Abstract
The efficacy and safety of olmesartan medoxomil (OM) vs active control (AC) monotherapy among elderly patients aged 60‐79 years (N = 4487) was evaluated by meta‐analysis (25 studies). In all patients, change from baseline to end point in blood pressure (BP) was significantly greater with OM vs AC (−19.5/−11.9 vs −16.8/−10.7 mm Hg). Greater proportions of OM‐ vs AC‐treated patients achieved BP goals. In patients with impaired renal function (estimated glomerular filtration rate <60 mL/min/1.73 m2), OM treatment resulted in a greater mean change from baseline in systolic BP vs AC (−21.2 vs −18.7 mm Hg, respectively) and a greater proportion of patients achieving BP goals. These parameters were similar in both groups for elderly patients with diabetes. OM was well tolerated with few adverse events. OM monotherapy can be used as an initial treatment for hypertension in elderly patients, including those with renal impairment or diabetes.
Keywords: elderly, hypertension, olmesartan medoxomil
1. INTRODUCTION
Hypertension is a chronic disease, which results in a significantly increased risk of cardiovascular events and mortality if left untreated.1, 2, 3 Hypertension affects 26% of adults across the globe.4 The prevalence of hypertension increases with age,5, 6 owing to age‐related changes in arterial structure and function, as well as the impacts of lifestyle factors.7, 8 According to the American Heart Association, the prevalence of hypertension from 2007 to 2012 among men and women aged 65‐74 years was 62.0% and 67.8%, respectively; for those aged ≥75 years, 76.4% and 79.9%, respectively.9
There is a lack of randomized clinical studies to help shape guideline recommendations for the choice of antihypertensive drugs in the elderly, with the exception of the Hypertension in the Very Elderly Trial (HYVET) in which a reduction in systolic blood pressure (SBP) to <150 mm Hg resulted in a reduction in the risk of stroke.10 The Eighth Joint National Committee (JNC 8) recommends that patients aged ≥60 years should pursue a goal of <150 mm Hg for seated SBP (SeSBP) and <90 mm Hg for seated diastolic blood pressure (SeDBP). The JNC 8 also suggests that patients who reach an SeSBP goal of <140 mm Hg with a well‐tolerated medication regimen be maintained on that existing therapy.11 JNC 8 does not make initial therapy recommendations specifically for the elderly; however, it does make recommendations for general patient populations that include the elderly, nonelderly, and those with chronic kidney disease or diabetes mellitus. These recommendations include initial treatment with an angiotensin‐converting enzyme (ACE) inhibitor, an angiotensin II receptor blocker (ARB), a calcium channel blocker (CCB), a diuretic (for nonblack patients), or a CCB or thiazide diuretic (for black patients). Joint guidelines from the American Society of Hypertension and the International Society of Hypertension recommend that patients aged ≥60 years initiate treatment with a CCB or thiazide diuretic, although these guidelines also recognize that ACE inhibitor and ARB treatments are usually effective as initial therapy for nonblack patients aged ≥60 years.12 The European Society of Hypertension (ESH) and European Society of Cardiology (ESC) recommend a SeSBP goal of 140‐150 mm Hg for patients aged ≤80 years with a baseline SeSBP of ≥160 mm Hg. In patients aged ≥80 years, who are in good physical and mental condition, a baseline SeSBP of ≥160 mm Hg is recommended.13 These guidelines also suggest that a SeSBP goal of <140 mm Hg be considered for fit elderly patients aged ≤80 years and recommend that blood pressure (BP) goals in the frail elderly be individualized on a patient‐by‐patient basis. ESH and ESC support a beneficial effect upon treatment of the elderly with an ACE inhibitor, ARB, β‐blocker, CCB, or thiazide diuretic.13 The recent Systolic Blood Pressure Intervention Trial (SPRINT) examined the impact of treating elderly patients to different BP goals; however, the protocol of this trial only encouraged, but did not mandate, the use of specific types of antihypertensive therapies. Furthermore, the study demonstrated that there were no discernible differences when treating frail vs fit elderly patients.14, 15, 16 Thus, the lack of homogenous treatments within the population leaves room for interpretation of the types of therapies to initiate in elderly populations.
In light of the variety of hypertension treatment options recommended for the elderly, a patient‐level meta‐analysis of studies in the olmesartan medoxomil clinical trials program was performed to better inform therapy decisions. This analysis sought to evaluate the BP‐lowering efficacy, safety, and tolerability of olmesartan monotherapy compared to other active control treatments when treating elderly persons with hypertension, including elderly patients with diabetes or mild renal impairment.
2. METHODS
Databases of completed studies within the olmesartan clinical trials program developed by Daiichi Sankyo were combined and evaluated for inclusion in a patient‐level meta‐analysis. Randomized, double‐blind, active‐controlled, phase 2 through phase 4 studies were reviewed for inclusion (n = 46). Studies were considered for inclusion in this analysis if they contained elderly male or female patients aged 60‐79 years and if they evaluated olmesartan monotherapy vs active control (non‐olmesartan) monotherapy with either an ARB, ACE inhibitor, β‐blocker, CCB, or a diuretic for the treatment of hypertension for ≥28 days, whether in the first period in a crossover study design or in a parallel study design. Patients in individual studies provided written informed consent, and the study protocol for each study was reviewed and approved by an institutional review board in accordance with local regulations and requirements at each site. Major efficacy end points included raw SeSBP and SeDBP values over time, changes from baseline in SeSBP and SeDBP over time, the estimated treatment difference between olmesartan and active control monotherapy, and the proportion of patients achieving goals for seated BP (SeBP) of <140/90 mm Hg and SeSBP of <140 mm Hg. Safety end points included the incidence of treatment‐emergent adverse events (TEAEs) and the incidence of individual TEAEs by preferred term and by primary system organ class.
