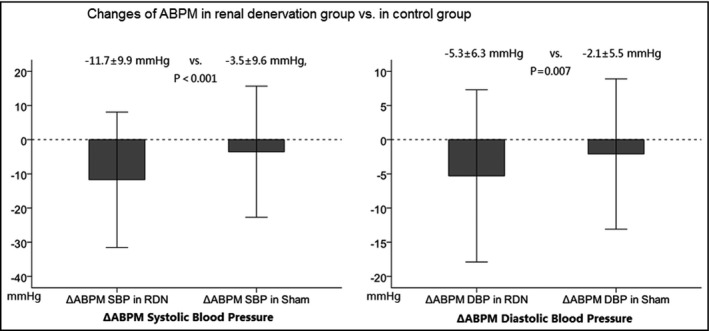
Comparison of change in ambulatory blood pressure monitoring (ABPM). DBP indicates diastolic blood pressure; RDN, renal denervation; SBP, systolic blood pressure; Δ, change
3.4. Secondary outcomes
3.4.1. Office BP, medications, and procedure‐related adverse events
Office BP was reported in 74 patients who underwent RDN. As shown in Table 4, the office BP at 6 months was significantly reduced as compared with that at baseline (office SBP: from 150.3±6.2 to 137.2±20.2 mm Hg [P<.001]; office DBP: from 83±11 to 75.8±11.8 mm Hg [P<0.001]).
Table 4.
Changes in Office Blood Pressure, Antihypertensive Drugs, and Procedure‐Related Adverse Events During Follow‐Up
| Study/First Author | No. (Group) | Baseline Office SBP, mm Hg | 6‐mo Follow‐up Office SBP, mm Hg | ΔOffice SBP, mm Hg | Baseline Office DBP, mm Hg | 6‐mo Follow‐up Office DBP, mm Hg | ΔOffice DBP, mm Hg | Patients With Reduced Antihypertensive Drugs, No. (%) | Serious Adverse Events Related to RDN, No. (%) |
|---|---|---|---|---|---|---|---|---|---|
| Kaltenbach10 | 20 (RDN) | 148.4±6.6 | 135±18.3 | −13.1±13.6 | 83.0±11.0 | 78±13.8 | −5.0±8.3 | 4 (20) | 0 (0) |
| Ott11 | 54 (RDN) | 151±6 | 138±21 | −13±16.6 | 83±11 | 75±11 | −7±6.96 | 20 (37) | 0 (0) |
| Desch12 | 35 (RDN) | — | — | — | — | — | — | — | 0 (0) |
| 36 (sham) | |||||||||
| Pooled | 74 (RDN) | 150.3±6.2 | 137.2±20.2 | −13±15.8 | 83±11 | 75.8±11.8 | −6.5±7.3 | 24 (32.4) | 0 (0) |
| 36 (sham) | — | — | — | — | — | — | — | — |
Abbreviations: DBP, diastolic blood pressure; RDN, renal denervation; SBP, systolic blood pressure; Δ, change.
Pooled analysis also showed that 32.4% (24 of 74) of patients had reduced the number of antihypertensive drugs. By 6‐month follow up, no severe procedure‐related adverse events and no exacerbation of renal function were found.
4. Discussion
4.1. Main findings
The presented pooled analysis showed that, in patients with mild RH, RDN led to a significant and clinically relevant reduction in both systolic (−11.7±9.9 mm Hg) and diastolic ABPM (−5.3±6.3 mm Hg), as relative to a less pronounced ABPM reduction in the control group. RDN was also associated with a significantly reduced office systolic and diastolic BP. Moreover, the pooled analysis showed that RDN can significantly reduce the pharmacotherapy burden among this patient group (32.4% reduction). These favorable effects of RDN were conferred without significantly increased risk of safety concerns.
4.2. Rationale to treat mild RH at early stage
Hypertension is a major risk factor for CV diseases. Better controlled BP can effectively prevent the development of its complications. The natural history from risk factors to clinical CV events evolves as a continuum, at any stage of which a targeted lowering of BP would be beneficial. A relative risk of clinical events can be largely reduced if intervention is initiated before irreversible organ damage or emergence of CV disease.13
A recent meta‐analysis of randomized trials demonstrated that an average BP reduction of 3.6/2.4 mm Hg was significantly associated with fewer stroke events and improved survival during 5 years of follow‐up in patients with BP in the grade 1 hypertension range without overt CV complications.14 A modest BP reduction in patients with mild hypertension can improve hard clinical outcomes, suggesting even greater absolute CV event reduction in higher‐risk populations.
