Abstract
In an 8‐week randomized trial of patients with mild or moderate hypertension, the authors investigated the efficacy and tolerability of initial high (5.0 mg/d) vs low (2.5 mg/d) doses of S‐(‐)‐amlodipine (equivalent to 5 and 10 mg of racemic amlodipine, respectively). In the S‐(‐)‐amlodipine 2.5‐mg group (n=263), 24‐hour ambulatory systolic/diastolic blood pressure (±standard deviation) decreased from 131.5±15.0/82.1±10.7 mm Hg at baseline to 126.0±13.5/78.5±9.5 mm Hg at 8 weeks of follow‐up by a least square mean (±standard error) change of 6.0±0.6/3.8±0.4 mm Hg. In the S‐(‐)‐amlodipine 5‐mg group (n=260), the corresponding changes were from 133.6±13.7/83.1±9.9 mm Hg to 125.0±12.0/78.2±8.9 mm Hg by 8.1±0.6/4.7±0.4 mm Hg, respectively. The between‐group differences in changes in 24‐hour systolic/diastolic blood pressure were 2.1/0.9 (P=.02/.17) mm Hg. Similar trends were observed for daytime and nighttime ambulatory and clinic blood pressure. The incidence rate was similar for all adverse events. An initial high dose of S‐(‐)‐amlodipine improved ambulatory blood pressure control with similar tolerability as an initial low dose in hypertension.
Keywords: ambulatory blood pressure, efficacy, initial dose, S‐(‐)‐amlodipine, tolerability
1. INTRODUCTION
For mild and moderate hypertension, current hypertension guidelines recommend initial low‐dose monotherapy of antihypertensive drugs.1, 2 If the goal blood pressure (BP) is not achieved with the low‐dose monotherapy within a few weeks, the initial therapy can either be uptitrated to a higher or maximum dose or combined with another drug of different mode of action. A major advantage of the initial low‐dose monotherapy approach is the possible low incidence of adverse effects.3 However, this approach may delay the goal‐attaining time, discouraging patients from achieving BP control, and negatively influence adherence to treatment. This may, to some extent, contribute to the low control rates in many countries.4, 5
There is a trend that long‐acting antihypertensive drugs, especially angiotensin receptor blockers such as telmisartan, be started at the maximum dosage.6 However, amlodipine, the most commonly used long‐acting calcium channel blocker, is usually started at the lower dosage of 5 mg daily or the even lower dosage of 2.5 mg daily.1, 2 A major concern is adverse effects, eg, ankle edema.7 In a recent study in Chinese patients with mild and moderate hypertension, two different dosages of the S‐(‐) enantiomer of amlodipine (2.5 and 5 mg) were compared as initial therapy.8 S‐(‐)‐amlodipine is the enantiomer with calcium channel affinity and BP‐lowering action.9 S‐(‐)‐amlodipine 2.5 mg and 5 mg are equivalent to 5 mg and 10 mg of racemic amlodipine, respectively.
In the present analysis,8 we investigated the ambulatory BP–lowering efficacy and tolerability of initial high (5 mg) vs low (2.5 mg) doses of S‐(‐)‐amlodipine in patients with mild and moderate hypertension.
2. METHODS
2.1. Study design
The present study was a multicenter, open‐label, randomized, parallel‐group trial (ClinicalTrials.gov number, NCT01131546) conducted from October 2010 to May 2011 in 22 hospitals across China. The sponsor of the trial, Simcere Pharmaceutical (Nanjing, Jiangsu Province, China), together with the principal investigator of the trial (Dayi Hu from Peking University People's Hospital), designed the study and supervised the conduct of the trial and collection of the data. The study complied with the International Conference on Harmonization Guidelines for Good Clinical Practice local regulations and the ethical principles of the Declaration of Helsinki. The study protocol was approved by the ethics committees of all participating hospitals. Written informed consent was obtained from all patients. The study protocol was previously published in detail in the Chinese literature with an English abstract.8 The trial included three groups (racemic amlodipine maleate 5 mg/d and S‐(‐)‐amlodipine besylate 2.5 mg/d or 5 mg/d) with two comparisons: amlodipine maleate 5 mg/d vs S‐(‐)‐amlodipine besylate 2.5 mg/d for efficacy equivalence and S‐(‐)‐amlodipine besylate 2.5 vs 5 mg/d for efficacy superiority of the high dose. The present analysis was restricted to the latter superiority trial and focused on ambulatory BP–lowering efficacy.
The study included two clinic visits during an 8‐week randomized treatment period for the assessment of efficacy and safety. After screening at the first clinic visit, eligible patients were immediately randomized in a 1:1 ratio to receive a once‐daily oral dose of S‐(‐)‐amlodipine 2.5 mg/d or 5 mg/d and followed up at the second clinic visit at 8 weeks. During the study treatment period, patients were instructed to take their study medication between 7 am and 10 am. Clinic BP measurement, laboratory tests of blood and urine, electrocardiography, and physical examinations were performed at the initial and terminating visits for all patients. Ambulatory BP monitoring (ABPM) was also performed at the two clinic visits if the patients were willing and could tolerate the measurement. The study medication was supplied free of charge for the entire study period by Simcere Pharmaceutical.