The primary statistical analysis was performed on the full analysis set of elderly patients across all included studies, defined as patients who took ≥1 dose of study medication and who had a non‐missing baseline and ≥1 non‐missing post‐baseline SeBP value. Dosages of olmesartan and active control were as specified in the individual study protocols, with scheduled dose increases or uptitration if necessary (Table S1). Additional analyses were performed on subpopulations of interest including elderly patients with an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, calculated according to the modification of diet in renal disease formula, and elderly patients with diabetes at baseline (defined as one of the following: medical history of diabetes, use of antidiabetic drugs, or glycated hemoglobin ≥6.5%). Changes in SeSBP and SeDBP over time were evaluated from baseline to study end point (week 12) using the last observation carried forward method. A two‐way analysis of covariance (ANCOVA) model was used to calculate the estimated treatment difference (olmesartan minus active control) of the absolute change on mean SeSBP and SeDBP at end point. The estimated treatment difference is presented as the least squares mean with a two‐sided 95% confidence interval (CI) and P value. ANCOVA analyses were performed on studies where at least 1 patient in any of the treatment groups was observed. Heterogeneity has been explored and the Q and I2 statistics are presented. For binary outcome variables, a Cochran‐Mantel‐Haenszel test, adjusting for individual studies, was used to compare treatment groups. All P values should be interpreted in a descriptive exploratory sense as no adjustments for multiplicity were implemented. Baseline demographics and the proportion of patients achieving BP goals are presented by descriptive statistics.
3. RESULTS
Of 46 studies in the olmesartan clinical development program, 21 did not meet the study inclusion criteria (did not include an active comparator with a single drug [n = 20 studies]; did not include an olmesartan‐only treatment arm [n = 9 studies]). Therefore, 25 were chosen for inclusion in the current meta‐analysis (Table S1).17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 The full analysis set included a total of 4487 elderly patients (olmesartan, n = 2374; active control, n = 2113; Table S1). A slightly greater proportion of patients were male (52.2%), with a mean age of 68.2 years, mean body mass index of 27.7 kg/m2, and mean SeBP of 162.7/97.0 mm Hg (Table 1). In the full analysis set, 17% of patients had diabetes and 21.7% had an eGFR <60 mL/min/1.73 m2. Baseline characteristics were largely similar in the subpopulations of elderly patients with an eGFR <60 mL/min/1.73 m2 and diabetes, with the exception of sex, in which the majority of patients with an eGFR <60 mL/min/1.73 m2 were female (61.0%).
Table 1.
Baseline demographics in the full analysis set
| Full analysis set | eGFR < 60 mL/min/1.73 m2 | Patients with diabetes | ||||
|---|---|---|---|---|---|---|
| Olmesartan (n = 2374) | Active control (n = 2113) | Olmesartan (n = 578) | Active control (n = 387) | Olmesartan (n = 399) | Active control (n = 362) | |
| Age, y | 68.3 ± 5.0 | 68.0 ± 5.0 | 69.3 ± 5.1 | 68.9 ± 4.9 | 68.9 ± 5.0 | 68.4 ± 4.9 |
| Body mass index, kg/m2 | 27.7 ± 4.3 | 27.8 ± 4.2 | 28.3 ± 4.5 | 28.2 ± 4.7 | 28.7 ± 4.5 | 28.6 ± 4.3 |
| Sex, n (%) | ||||||
| Male | 1217 (51.3) | 1124 (53.2) | 230 (39.8) | 146 (37.7) | 227 (56.9) | 208 (57.5) |
| Female | 1157 (48.7) | 989 (46.8) | 348 (60.2) | 241 (62.3) | 172 (43.1) | 154 (42.5) |
| Race, n (%) | ||||||
| Asian | 227 (9.6) | 216 (10.2) | 17 (2.9) | 13 (3.4) | 31 (7.8) | 31 (8.6) |
| Black or African American | 60 (2.5) | 78 (3.7) | 22 (3.8) | 19 (4.9) | 16 (4.0) | 20 (5.5) |
| Hispanic or Latino | 24 (1.0) | 41 (1.9) | 0 (0.0) | 1 (0.3) | 2 (0.5) | 4 (1.1) |
| White | 2061 (86.8) | 1777 (84.1) | 539 (93.3) | 354 (91.5) | 349 (87.5) | 307 (84.8) |
| Other | 2 (0.1) | 1 (<0.1) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 0 (0.0) |
| SeSBP, mm Hg | 162.9 ± 13.0 | 162.5 ± 13.1 | 164.1 ± 13.3 | 163.4 ± 13.9 | 163.3 ± 13.1 | 163.1 ± 13.4 |
| SeDBP, mm Hg | 96.9 ± 8.4 | 97.2 ± 8.2 | 95.9 ± 9.0 | 96.9 ± 8.7 | 95.2 ± 9.3 | 95.2 ± 8.4 |
| Diabetes, n (%) | 401 (16.9) | 362 (17.1) | 114 (19.7) | 75 (19.4) | 399 (100.0) | 362 (100.0) |
| eGFR, n (%) | ||||||
| >90 mL/min/1.73 m2 | 456 (19.2) | 489 (23.1) | 0 (0.0) | 0 (0.0) | 77 (19.3) | 74 (20.4) |
| >60 to ≤90 mL/min/1.73 m2 | 1319 (55.6) | 1213 (57.4) | 0 (0.0) | 0 (0.0) | 204 (51.1) | 208 (57.5) |
| >45 to ≤60 mL/min/1.73 m2 | 489 (20.6) | 340 (16.1) | 488 (84.4) | 334 (86.3) | 93 (23.3) | 65 (18.0) |
| >30 to ≤45 mL/min/1.73 m2 | 75 (3.2) | 50 (2.4) | 75 (13.0) | 50 (12.9) | 19 (4.8) | 11 (3.0) |
| >15 to ≤30 mL/min/1.73 m2 | 15 (0.6) | 3 (0.1) | 15 (2.6) | 3 (0.8) | 2 (0.5) | 0 (0.0) |
| Missing data | 20 (0.8) | 18 (0.9) | 0 (0.0) | 0 (0.0) | 4 (1.0) | 4 (1.1) |
eGFR, estimated glomerular filtration rate; SeDBP, seated diastolic blood pressure; SeSBP, seated systolic blood pressure.