The sympathetic nervous system steers BP by regulating cardiac output and systemic vascular resistance. Numerous studies have demonstrated that a state of sympathetic overdrive proceeds and favors the development of hypertension. Moreover, sympathetic overactivity appears to be more pronounced in younger than in elderly hypertensive patients, supporting the concept that a sympathetic mechanism is involved, particularly in the initiation of the hypertensive state in the early stage.15
4.3. RDN to treat RH
Nonadherence to pharmacotherapy is common in hypertensive patients.16 RDN has been proposed as a novel technique to modulate sympathetic activity and treat RH. Encouraging results of its BP‐lowering efficacy have been shown in many clinical trials.17, 18, 19, 20, 21, 22, 23, 24 Economic analysis also suggests that RDN would be a cost‐effective strategy for RH.25, 26
Although the recent Renal Denervation in Patients With Uncontrolled Hypertension (SYMPLICITY HTN‐3) trial did not find a significant reduction of BP after 12 months of follow‐up when compared with sham‐control, the different patient enrollment, ineffective denervation procedure, a presumed Hawthorne effect, and changeable medication adherence were extensively discussed as causes for such neutral results.27 The failure of the SYMPLICITY HTN‐3 trial will likely not declare the end of this new technique.28 Given the pivotal role of the sympathetic nervous system in the pathophysiology of hypertension, RDN researchers may continue to study the anatomic distribution features of renal sympathetic nerves to improve “mapping” and “ablation” techniques and achieve effective and safe denervation, to find out possible and practical predictors of successful ablation, and to identify more eligible patient groups.28, 29, 30, 31, 32
Most recently, the multicenter, prospective, randomized Renal Denervation for Hypertension (DENERHTN) trial, in which patients with well‐defined RH were carefully enrolled, continued to show favorable results of RDN over standard stepped‐care antihypertensive pharmacotherapy in BP reduction.17 The global RDN registry is a prospective multicenter registry that aims to evaluate the efficacy and safety of RDN for RH in a global clinical setting. The first report from this registry33 showed a significant reduction of office and 24‐hour BP at 6 months among real‐world patients undergoing RDN. Moreover, the Renal Denervation for Management of Drug‐Resistant Hypertension (INSPiRED) trial (NCT01505010) is currently underway and further results are expected.
4.4. RDN to treat mild RH
There were two prospective observational studies included in the pooled analysis.10, 11 Both found a reduction in BP in patients with mild RH treated by RDN. Only one randomized trial reported the outcome of RDN in patients with mild RH.12 After intention‐to‐treat analysis, this small study did not show a significant decrease in ABPM by RDN at 6‐month follow‐up; however, the per‐protocol analysis of patients who actually received the RDN intervention demonstrated significant reduction in ABPM by RDN (P=.042) as compared with patients who did not undergo RDN intervention.12
Other aspects should also be emphasized. First, per intention‐to‐treat analysis, the RDN group exhibited a trend toward ABPM reduction (−7.0 mm Hg in the RDN group, vs −3.5 mm Hg in the control group). Second, as the investigators mentioned, the sample size randomized to each group was small (30 per group) and the number of patients who actually accepted RDN may have been even smaller.
4.5. Summary, significance, and limitations
The present study focused on the effect of RDN in RH patients with mild elevated BP. Combining data showed that patients who received RDN treatment had significantly reduced ABPM values when compared with baseline values or with the control group, supporting an effective role of RDN in mild RH and suggesting that patients with mild RH may be a targeted population group for RDN intervention.
Consistent with previous clinical trials and data, the RDN procedure was well tolerated and safe in treated patients. No severe procedure‐related adverse events and no exacerbation of renal function were found during study follow‐up.
Moreover, the pooled analysis also showed a significant reduction in the number of antihypertensive drugs in patients treated with RDN. This outcome measurement seems to be in line with recent economic analysis, which demonstrates that RDN may be a cost‐effective strategy to treat RH.25, 26
By using pooled‐analysis techniques, efforts were made to minimize the intergroup heterogeneity in our study. However, based on a limited number of randomized trials, the power of the present study was suboptimal. Intrinsic variations between studies should be noted, and bias introduced by potential confounders could not be fully ruled out. In viewing 6‐month data, further studies with long‐term follow‐up are warranted. Currently, RDN intervention remains at a stage of “blinded” ablation; hence, novel technologies to achieve “targeted” ablation end points are urgently needed.