2.2. Study population
Eligible patients were men and women aged 18 to 75 years with mild or moderate hypertension. Previously untreated patients were required to have a systolic/diastolic BP of 140 to 179/90 to 109 mm Hg (mean of three readings at the initial clinic visit). Patients with previously treated hypertension but uncontrolled BP (systolic/diastolic BP of 140–179/90–109 mm Hg) with antihypertensive monotherapy could enter the trial if they were willing to switch their previous treatment to the study treatment.
Exclusion criteria included secondary hypertension; severe hypertension (systolic BP ≥180 mm Hg or diastolic BP ≥110 mm Hg); use of medication that may affect BP (monoamine oxidase inhibitors, anesthetics, antidepressant drugs of the third or fourth ring, corticosteroids, thyroid hormones, nasal decongestant drugs containing high doses of sympathomimetic medicine); allergy to dihydropyridine calcium channel blockers; acute cardiovascular events within 3 months prior to enrollment; congestive heart failure, unstable angina, or severe cardiac arrhythmia; hepatic impairment (aspartate or alanine aminotransferase two or more times the upper limit of normal for the respective institution); renal impairment (serum creatinine ≥2.5 mg/dL); cancer; drug abuse; mental disorders; taking contraceptives or being of childbearing potential; participation in another investigational drug trial within 3 months prior to enrollment; and an investigator's opinion that the patient would be inappropriate for the study.
Diabetes mellitus was not an exclusion criterion, regardless of whether it was self‐reported, documented in the medical records, or defined as a fasting plasma glucose of at least 126 mg/dL (7.0 mmol/L) or as the use of antidiabetic drugs.
2.3. Efficacy and safety evaluations
The primary efficacy variable was the change from baseline in ambulatory systolic/diastolic BP. Secondary efficacy variables included change from baseline in clinic systolic/diastolic BP and control rate of clinic systolic/diastolic BP (<140/90 mm Hg, respectively). Safety evaluations included adverse events and serious adverse events, including any clinically significant abnormalities on physical examination or laboratory tests. Information about symptoms, severity, relation to the study medication, intervention, and outcome were documented for all adverse events.
2.4. BP measurement
Clinic BP was measured between 8 am and 10 am using a standard mercury sphygmomanometer after the patients had rested for at least 5 minutes in the sitting position. A cuff with an appropriately sized bladder was used. On each occasion, three consecutive readings were taken with an interval of 1 minute and averaged for analysis.
ABPM was performed with the ambulatory BP monitors and the protocol of each participating hospital. Ambulatory BP monitors were programmed to obtain ambulatory BP readings during daytime/nighttime at 20/30 minutes (n=364) in 11 hospitals, 30/60 minutes (n=92) in four hospitals, 30/30 minutes (n=41) in three hospitals, 20/60 minutes (n=13) in one hospital, and 60/60‐minute intervals (n=21) in one hospital. On the monitoring day, the study participants were instructed to follow their usual daily activities, avoid vigorous exercise, and remain still with the forearm extended during each BP measurement. ABPM data were collected on case report forms with the hours but not the minutes or seconds of the measurement time. In the analysis, daytime was defined as the period from 8 am to 9 pm and nighttime as the period from 10 pm to 7 am. A recording was considered valid and included in the analysis if there were at least 14 BP readings in the daytime and at least seven in the nighttime.10
2.5. Statistical analysis
We used SAS software version 9.2 (SAS Institute Inc) for statistical analysis. Sample size estimation was based on the hypothesis of efficacy equivalence between amlodipine maleate 5 mg/d vs S‐(‐)‐amlodipine besylate 2.5 mg/d, with a projected clinic BP control rate of 30% and boundary of 10%, an α level of 0.05, and 80% of power. The number of patients per group was estimated to be 330. Assuming a dropout rate of 10%, approximately 360 patients per group would be required. For the superiority trial of high‐ vs low‐dose S‐(‐)‐amlodipine, with a projected clinic BP control rate of 45% and 30%, respectively, only 160 patients per group would be required. However, to have an equal sample size in all three groups, 360 patients per group were enrolled.8
The efficacy analysis was performed in all randomized patients who had a valid ambulatory recording both at baseline and at the end of the study. The safety analysis was performed in the patients who took at least one dose of study treatment. Means and proportions at baseline were compared by Student t test and χ2 test, respectively. We performed analysis of covariance to compute the least square mean (±standard error) change from baseline and the between‐group differences (95% CIs), with baseline values as covariates. The change from baseline was computed by subtracting the values at the end of follow‐up from those at baseline. Positive values therefore indicate a decrease from baseline. The between‐group difference was computed by subtracting the change in the S‐(‐)‐amlodipine 2.5‐mg/d group from that in the S‐(‐)‐amlodipine 5.0‐mg/d group. Positive values therefore indicate a larger reduction in the S‐(‐)‐amlodipine 5.0‐mg/d group.