Data are shown as mean ± standard deviation unless otherwise stated.
3.1. Efficacy
In the full analysis set, SeSBP (Figure 1A) and SeDBP (Figure 1B) were decreased in a similar fashion over time in both treatment groups, with the majority of the decrease in BP observed by week 2. Additional reductions were observed at weeks 4 through 12 and were generally sustained at the end point observation. The change in SeBP from baseline to end point at week 12 was greater in the olmesartan monotherapy group (−19.5/−11.9 mm Hg) compared with the active control group (−16.8/−10.7 mm Hg; Figure 1C,D).
Figure 1.
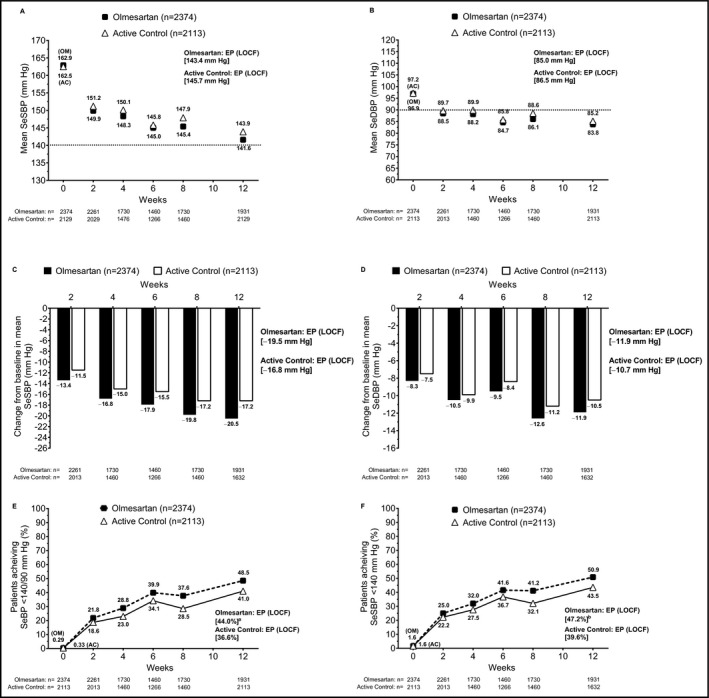
Effect of drug treatment on the elderly patient population in the full analysis set. (A) Mean SeSBP over time; (B) mean SeDBP over time; changes from baseline in mean (C) SeSBP and (D) SeDBP over time; achievement of (E) SeBP <140/90 mm Hg and (F) SeSBP <140 mm Hg from baseline through study end point (LOCF). aP = .0011 for OM vs AC based on Cochran‐Mantel‐Haenszel test. bP = .0004 for OM vs AC based on Cochran‐Mantel‐Haenszel test. AC, active control; EP, end point; LOCF, last observation carried forward; OM, olmesartan medoxomil; SeBP, seated blood pressure; SeDBP, seated diastolic blood pressure; SeSBP, seated systolic blood pressure
The rates of BP goal achievement among elderly patients increased over time in both treatment groups. Compared with active control, a greater proportion of patients treated with olmesartan achieved the SeBP goal of <140/90 mm Hg (36.6% vs 44.0%, respectively; Figure 1E) and the SeSBP goal of <140 mm Hg (39.6% vs 47.2%, respectively; Figure 1F) at end point.
The estimated treatment difference in the absolute change in mean SeSBP for olmesartan vs active control at end point was significantly in favor of olmesartan at −1.35 mm Hg (95% CI, −2.52 to −0.19; Figure 2; Table S1). When comparing the estimated treatment differences for changes in mean SeSBP at end point for each individual active control comparator, olmesartan monotherapy was significantly more effective at lowering SeSBP than ACE inhibitor (estimated treatment difference, −3.54 mm Hg; 95% CI, −5.54 to −1.54) and β‐blocker monotherapy (−3.43 mm Hg; 95% CI, −6.03 to −0.84; Table S2). Olmesartan was as effective at reducing SeSBP as comparator ARB (estimated treatment difference, −1.38 mm Hg; 95% CI, −3.56 to 0.79), CCB (1.77 mm Hg; 95% CI, −0.51 to 4.04), and diuretic therapy (−2.64 mm Hg; 95% CI, −6.83 to 1.55).
Figure 2.