5. Conclusions
The present study suggests that RDN seems feasible to treat mild RH. Further research of RDN in this patient group is needed.
Conflicts of Interest
There are no conflicts of interest to declare regarding the content of this study.
Supporting information
Acknowledgment
We thank all the staff and participants in this study. This study is part of a Collaborative Postdoc Program Project on Renal Denervation, Pacemed, Rio de Janeiro. Dr Chen acknowledges the fellowship of the European Heart Rhythm Association/European Society of Cardiology.
Chen S, Kiuchi MG, Acou W‐J, et al. Feasibility of catheter ablation renal denervation in “mild” resistant hypertension. J Clin Hypertens. 2017;19:361–368. doi: 10.1111/jch.12988
Dr M. G. Kiuchi is the co‐first researcher.
*Dr. Chen and Dr. Kiuchi contributed equally to this research.
Contributor Information
Shaojie Chen, Email: drsjchen@126.com.
Tetsuaki Kiuchi, Email: marciokiuchi@gmail.com.
References
- 1. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. [DOI] [PubMed] [Google Scholar]
- 2. Mancia G, Fagard R, Narkiewicz K. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 3. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 4. de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898–902. [DOI] [PubMed] [Google Scholar]
- 5. Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5 suppl 1):S1–S290. [PubMed] [Google Scholar]
- 6. Mahfoud F, Lüscher TF, Andersson B, et al. Expert consensus document from the European Society of Cardiology on catheter‐based renal denervation. Eur Heart J. 2013;34:2149–2157. [DOI] [PubMed] [Google Scholar]
- 7. Schlaich MP, Schmieder RE, Bakris G, et al. International expert consensus statement: percutaneous transluminal renal denervation for the treatment of resistant hypertension. J Am Coll Cardiol. 2013;62:2031–2045. [DOI] [PubMed] [Google Scholar]
- 8. McAdam‐Marx C, Ye X, Sung JC, Brixner DI, Kahler KH. Results of a retrospective, observational pilot study using electronic medical records to assess the prevalence and characteristics of patients with resistant hypertension in an ambulatory care setting. Clin Ther. 2009;31:1116–1123. [DOI] [PubMed] [Google Scholar]
- 9. Persell SD. Prevalence of resistant hypertension in the United States, 2003‐2008. Hypertension. 2011;57:1076–1080. [DOI] [PubMed] [Google Scholar]
- 10. Kaltenbach B, Franke J, Bertog SC, Steinberg DH, Hofmann I, Sievert H. Renal sympathetic denervation as second‐line therapy in mild resistant hypertension: a pilot study. Catheter Cardiovasc Interv. 2013;81:335–339. [DOI] [PubMed] [Google Scholar]
- 11. Ott C, Mahfoud F, Schmid A, et al. Renal denervation in moderate treatment‐resistant hypertension. J Am Coll Cardiol. 2013;62:1880–1886. [DOI] [PubMed] [Google Scholar]
- 12. Desch S, Okon T, Heinemann D, et al. Randomized sham‐controlled trial of renal sympathetic denervation in mild resistant hypertension. Hypertension. 2015;65:1202–1208. [DOI] [PubMed] [Google Scholar]
- 13. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 3. Effects in patients at different levels of cardiovascular risk—overview and meta‐analyses of randomized trials. J Hypertens. 2014;32:2305–2314. [DOI] [PubMed] [Google Scholar]
- 14. Sundström J, Arima H, Jackson R, et al. Effects of blood pressure reduction in mild hypertension. A systematic review and meta‐analysis. Ann Intern Med. 2015;162:184–191. [DOI] [PubMed] [Google Scholar]
- 15. Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. 2015;116:976–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tomaszewski M, White C, Patel P, et al. High rates of non‐adherence to antihypertensive treatment revealed by high‐performance liquid chromatography‐tandem mass spectrometry (HP LC‐MS/MS) urine analysis. Heart. 2014;100:855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Azizi M, Sapoval M, Gosse P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open‐label, randomised controlled trial. Lancet. 2015;385:1957–1965. [DOI] [PubMed] [Google Scholar]