We performed subgroup analysis in previously treated and untreated hypertensive patients separately and in patients with sustained hypertension and those with white‐coat hypertension at baseline. Sustained hypertension was defined as a clinic BP of ≥140 mm Hg systolic or ≥90 mm Hg diastolic and a daytime BP of ≥135 mm Hg systolic or ≥85 mm Hg diastolic, irrespective of treatment status. White‐coat hypertension was defined as a clinic BP of ≥140 mm Hg systolic or ≥90 mm Hg diastolic and a daytime ambulatory BP of <135 mm Hg systolic and <85 mm Hg diastolic, irrespective of treatment status.
3. RESULTS
3.1. Characteristics of the study participants
Of the 701 randomized patients in the S‐(‐)‐amlodipine 2.5‐mg (n=351) and S‐(‐)‐amlodipine 5‐mg (n=350) groups, one patient in the S‐(‐)‐amlodipine 2.5‐mg group did not take any study medication, leaving 700 patients in the safety analysis. In both groups combined, 634 patients completed the study and 523 patients were included in the efficacy analysis (Figure 1). Of these 523 patients, 263 and 260 were in the S‐(‐)‐amlodipine 2.5‐mg and S‐(‐)‐amlodipine 5‐mg groups, respectively, with 97.0% and 99.2% of patients who used >90% of their study medications, respectively, and none and 0.4% of patients who used other antihypertensive drugs, respectively, during the 8‐week follow‐up period. The baseline characteristics were comparable between the 2.5‐mg daily and 5‐mg daily S‐(‐)‐amlodipine treatment groups (Table 1).
Figure 1.
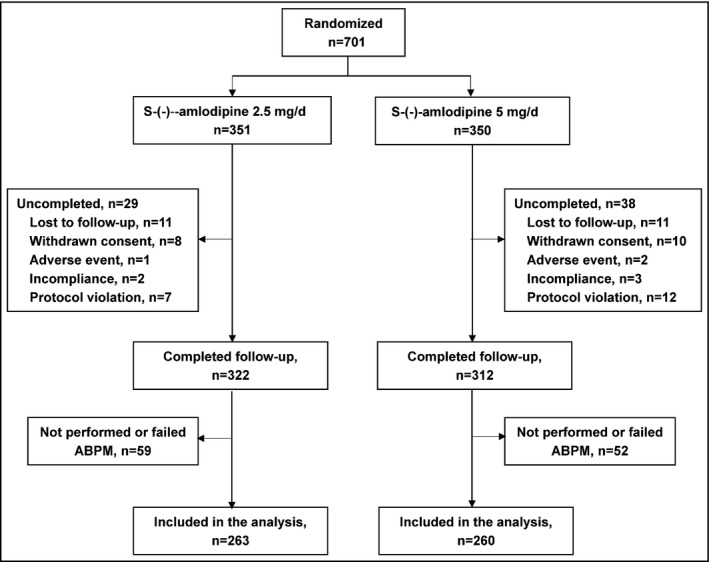
Flow of study patients. ABPM indicates ambulatory blood pressure monitoring
Table 1.
Characteristics of the study participants at baseline
| Characteristic | S‐(‐)‐amlodipine 2.5 mg/d (n=263) | S‐(‐)‐amlodipine 5 mg/d (n=260) | P value |
|---|---|---|---|
| Women, No. (%) | 134 (51.0) | 139 (53.5) | .57 |
| Age, y | 56.6±10.5 | 57.3±10.4 | .42 |
| Body mass index, kg/m² | 24.8±3.2 | 25.0±2.7 | .34 |
| Current smoking, No. (%) | 62 (23.6) | 53 (20.4) | .38 |
| Alcohol intake, No. (%) | 67 (25.5) | 67 (25.8) | .94 |
| Diabetes mellitus, No. (%) | 36 (13.7) | 33 (12.7) | .74 |
| Previous antihypertensive treatment, No. (%) | 183 (69.6) | 178 (68.5) | .78 |
| Calcium channel blockers | 88 (48.1) | 84 (47.2) | .86 |
| Angiotensin‐converting enzyme inhibitors | 33 (18.0) | 26 (14.6) | .38 |
| Angiotensin receptor blockers | 24 (13.1) | 30 (16.9) | .32 |
| β‐Blockers | 10 (5.5) | 11 (6.2) | .77 |
| Diuretics | 3 (1.6) | 9 (5.1) | .08 |
| Other | 25 (13.7) | 21 (11.8) | .70 |
| Clinic BP, mm Hg | |||
| Systolic | 150.6±10.0 | 151.3±9.8 | .43 |
| Diastolic | 94.5±8.1 | 94.9±7.7 | .53 |
| Clinic pulse rate, beats per min | 71.0±8.2 | 71.6±12.0 | .52 |
| Ambulatory BP, mm Hg | |||
| 24‐h systolic | 131.5±15.0 | 133.6±13.7 | .10 |
| 24‐h diastolic | 82.0±10.7 | 83.1±9.9 | .24 |
| Daytime systolic | 134.2±15.8 | 135.9±13.6 | .19 |
| Daytime diastolic | 84.1±11.4 | 84.9±10.3 | .38 |
| Nighttime systolic | 126.2±15.3 | 129.1±15.8 | .03 |
| Nighttime diastolic | 78.2±10.6 | 79.6±10.5 | .12 |
Abbreviation: BP, blood pressure. Values are expressed as mean±standard deviation or number of patients (percentage). For the definition of diabetes mellitus, see Section 2. The P value is for the comparison between the S‐(‐)‐amlodipine 2.5‐mg/d and 5‐mg/d groups.