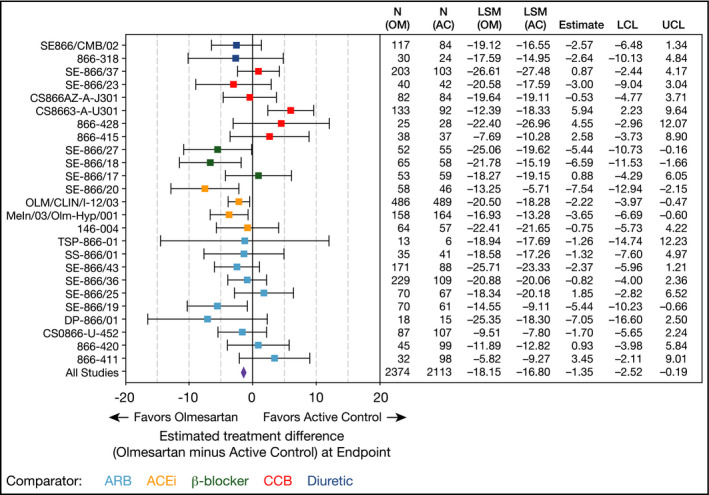
Two‐way analysis of covariance of absolute change on mean seated systolic blood‐pressure from baseline to end point in the full analysis set (wk 12, last observation carried forward). For the estimated treatment difference (all studies), the exploratory P value is .023 and heterogeneity values are Q = 47.138, P = .003, and I 2 = 49.086. AC, active control; ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; CI, confidence interval; LCL, lower confidence limit; LSM, least‐squares mean; OM, olmesartan medoxomil; UCL, upper confidence limit
In the subpopulation of elderly patients with an eGFR <60 mL/min/1.73 m2 (olmesartan, n = 578; active control, n = 387), both olmesartan and active control monotherapy were effective at lowering SeSBP over time, similar to results observed in the full analysis set. Reductions in SeSBP were observed by week 2 and additional reductions were seen at weeks 4 through 12, which were sustained at end point (Figure 3A). In this subpopulation, the change from baseline to end point in mean SeBP was −21.2 mm Hg for olmesartan vs −18.7 mm Hg for active control (Figure 3B). Similar trends were observed when examining diastolic BP (Figure S1).
Figure 3.
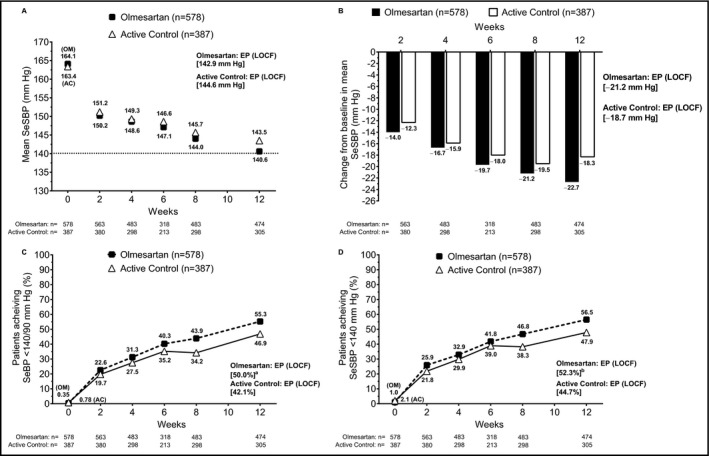
Effect of drug treatment on the elderly patient population with an estimated glomerular filtration rate <60 mL/min/1.73 m2. (A) Mean SeSBP over time; (B) change from baseline in mean SeSBP over time; achievement of (C) SeBP <140/90 mm Hg and (D) SeSBP <140 mm Hg from baseline through study end point (LOCF). aP = .2301 for OM vs AC based on Cochran‐Mantel‐Haenszel test. bP = .2470 for OM vs AC based on Cochran‐Mantel‐Haenszel test. AC, active control; EP, end point; LOCF, last observation carried forward; OM, olmesartan medoxomil; SeBP, seated blood pressure; SeSBP, seated systolic blood pressure
The estimated treatment difference in the absolute change from baseline to end point in mean SeSBP was similar to active control for patients with an eGFR <60 mL/min/1.73 m2 (0.69 mm Hg; 95% CI, −3.44 to 4.81). The SeBP goal (<140/90 mm Hg) was achieved by a greater proportion of patients receiving olmesartan (50.0%) compared with active control (42.1%; Figure 3C). A similar observation was made when evaluating the SeSBP goal achievement of <140 mm Hg in this subpopulation (52.3% vs 44.7%, respectively; Figure 3D).
The subpopulation of elderly patients with diabetes included 399 and 362 patients in the olmesartan and active control groups, respectively. Mean SeSBP at end point was similar for both treatment groups in this population (144.8 vs 144.1 mm Hg for the olmesartan and active control groups, respectively; Figure 4A), as was the mean change from baseline in SeSBP (−18.4 vs −19.0 mm Hg; Figure 4B). The similarity in results between groups observed for SeSBP was mirrored in the results for SeDBP (Figure S2).
Figure 4.