- 18. Symplicity HTN‐2 Investigators , Esler MD, Krum H, Sobotka PA, et al. Renal sympathetic denervation in patients with treatment‐resistant hypertension (The Symplicity HTN‐2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. [DOI] [PubMed] [Google Scholar]
- 19. Krum H, Schlaich MP, Sobotka PA, et al. Percutaneous renal denervation in patients with treatment‐resistant hypertension: final 3‐year report of the Symplicity HTN‐1 study. Lancet. 2014;383:622–629. [DOI] [PubMed] [Google Scholar]
- 20. Kiuchi MG, Maia GL, De Queiroz Carreira MA, et al. Effects of renal denervation with a standard irrigated cardiac ablation catheter on blood pressure and renal function in patients with chronic kidney disease and resistant hypertension. Eur Heart J. 2013;34:2114–2121. [DOI] [PubMed] [Google Scholar]
- 21. Kiuchi MG, Chen S, Andrea BR, et al. Renal sympathetic denervation in patients with hypertension and chronic kidney disease: does improvement in renal function follow blood pressure control? J Clin Hypertens (Greenwich). 2014;16:794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kiuchi MG, Chen S, Graciano ML, et al. Acute effect of renal sympathetic denervation on blood pressure in refractory hypertensive patients with chronic kidney disease. Int J Cardiol. 2015;190:29–31. [DOI] [PubMed] [Google Scholar]
- 23. Kiuchi MG, Graciano ML, Carreira MA, Kiuchi T, Chen S, Lugon JR. Long‐term effects of renal sympathetic denervation on hypertensive patients with mild to moderate chronic kidney disease. J Clin Hypertens (Greenwich). 2016;18:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kiuchi MG, Mion D Jr, Graciano ML, et al. Proof of concept study: improvement of echocardiographic parameters after renal sympathetic denervation in CKD refractory hypertensive patients. Int J Cardiol. 2016;207:6–12. [DOI] [PubMed] [Google Scholar]
- 25. Dorenkamp M, Bonaventura K, Leber AW, et al. Potential lifetime cost‐effectiveness of catheter‐based renal sympathetic denervation in patients with resistant hypertension. Eur Heart J. 2013;34:451–461. [DOI] [PubMed] [Google Scholar]
- 26. Geisler BP, Egan BM, Cohen JT, et al. Cost‐effectiveness and clinical effectiveness of catheter‐based renal denervation for resistant hypertension. J Am Coll Cardiol. 2012;60:1271–1277. [DOI] [PubMed] [Google Scholar]
- 27. Bakris GL, Townsend RR, Flack JM, et al. 12‐month blood pressure results of catheter‐based renal artery denervation for resistant hypertension: the SYMPLICITY HTN‐3 trial. J Am Coll Cardiol. 2015;65:1314–1321. [DOI] [PubMed] [Google Scholar]
- 28. Esler M. Illusions of truths in the Symplicity HTN‐3 trial: generic design strengths but neuroscience failings. J Am Soc Hypertens. 2014;8:593–598. [DOI] [PubMed] [Google Scholar]
- 29. Wang Z, Chen S, Zhou T, et al. Comparison of saline‐irrigated catheter vs. temperature‐controlled catheter for renal denervation in a canine model. Am J Hypertens. 2015;28:1434–1443. [DOI] [PubMed] [Google Scholar]
- 30. Lu J, Wang Z, Zhou T, et al. Selective proximal renal denervation guided by autonomic responses evoked via high‐frequency stimulation in a preclinical canine model. Circ Cardiovasc Interv. 2015;8(6). pii: e001847. [DOI] [PubMed] [Google Scholar]
- 31. Kiuchi MG, Graciano ML, Chen S, et al. Improvement of the renal function after renal sympathetic denervation in refractory hypertensive patients with chronic kidney disease: possible predictors. Int J Cardiol. 2015;199:10–12. [DOI] [PubMed] [Google Scholar]
- 32. Kiuchi MG, Chen S, Hoye NA. Acute vasodilation caused by different strategies of renal sympathetic denervation for right and left renal arteries. Ann Vasc Surg. 2017;38:345–347. [DOI] [PubMed] [Google Scholar]
- 33. Böhm M, Mahfoud F, Ukena C, et al. First report of the Global SYMPLICITY Registry on the effect of renal artery denervation in patients with uncontrolled hypertension. Hypertension. 2015;65:766–774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