3.2. Antihypertensive efficacy
In the S‐(‐)‐amlodipine 2.5‐mg treatment group (n=263), 24‐hour systolic/diastolic BP (±standard deviation) decreased from 131.5±15.0/82.0±10.7 mm Hg at baseline to 126.0±13.5/78.5±9.5 mm Hg at 8 weeks of follow‐up by a least square mean (±standard error) change of 6.0±0.6/3.8±0.4 mm Hg (Figure 2 and Tables 1 and 2). The corresponding changes in the S‐(‐)‐amlodipine 5.0‐mg treatment group (n=260) were from 133.6±13.7/83.1±9.9 mm Hg to 125.0±12.0/78.2±8.9 mm Hg by 8.1±0.6/4.7±0.4 mm Hg, respectively. The between‐group least square mean differences in the changes in 24‐hour ambulatory systolic and diastolic BP were 2.1 mm Hg (95% CI, 0.4–3.8; P=.02) and 0.9 mm Hg (95% CI, −0.2 to 2.0; P=.17), respectively (Table 2). Similar trends were observed for daytime and nighttime ambulatory BP and clinic BP (Table 2). Clinic pulse rate slightly and significantly (P≤.04) decreased from baseline to the end of follow‐up in both S‐(‐)‐amlodipine 2.5‐mg (1.3±0.5 beats per min) and 5.0‐mg treatment groups (1.6±0.5 beats per min), with no difference between the two groups (P=.60).
Figure 2.
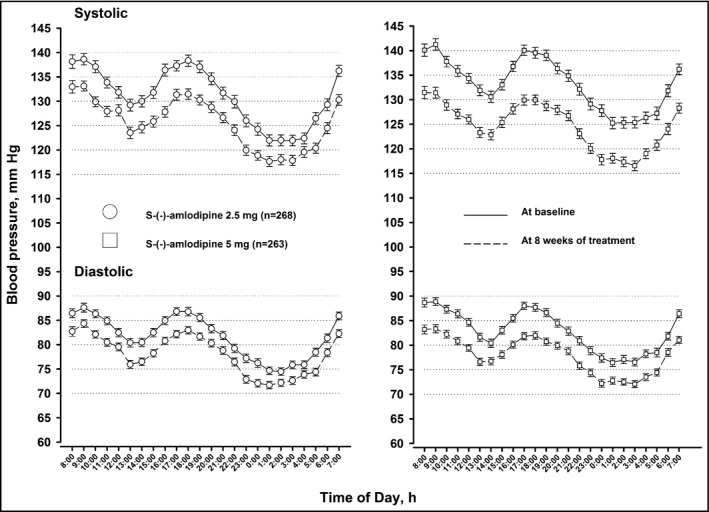
Twenty‐four‐hour blood pressure profile at baseline and at 8 weeks of treatment with S‐(‐)‐amlodipine 2.5 mg/d or 5 mg/d. Symbols denote hourly mean. Vertical lines denote standard error
Table 2.
Least square mean change at 8 weeks of treatment from baseline in clinic and ambulatory BP and pulse rate in all patients
| S‐(‐)‐amlodipine 2.5 mg/d (n=263) | S‐(‐)‐amlodipine 5 mg/d (n=260) | Least square mean difference (95% CI) | P value | |
|---|---|---|---|---|
| Clinic BP, mm Hg | ||||
| Systolic | 20.6±0.6 | 23.6±0.6 | 3.0 (1.3–4.7) | .0005 |
| Diastolic | 12.7±0.4 | 14.3±0.4 | 1.6 (0.5–2.7) | .01 |
| Clinic pulse rate, beats per min | 1.3±0.5 | 1.6±0.5 | 0.3 (−1.1 to 1.7) | .60 |
| Ambulatory BP, mm Hg | ||||
| 24‐h systolic | 6.0±0.6 | 8.1±0.6 | 2.1 (0.4–3.8) | .02 |
| 24‐h diastolic | 3.8±0.4 | 4.7±0.4 | 0.9 (−0.2 to 2.0) | .17 |
| Daytime systolic | 6.3±0.7 | 8.3±0.7 | 2.0 (0.1–3.9) | .04 |
| Daytime diastolic | 3.9±0.5 | 4.8±0.5 | 0.9 (−0.5 to 2.3) | .18 |
| Nighttime systolic | 5.6±0.7 | 7.6±0.7 | 2.0 (0.1–3.9) | .04 |
| Nighttime diastolic | 3.8±0.5 | 4.3±0.5 | 0.5 (−0.9 to 1.9) | .37 |
Abbreviation: BP, blood pressure.