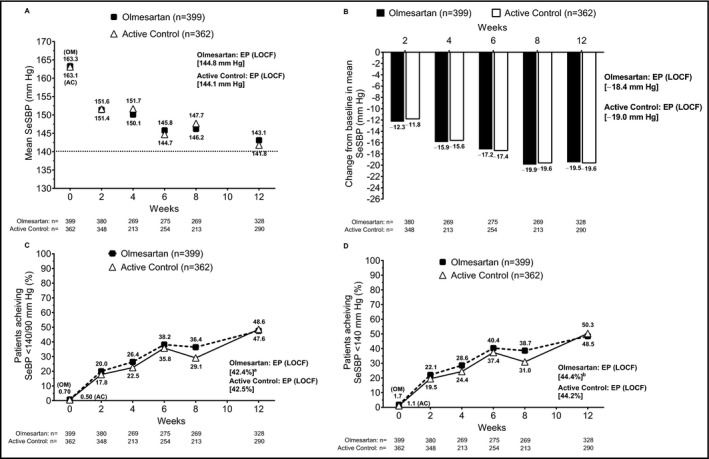
Effect of drug treatment on the elderly patient population with diabetes. (A) Mean SeSBP over time; (B) change from baseline in mean SeSBP over time; achievement of (C) SeBP <140/90 mm Hg and (D) SeSBP <140 mm Hg from baseline through study end point (LOCF). aP = .4478 for OM vs AC based on Cochran‐Mantel‐Haenszel test. bP = .5853 for OM vs AC based on Cochran‐Mantel‐Haenszel test. AC, active control; EP, end point; LOCF, last observation carried forward; OM, olmesartan medoxomil; SeBP, seated blood pressure; SeSBP, seated systolic blood pressure
The estimated treatment difference in the absolute change from baseline to end point in mean SeSBP by ANCOVA was not significantly different from active control in the subpopulation of elderly patients with diabetes (3.59 mm Hg; 95% CI, −0.03 to 7.22). At end point, a similar proportion of patients in the olmesartan and active control treatment groups achieved the goals of SeBP <140/90 mm Hg (42.4% vs 42.5%, respectively; Figure 4C) and SeSBP <140 mm Hg (44.4% vs 44.2%; Figure 4D).
3.2. Safety
In the full analysis set population, 33.7% and 31.9% of elderly patients in the olmesartan and active control treatment groups, respectively, experienced ≥1 TEAE (Table 2). Incidences of drug‐related TEAEs, serious TEAEs, and serious drug‐related TEAEs were similar between treatment groups. The incidence of the top 5 most frequent individual TEAEs (≥1% incidence) was also similar between treatment groups and included headache (3.3% vs 3.0%), nasopharyngitis (2.3% vs 2.5%), dizziness (2.3% vs 2.1%), cough (1.1% vs 2.2%), and back pain (1.5% vs 1.3%) in the olmesartan vs active control treatment groups, respectively. The incidence of hypotension and orthostatic hypotension was infrequent but numerically greater for olmesartan (hypotension, 0.4% vs 0.1% for olmesartan vs active control, respectively; orthostatic hypotension, 0.1% vs 0.0%). Renal and urinary TEAEs were also infrequently reported and included hematuria (0.3% vs 0.4%), pollakiuria (0.2% vs 0.3%), and proteinuria (0.2% vs 0.1%) in the olmesartan and active control groups, respectively. There were 2 deaths in the olmesartan treatment group and 1 death in the active control group.
Table 2.
Incidence of treatment‐emergent adverse events
| Variable, n (%) | Full analysis set | eGFR <60 mL/min/1.73 m2 | Patients with diabetes | |||
|---|---|---|---|---|---|---|
| Olmesartan (n = 2374) | Active control (n = 2112) | Olmesartan (n = 578) | Active control (n = 387) | Olmesartan (n = 399) | Active control (n = 362) | |
| TEAEs | 801 (33.7) | 674 (31.9) | 211 (36.5) | 130 (33.6) | 144 (36.1) | 113 (31.2) |
| Serious TEAEs | 34 (1.4) | 25 (1.2) | 9 (1.6) | 11 (2.8) | 3 (0.8) | 4 (1.1) |
| Drug‐related TEAEs | 222 (9.4) | 215 (10.2) | 47 (8.1) | 47 (12.1) | 32 (8.0) | 25 (6.9) |
| Serious drug‐related TEAEs | 4 (0.2) | 4 (0.2) | 0 (0.0) | 4 (1.0) | 0 (0.0) | 0 (0.0) |
| Deaths | 2 (0.1) | 1 (0.1) | 0 (0.0) | 1 (0.3) | 0 (0.0) | 0 (0.0) |
| Individual TEAEs (≥1% incidence)a | ||||||
| Headache | 79 (3.3) | 63 (3.0) | 17 (2.9) | 11 (2.8) | 12 (3.0) | 10 (2.8) |
| Nasopharyngitis | 54 (2.3) | 52 (2.5) | 7 (1.2) | 4 (1.0) | 9 (2.3) | 3 (0.8) |
| Dizziness | 54 (2.3) | 44 (2.1) | 15 (2.6) | 11 (2.8) | 8 (2.0) | 6 (1.7) |
| Cough | 27 (1.1) | 47 (2.2) | 7 (1.2) | 6 (1.6) | 3 (0.8) | 2 (0.6) |
| Back pain | 36 (1.5) | 27 (1.3) | 8 (1.4) | 4 (1.0) | 6 (1.5) | 4 (1.1) |
| Peripheral edema | 27 (1.1) | 29 (1.4) | 6 (1.0) | 8 (2.1) | 5 (1.3) | 9 (2.5) |
| Bronchitis | 26 (1.1) | 20 (1.0) | 6 (1.0) | 7 (1.8) | 3 (0.8) | 8 (2.2) |
| Diarrhea | 29 (1.2) | 15 (0.7) | 0 (0.0) | 3 (0.8) | 7 (1.8) | 2 (0.6) |
| Upper respiratory tract infection | 18 (0.8) | 24 (1.1) | 2 (0.4) | 2 (0.5) | 1 (0.3) | 6 (1.7) |
| Arthralgia | 19 (0.8) | 21 (1.0) | 3 (0.5) | 2 (0.5) | 5 (1.3) | 2 (0.6) |
eGFR, estimated glomerular filtration rate; TEAE, treatment‐emergent adverse event.
Preferred term by primary system organ class.