Values are expressed as least square mean±standard error unless otherwise indicated. We performed analysis of covariance to calculate the least square mean (±standard error) change from baseline and the between‐group difference (95% CIs) with the baseline values as covariates. The change from baseline was computed by subtracting the values at the end of follow‐up from those at baseline. Positive values therefore indicate a decrease from baseline. The between‐group difference was computed by subtracting the change in the S‐(‐)‐amlodipine 2.5‐mg/d group from that in the S‐(‐)‐amlodipine 5.0‐mg/d group. Positive values therefore indicate a larger reduction in the S‐(‐)‐amlodipine 5.0‐mg/d group. The P value is for the comparison between the S‐(‐)‐amlodipine 2.5‐mg/d and 5‐mg/d groups.
The control rate was significantly (P≤.003) higher in the S‐(‐)‐amlodipine 5.0‐mg than the 2.5‐mg treatment group for clinic systolic BP (90.8% vs 81.8%) and diastolic BP (94.2% vs 84.0%) and both (87.3% vs 75.7%).
We performed further subgroup analysis in previously untreated (n=162) and treated hypertensive patients (n=361, Table 3) and in patients with sustained (n=321) and white‐coat (n=202, Table 4 hypertension. Changes from baseline to 8 weeks of follow‐up for clinic BP were similar in previously untreated and treated hypertensive patients (P≥.07), except for systolic BP at high dosage (P<.0001). However, changes from baseline to 8 weeks of follow‐up for ambulatory BP were significantly greater in previously treated hypertensive patients (P≤.04), except for nighttime systolic BP at high dosage (P=.48). The between‐group differences in favor of the high dose of S‐(‐)‐amlodipine were greater in previously treated than untreated hypertensive patients. Statistical significance was achieved for clinic systolic and diastolic BP and nighttime systolic BP (P≤.03, Table 3). Changes from baseline to 8 weeks of follow‐up were significantly greater for ambulatory (P≤.0001) but not clinic (P≥.06) BP in patients with sustained hypertension than those with white‐coat hypertension, regardless of the dosage of S‐(‐)‐amlodipine. The between‐group differences in favor of the high dose of S‐(‐)‐amlodipine were significantly greater in patients with sustained hypertension for clinic BP and ambulatory systolic BP (P≤.01) but not for ambulatory diastolic BP (P≥.06, Table 4).
Table 3.
Least square mean change at 8 weeks of treatment from baseline in clinic and ambulatory BP and pulse rate in previously treated and untreated hypertensive patients separately
| S‐(‐)‐amlodipine 2.5 mg/d (n=263) | S‐(‐)‐amlodipine 5 mg/d (n=260) | Least square mean difference (95% CI) | P value | |
|---|---|---|---|---|
| Previously untreated, No. | 80 | 82 | ||
| Clinic BP, mm Hg | ||||
| Systolic | 20.7±1.0 | 21.3±1.0 | 0.6 (−2.2 to 3.4) | .71 |
| Diastolic | 13.5±0.8 | 13.6±0.8 | 0.1 (−2.1 to 2.3) | .92 |
| Clinic pulse rate, beats per min | 1.2±0.8 | 2.6±0.8 | 1.4 (−0.8 to 3.6) | .23 |
| Ambulatory BP, mm Hg | ||||
| 24‐h systolic | 8.7±1.2 | 9.5±1.2 | 0.8 (−2.5 to 4.1) | .64 |
| 24‐h diastolic | 6.4±0.9 | 6.6±0.9 | 0.2 (−2.3 to 2.7) | .89 |
| Daytime systolic | 9.3±1.3 | 10.4±1.3 | 1.1 (−2.5 to 4.7) | .53 |
| Daytime diastolic | 6.9±0.9 | 7.1±0.9 | 0.2 (−2.3 to 2.7) | .88 |
| Nighttime systolic | 8.2±1.3 | 8.1±1.3 | −0.1 (−3.7 to 3.5) | .97 |
| Nighttime diastolic | 6.0±0.9 | 5.9±0.9 | −0.1 (−2.6 to 2.4) | .95 |
| Previously treated, No. | 183 | 178 | ||
| Clinic BP, mm Hg | ||||
| Systolic | 20.6±0.7 | 24.6±0.7 | 4.0 (2.1–5.9) | <.0001 |
| Diastolic | 12.4±0.5 | 14.6±0.5 | 2.2 (0.8–3.6) | .004 |
| Clinic pulse rate, beats per min | 1.3±0.6 | 1.2±0.6 | −0.1 (−1.8 to 1.6) | .90 |
| Ambulatory BP, mm Hg | ||||
| 24‐h systolic | 4.8±0.8 | 7.5±0.8 | 2.7 (0.5–4.9) | .01 |
| 24‐h diastolic | 2.6±0.5 | 3.8±0.5 | 1.2 (−0.2 to 2.6) | .07 |
| Daytime systolic | 4.9±0.8 | 7.4±0.8 | 2.5 (0.3–4.7) | .03 |
| Daytime diastolic | 2.5±0.5 | 3.9±0.5 | 1.4 (−0.2 to 2.9) | .08 |
| Nighttime systolic | 4.5±0.8 | 7.4±0.8 | 2.9 (0.7–5.1) | .01 |
| Nighttime diastolic | 2.6±0.5 | 3.6±0.5 | 1.0 (−0.4 to 2.4) | .22 |
Abbreviation: BP, blood pressure.
Values are expressed as least square mean±standard error unless otherwise indicated. We performed analysis of covariance to calculate the least square mean (±standard error) change from baseline and the between‐group difference (95% CIs) with the baseline values as covariates. The change from baseline was computed by subtracting the values at the end of follow‐up from those at baseline. Positive values therefore indicate a decrease from baseline. The between‐group difference was computed by subtracting the change in the S‐(‐)‐amlodipine 2.5‐mg/d group from that in the S‐(‐)‐amlodipine 5.0‐mg/d group. Positive values therefore indicate a larger reduction in the S‐(‐)‐amlodipine 5.0‐mg/d group. The P value is for the comparison between the S‐(‐)‐amlodipine 2.5‐mg/d and 5‐mg/d groups.
Table 4.
Least square mean change at 8 weeks of treatment from baseline in clinic and ambulatory BP and pulse rate in patients with sustained hypertension and those with white‐coat hypertension
| S‐(‐)‐amlodipine 2.5 mg/d (n=263) | S‐(‐)‐amlodipine 5 mg/d (n=260) | Least square mean difference (95% CI) | P value | |
|---|---|---|---|---|
| Sustained hypertension | n=154 | n=167 | ||
| Clinic BP, mm Hg | ||||
| Systolic | 20.1±0.8 | 24.3±0.8 | 4.2 (2.0–6.4) | .0002 |
| Diastolic | 12.4±0.6 | 14.8±0.6 | 2.4 (0.7–4.1) | .007 |
| Clinic pulse rate, beats per min | 0.7±0.5 | 1.8±0.5 | 1.1 (−0.3 to 2.5) | .19 |
| Ambulatory BP, mm Hg | ||||
| 24‐h systolic | 9.3±0.9 | 12.9±0.8 | 3.6 (1.2–6.0) | .003 |
| 24‐h diastolic | 6.0±0.6 | 7.5±0.6 | 1.5 (−1.7 to 3.2) | .09 |
| Daytime systolic | 10.0±1.0 | 13.3±0.9 | 3.3 (0.7–5.9) | .01 |
| Daytime diastolic | 6.2±0.7 | 7.7±0.7 | 1.5 (−0.4 to 3.4) | .11 |
| Nighttime systolic | 8.0±0.9 | 12.2±0.9 | 4.2 (1.7–6.7) | .0009 |
| Nighttime diastolic | 5.6±0.7 | 6.9±0.6 | 1.3 (−0.5 to 3.1) | .15 |
| White‐coat hypertension | n=109 | n=93 | ||
| Clinic BP, mm Hg | ||||
| Systolic | 21.2±0.8 | 22.6±0.9 | 1.4 (−1.0 to 3.8) | .26 |
| Diastolic | 12.9±0.6 | 13.8±0.6 | 0.9 (−0.8 to 2.6) | .30 |
| Clinic pulse rate, beats per min | 2.1±0.7 | 1.2±0.8 | −0.9 (−3.0 to 1.2) | .43 |
| Ambulatory BP, mm Hg | ||||
| 24‐h systolic | 0.61±0.9 | 0.18±1.0 | −0.4 (−3.1 to 2.2) | .75 |
| 24‐h diastolic | 0.25±0.6 | 0.11±0.6 | −0.1 (−1.8 to 1.5) | .87 |
| Daytime systolic | 0.26±1.0 | 0.19±1.0 | −0.1 (−2.9 to 2.7) | .96 |
| Daytime diastolic | 0.24±0.6 | 0.11±0.7 | −0.1 (−1.9 to 1.7) | .89 |
| Nighttime systolic | 1.70±1.0 | 0.13±1.1 | −1.6 (−4.5 to 1.3) | .30 |
| Nighttime diastolic | 0.57±0.7 | 0.10±0.7 | −0.5 (−2.4 to 1.5) | .64 |
Values are expressed as least square mean±standard error unless otherwise indicated. We performed analysis of covariance to calculate the least square mean (±standard error) change from baseline and the between‐group difference (95% CIs) with the baseline values as covariates. The change from baseline was computed by subtracting the values at the end of follow‐up from those at baseline. Positive values therefore indicate decrease from baseline. The between‐group difference was computed by subtracting the change in the S‐(‐)‐amlodipine 2.5‐mg/d group from that in the S‐(‐)‐amlodipine 5.0‐mg/d group. Positive values therefore indicate a larger reduction in the S‐(‐)‐amlodipine 5.0‐mg/d group. The P value is for the comparison between the S‐(‐)‐amlodipine 2.5‐mg/d and 5‐mg/d groups. Sustained hypertension was defined as a clinic blood pressure (BP) of ≥140 mm Hg systolic or ≥90 mm Hg diastolic and daytime BP of ≥135 mm Hg systolic or ≥85 mm Hg diastolic at baseline.