Safety results were largely similar in the subpopulations of interest. The incidence of ≥1 TEAE was numerically higher in the olmesartan treatment group vs the active control group among elderly patients with an eGFR <60 mL/min/1.73 m2 and among those with diabetes (Table 2). Serious TEAEs were more frequent in the active control group for both subpopulations of interest. For both subpopulations of interest, the incidences of individual TEAEs were similar between treatment groups. The maximum difference in incidence was no more than 1.5% between groups for any individual TEAE in either subpopulation examined.
4. DISCUSSION
In an environment where clinical practice guidelines avoid making firm recommendations on the best initial choice of antihypertensive therapy,11, 12, 13 this analysis provides insight into the overall efficacy and safety of olmesartan and other commonly recommended medications for the treatment of hypertension in the elderly. Treatment with olmesartan monotherapy resulted in a greater reduction in SeSBP from baseline to end point in the full analysis set compared with the overall active control treatment group. Olmesartan was also efficacious in the subpopulation of elderly patients with mild renal impairment and those with diabetes. Considering the enhanced or similar efficacy of olmesartan in comparison with other antihypertensive treatments that are considered as initial therapy options among the elderly, the results presented herein suggest that olmesartan may likewise be suitable as an initial treatment option in this population.
The current meta‐analysis allowed for evaluation of olmesartan monotherapy vs individual active control treatments, as well as the combined effect of all active control comparators. When analyzed by individual active control treatments, olmesartan monotherapy was shown to be more effective at lowering SeSBP than ACE inhibitors and β‐blockers and as effective at lowering BP compared with ARBs, CCBs, and diuretics. Whereas olmesartan was shown to be numerically superior to each individual comparator or the overall active control group, the estimated treatment difference in all circumstances ranged from 1.4 tp 3.5 mm Hg. This difference may not be clinically relevant when considering the very high SeSBP (≥160 mm Hg) that is often seen in elderly patients; in this analysis, the baseline SeSBP in the full analysis set was 162.7 mm Hg.
The favorable efficacy results of olmesartan monotherapy provide a foundation for exploring the efficacy of olmesartan combination therapy in elderly patients. Despite the high baseline SeBP in the current meta‐analysis, almost one‐half of patients treated with olmesartan monotherapy in the full analysis set achieved the BP goal of <140/90 mm Hg, and the use of combination therapy is expected to further increase BP goal achievement rates. Previous studies have demonstrated greater BP‐lowering efficacy for olmesartan dual combination therapy vs monotherapy in a variety of patient populations.20, 21, 32, 33 In this meta‐analysis of elderly patients, the greater reduction in BP from baseline to end point and higher proportion of patients with SeBP <140/90 mm Hg for olmesartan monotherapy than the overall active control group suggests that olmesartan‐based combinations may be more effective than those with a different antihypertensive drug. This has been observed in a cross‐study comparison of several ARBs in combination with hydrochlorothiazide.34 However, additional evidence is needed to evaluate the efficacy and safety of olmesartan dual combination therapy in elderly patients, including those with mild renal impairment or diabetes. A meta‐analysis evaluating the efficacy and safety of olmesartan dual combination therapy vs monotherapy in elderly patients is currently being conducted in the same study population and those results are forthcoming in a separate manuscript.
Results in the subpopulation of elderly patients with an eGFR <60 mL/min/1.73 m2 mostly mirrored those efficacy findings in the full analysis set. Interestingly, greater proportions of elderly patients with mild renal impairment achieved overall BP and SeSBP goals (50.0% and 52.3%, respectively) vs patients in the full analysis set (44.0% and 47.2%, respectively), despite similar starting baseline BP values. Olmesartan monotherapy demonstrated efficacy in reducing BP in the subpopulation of elderly patients with diabetes; although the efficacy was not significantly different from that observed with active control therapy, the reduction in SBP was numerically greater for active control. These results may be a consequence of the greater need for combination therapy to achieve BP goals in patients with diabetes.35, 36
The recent randomized controlled SPRINT trial has expanded the dialogue regarding the treatment of hypertension.14 Analyses among the study population aged ≥75 years has demonstrated a significant improvement in hard outcomes of interest (fewer fatal and nonfatal cardiovascular events and deaths) among patients who were treated to an intensive goal of <120 mm Hg, compared with those treated to a standard goal of <140 mm Hg.15 Most of the studies included for analysis in the integrated database targeted a BP goal of <140/90 mm Hg according to current guidelines; therefore, we selected the same BP goal for evaluation here. Olmesartan was found to be superior to, or as effective as, other antihypertensive treatment options when treating to a goal of <140 mm Hg in the full analysis set and the subpopulations analyzed. Post hoc analyses also examined the SeSBP goal of <130 mm Hg and found similar results among all examined populations (data not shown). The data presented herein provide an additional level of detail for clinicians treating elderly patients with comorbidities and suggest that olmesartan could be considered alongside the active control comparators used in this study as an initial treatment option.
Safety is an important consideration, particularly in an aging population. The safety of olmesartan treatment in elderly patients was similar to the findings in the active control treatment group with respect to the incidence of overall TEAEs and individual TEAEs. A recent meta‐analysis of 16 randomized trials in elderly patients treated with ARB therapy indicated that ARBs were associated with a significantly increased risk of acute kidney injury, hypotension, and hyperkalemia.37 Hypotension occurred in less than 0.4% of patients treated with olmesartan in this analysis and neither acute kidney injury nor hyperkalemia was reported in both treatment groups. The safety profile of olmesartan in the general population of elderly patients was similar to that observed in the subpopulations of patients with diabetes and mild renal impairment. Overall, the favorable safety profile of olmesartan among all examined populations offers a potential advantage over other antihypertensive therapies.