We performed additional sensitivity analysis in 487 patients who had at least 20 daytime readings and seven nighttime readings, as recommended in the 2013 position paper11 and 2014 European Society of Hypertension practice guidelines12 for ABPM. The results remained unaltered.
3.3. Safety
Safety was assessed in 700 patients who took at least one pill of the study medication. The incidence rate was similar in the two groups for all adverse events (20.0% [n=70] vs 17.7% [n=62]; P=.50) (Table 5). In general, the incidence rate was low and the severity was mild for all adverse events. Only one serious adverse event was reported in the study, which was in the 2.5‐mg S‐(‐)‐amlodipine group. No death was reported during the study in either group.
Table 5.
Incidence of adverse events
| S‐(‐)‐amlodipine 2.5 mg (n=350) | S‐(‐)‐amlodipine 5 mg (n=350) | P value | |
|---|---|---|---|
| Symptoms or diseases | |||
| Ankle edema | 3 | 2 | |
| Headache | 3 | 3 | |
| Dizziness | 2 | 3 | |
| Fractures | 2 | 0 | |
| Flushing | 1 | 1 | |
| Palpitation | 1 | 0 | |
| Chest distress | 0 | 1 | |
| Pneumonia | 0 | 1 | |
| Upper respiratory tract infection | 0 | 1 | |
| Urinary tract infection | 0 | 1 | |
| Electrocardiographic abnormalities | |||
| T‐wave abnormalities | 2 | 3 | |
| Ventricular ectopic beats | 0 | 2 | |
| Bradycardia | 0 | 2 | |
| ST‐segment depression | 1 | 0 | |
| PR interval prolongation | 0 | 1 | |
| Left axis deviation | 1 | 0 | |
| Sick sinus syndrome | 1 | 0 | |
| Blood biochemical abnormalities | |||
| Blood glucose elevation | 10 | 8 | |
| Triglycerides elevation | 3 | 6 | |
| Transaminase elevation | 5 | 3 | |
| Total cholesterol elevation | 3 | 4 | |
| HDL cholesterol decline | 2 | 5 | |
| LDL cholesterol elevation | 0 | 3 | |
| Total bilirubin elevation | 0 | 2 | |
| C‐reactive protein elevation | 0 | 1 | |
| Blood glucose decline | 0 | 1 | |
| Routine blood test abnormalities | |||
| Anemia | 2 | 0 | |
| Thrombocythemia | 0 | 2 | |
| Lymphocytosis | 1 | 0 | |
| Polycythemia | 1 | 0 | |
| Hemoglobin elevation | 1 | 0 | |
| Routine urine test abnormalities | |||
| Hematuria | 3 | 6 | |
| Proteinuria | 5 | 4 | |
| Urine white blood cell count | 4 | 3 | |
| Glucosuria | 5 | 1 | |
| Total | 62 (17.7) | 70 (20.0) | .50 |
Values are expressed as number of patients (percentage). The P value is for the comparison between the S‐(‐)‐amlodipine 2.5‐mg/d and 5.0‐mg/d groups.