5. STUDY LIMITATIONS AND STRENGTHS
One of the strengths of this meta‐analysis lies in the inclusion of both published17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 and unpublished randomized controlled trials; however, all studies were industry‐sponsored from a single pharmaceutical group. Due to the number of studies included, the study included a large number of patients. One limitation of the current study is the degree of heterogeneity that exists among individual study designs, in which the dosing, dose uptitration timing, and follow‐up times vary slightly over the large number of studies included. Due to the degree of heterogeneity of the included studies, the sample sizes of the sub‐analyses were considerably smaller than that of the overall full analysis set and thus weakened the statistical power to make comparisons. Furthermore, only 3 studies that included an active comparator of β‐blockers qualified for inclusion in this meta‐analysis. To address the issue of missing data, the current analysis focused on last observation carried forward effects and, as such, may have imparted bias in the results. It has been postulated that regression to the mean is a possible explanation for the rapid drop in BP observed in the first 2 weeks of study in both the OM and active control groups; however, the results of this study can neither confirm nor deny this possibility. Lastly, we analyzed and evaluated efficacy and safety during the limited treatment period of 12 weeks and the individual component studies included in the analysis were not designed to inform on major safety outcomes of interest in patients with hypertension.
6. CONCLUSIONS
On the basis of BP‐lowering efficacy, goal achievement, and safety, it can be strongly argued that olmesartan belongs as an initial treatment choice in the management of hypertension in elderly patients, alongside the other pharmacologic classes evaluated in this analysis. This interpretation extends to those patients who have mild renal impairment or diabetes.
CONFLICTS OF INTEREST
JR is an advisory board member and lecturer for Boehringer Ingelheim, Daiichi Sankyo, and Menarini International and is a lecturer for Novartis. MAW has served as a consultant for Allergan, Daiichi Sankyo, and Novartis and as a speaker for Menarini and Merck Sharp & Dohme. P‐ER is an employee of Daiichi Sankyo. J‐GW reports receiving research grants from Bayer and Pfizer and lecture and consulting fees from Bayer, Daiichi Sankyo, Merck Sharp & Dohme, Novartis, Pfizer, Sanofi, and Servier.
AUTHOR CONTRIBUTIONS
JR reviewed and edited the manuscript and provided substantial feedback on content. MAW contributed original ideas to guide the analysis, made recommendations for the content, and participated in reviewing and editing the manuscript. P‐ER performed the identification of studies for the integrated database and for the subgroups in the meta‐analysis, performed statistical analyses, and provided feedback in the drafting and editing of the manuscript. J‐GW participated in the interpretation of the data, and reviewed and edited the manuscript.
Supporting information
Redon J, Weber MA, Reimitz P‐E, Wang J‐G. Comparative effectiveness of an angiotensin receptor blocker, olmesartan medoxomil, in older hypertensive patients. J Clin Hypertens. 2018;20:356–365. 10.1111/jch.13183
Funding information
Jessica Deckman, PhD, CMPP, Raewyn Poole, MSc, and Robert Schupp, PharmD, CMPP, of inScience Communications, Springer Healthcare (Philadelphia, PA), provided medical writing support funded by Daiichi Sankyo, Inc.
REFERENCES
- 1. Ford ES. Trends in mortality from all causes and cardiovascular disease among hypertensive and nonhypertensive adults in the United States. Circulation. 2011;123:1737‐1744. [DOI] [PubMed] [Google Scholar]
- 2. Muntner P, Davis BR, Cushman WC, et al. Treatment‐resistant hypertension and the incidence of cardiovascular disease and end‐stage renal disease: results from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension. 2014;64:1012‐1021. [DOI] [PubMed] [Google Scholar]
- 3. Verdecchia P, Reboldi G, Angeli F, et al. Systolic and diastolic blood pressure changes in relation with myocardial infarction and stroke in patients with coronary artery disease. Hypertension. 2015;65:108‐114. [DOI] [PubMed] [Google Scholar]
- 4. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217‐223. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization . A global brief on hypertension. Geneva, Switzerland, 2013. http://ish-world.com/downloads/pdf/global_brief_hypertension.pdf. Accessed March 3, 2016. [Google Scholar]
- 6. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief. 2013;133:1‐8. [PubMed] [Google Scholar]
- 7. Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation. 2011;123:243‐2506. [DOI] [PubMed] [Google Scholar]
- 8. Harvey A, Montezano AC, Touyz RM. Vascular biology of ageing–implications in hypertension. J Mol Cell Cardiol. 2015;83:112‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2016 update: a report from the American Heart Association. Circulation. 2016;133:e38‐e360. [DOI] [PubMed] [Google Scholar]
- 10. Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887‐1898. [DOI] [PubMed] [Google Scholar]
- 11. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507‐520. [DOI] [PubMed] [Google Scholar]
- 12. Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens. 2014;16:14‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159‐2219. [DOI] [PubMed] [Google Scholar]
- 14. SPRINT Research Group , Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673‐2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chobanian AV. SPRINT results in older patients: how low to go? JAMA. 2016;315:2669‐2670. [DOI] [PubMed] [Google Scholar]
- 17. Ball KJ, Williams PA, Stumpe KO. Relative efficacy of an angiotensin II antagonist compared with other antihypertensive agents. Olmesartan medoxomil versus antihypertensives. J Hypertens Suppl. 2001;19:S49‐S56. [DOI] [PubMed] [Google Scholar]
- 18. Brunner HR, Stumpe KO, Januszewicz A. Antihypertensive efficacy of olmesartan medoxomil and candesartan cilexetil assessed by 24‐hour ambulatory blood pressure monitoring in patients with essential hypertension. Clin Drug Investig. 2003;23:419‐430. [DOI] [PubMed] [Google Scholar]
- 19. Chrysant SG, Marbury TC, Robinson TD. Antihypertensive efficacy and safety of olmesartan medoxomil compared with amlodipine for mild‐to‐moderate hypertension. J Hum Hypertens. 2003;17:425‐432. [DOI] [PubMed] [Google Scholar]
- 20. Chrysant SG, Melino M, Karki S, Lee J, Heyrman R. The combination of olmesartan medoxomil and amlodipine besylate in controlling high blood pressure: COACH, a randomized, double‐blind, placebo‐controlled, 8‐week factorial efficacy and safety study. Clin Ther. 2008;30:587‐604. [DOI] [PubMed] [Google Scholar]
- 21. Chrysant SG, Weber MA, Wang AC, Hinman DJ. Evaluation of antihypertensive therapy with the combination of olmesartan medoxomil and hydrochlorothiazide. Am J Hypertens. 2004;17:252‐259. [DOI] [PubMed] [Google Scholar]
- 22. Giles TD, Oparil S, Silfani TN, Wang A, Walker JF. Comparison of increasing doses of olmesartan medoxomil, losartan potassium, and valsartan in patients with essential hypertension. J Clin Hypertens (Greenwich). 2007;9:187‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heagerty AM, Mallion JM. Olmesartan medoxomil in elderly patients with essential or isolated systolic hypertension: efficacy and safety data from clinical trials. Drugs Aging. 2009;26:61‐76. [DOI] [PubMed] [Google Scholar]
- 24. Malacco E, Omboni S, Volpe M, Auteri A, Zanchetti A, Group ES. Antihypertensive efficacy and safety of olmesartan medoxomil and ramipril in elderly patients with mild to moderate essential hypertension: the ESPORT study. J Hypertens. 2010;28:2342‐2350. [DOI] [PubMed] [Google Scholar]
- 25. Mallion JM, Heagerty A, Laeis P. Systolic blood pressure reduction with olmesartan medoxomil versus nitrendipine in elderly patients with isolated systolic hypertension. J Hypertens. 2007;25:2168‐2177. [DOI] [PubMed] [Google Scholar]
- 26. Ogihara T, Saruta T, Shimada K, Kuramoto K. A randomized, double‐blind, four‐arm parallel‐group study of the efficacy and safety of azelnidipine and olmesartan medoxomil combination therapy compared with each monotherapy in Japanese patients with essential hypertension: the REZALT study. Hypertens Res. 2009;32:1148‐1154. [DOI] [PubMed] [Google Scholar]
- 27. Oparil S, Williams D, Chrysant SG, Marbury TC, Neutel J. Comparative efficacy of olmesartan, losartan, valsartan, and irbesartan in the control of essential hypertension. J Clin Hypertens (Greenwich). 2001;3:283‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Punzi HA, Lewin A, Li W, Chavanu KJ. Efficacy/safety of olmesartan medoxomil versus losartan potassium in naive versus previously treated subjects with hypertension. Adv Ther. 2012;29:524‐537. [DOI] [PubMed] [Google Scholar]
- 29. Rosendorff C, Dubiel R, Xu J, Chavanu KJ. Comparison of olmesartan medoxomil versus amlodipine besylate on regression of ventricular and vascular hypertrophy. Am J Cardiol. 2009;104:359‐365. [DOI] [PubMed] [Google Scholar]
- 30. Stumpe KO, Agabiti‐Rosei E, Zielinski T, et al. Carotid intima‐media thickness and plaque volume changes following 2‐year angiotensin II‐receptor blockade. The Multicentre Olmesartan atherosclerosis Regression Evaluation (MORE) study. Ther Adv . Cardiovasc Dis. 2007;1:97‐106. [DOI] [PubMed] [Google Scholar]
- 31. Stumpe KO, Ludwig M. Antihypertensive efficacy of olmesartan compared with other antihypertensive drugs. J Hum Hypertens. 2002;16(Suppl 2):S24‐S28. [DOI] [PubMed] [Google Scholar]
- 32. Chrysant SG, Lee J, Melino M, Karki S, Heyrman R. Efficacy and tolerability of amlodipine plus olmesartan medoxomil in patients with difficult‐to‐treat hypertension. J Hum Hypertens. 2010;24:730‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shimada K, Ogihara T, Saruta T, Kuramoto K. Effects of combination olmesartan medoxomil plus azelnidipine versus monotherapy with either agent on 24‐hour ambulatory blood pressure and pulse rate in Japanese patients with essential hypertension: additional results from the REZALT study. Clin Ther. 2010;32:861‐881. [DOI] [PubMed] [Google Scholar]
- 34. Ram CV. Antihypertensive efficacy of angiotensin receptor blockers in combination with hydrochlorothiazide: a review of the factorial‐design studies. J Clin Hypertens. 2004;6:569‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206‐1252. [DOI] [PubMed] [Google Scholar]
- 36. Kereiakes DJ, Chrysant SG, Izzo JL Jr, et al. Olmesartan/amlodipine/hydrochlorothiazide in participants with hypertension and diabetes, chronic kidney disease, or chronic cardiovascular disease: a subanalysis of the multicenter, randomized, double‐blind, parallel‐group TRINITY study. Cardiovasc Diabetol. 2012;11:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Elgendy IY, Huo T, Chik V, Pepine CJ, Bavry AA. Efficacy and safety of angiotensin receptor blockers in older patients: a meta‐analysis of randomized trials. Am J Hypertens. 2015;28:576‐585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