4. DISCUSSION
Our randomized study demonstrated that an initial high dose (5 mg) of S‐(‐)‐amlodipine significantly improved BP control on both ambulatory and clinic measurements, especially systolic BP, with similar tolerability as the initial low dose (2.5 mg). The advantage of the initial high dose is approximately 2 mm Hg in ambulatory systolic BP in all patients during 24 hours, in the daytime and at night. According to the epidemiological estimation of risks associated with 24‐hour, daytime, and nighttime BP, the expected risk reductions could be 4.4% to 5.1% for stroke, 1.9% to 2.7% for coronary events, and 3.5% to 3.7% for all cardiovascular events.13
To the best of our knowledge, our study was the first to compare high with low initial dose of amlodipine for ambulatory BP–lowering efficacy and tolerability in Asians. Nonetheless, our study can be compared with a recent study in 562 North American patients with a clinic diastolic BP of 95 to 115 mm Hg.14 In this 8‐week, 4×4 factorial design trial, patients were randomly assigned to receive initial placebo, telmisartan (20, 40, or 80 mg), and/or amlodipine (2.5, 5, or 10 mg). The between‐group (5 mg [n=52] vs 10 mg [n=58] amlodipine monotherapy) mean differences in the changes in 24‐hour systolic/diastolic BP were 2.6/1.5 mm Hg in all patients and 2.6/2.3 mm Hg in patients with stage 2 diastolic hypertension. The corresponding between‐group mean differences for telmisartan monotherapy (40 mg [n=52] vs 80 mg [n=58]) were comparable for all patients (2.6/1.5 mm Hg) and patients with stage 2 diastolic hypertension (3.9/1.8 mm Hg).14 In spite of similar efficacy results between this trial14 and our study, the incidence of peripheral edema was much higher in the amlodipine (racemic) 10‐mg/d monotherapy group (13.8%) than the lower‐dose monotherapy groups (rate not reported) and the telmisartan/amlodipine combination groups (overall pooled 5.2%, P<.0001).15 Taken the efficacy and tolerability results of this14, 15 and our study results together, an initial high dose of amlodipine is probably more appropriate for Asian patients.
Although the between‐group differences were similar for clinic and ambulatory BP, the relative reductions from baseline were much smaller for ambulatory than clinic BP (difference between ambulatory and clinic pressure reductions, 14.3/8.8 and 15.3/9.5 mm Hg in the S‐(‐)‐amlodipine 2.5‐ and 5‐mg daily groups, respectively). This difference to a large extent can be attributed to the white‐coat effect on BP measurements at baseline.11, 12, 16 Indeed, the differences between the clinic and daytime ambulatory systolic/diastolic BP at baseline were 14.8/9.1 mm Hg. The ambulatory BP was low at baseline. On the other hand, the placebo effect can also explain at least a part of the much larger mean reductions from baseline in clinic than ambulatory BP.11, 12, 17 These observations provided further evidence that ABPM may be devoid of white‐coat and/or placebo effect,11, 12 and therefore may improve cardiovascular outcomes in patients with treated hypertension.18, 19, 20, 21, 22, 23
Our study on ambulatory BP measurement also provides further evidence on the 24‐hour effect of amlodipine at both low and high doses. The relative changes from baseline were similar for daytime and nighttime BP. The between‐group differences were also similar for daytime and nighttime BP. These findings are in line with the results of several previous studies on the ambulatory BP–lowering efficacy of amlodipine monotherapy in patients with mild or moderate hypertension.24, 25, 26 Indeed, daytime and nighttime ambulatory BPs were reduced with 5‐mg amlodipine monotherapy by a mean of 13/7 mm Hg and 12/7 mm Hg in 23 patients with both clinic (≥140/90 mm Hg and <120 mm Hg diastolic) and ambulatory daytime hypertension (≥135/85 mm Hg),24 respectively, and by a mean of 17.6/8.9 mm Hg and 17.5/8.9 mm Hg in 359 patients with stage 1 and 2 clinic hypertension,25 respectively. The corresponding BP reductions with 2.5‐mg to 10‐mg amlodipine treatment were 12/8 mm Hg and 11/8 mm Hg, respectively, in 43 patients with clinic hypertension (systolic 140–200 mm Hg and/or diastolic 90–110 mm Hg), type 2 diabetes mellitus, and overt nephropathy.26
5. STUDY STRENGTHS AND LIMITATIONS
Our study should be interpreted within the context of its strengths and limitations. The sample size was relatively large for an ABPM study. Nonetheless, our study had an open design and short duration of follow‐up. These design features may lead to underestimation of treatment effect. Second, our study did not include a washout period before randomization. Previously treated study participants (69.0%) directly switched their previous antihypertensive treatment to the study medication. Previously untreated study participants may have had insufficient evaluation of their BP level. This design feature may also influence the estimation of treatment effect. Third, a large proportion (17.5%) of patients had to be excluded from the efficacy analysis because they did not have valid ambulatory BP recordings either at baseline or at the end of follow‐up. This might have caused exclusion of incompliant patients who had reduced drug adherence during follow‐up. Finally, during the 8‐week follow‐up period, the randomized participants had only one clinic visit at the end of the trial. Thus, the time to treatment target achievement could not be properly evaluated.
6. CONCLUSIONS
Our study demonstrated superiority of an initial high dose of S‐(‐)‐amlodipine in ambulatory BP lowering with similar tolerability as an initial low dose. This approach may improve BP control and, if widely used in clinical practice, should increase the control rate of hypertension. Nonetheless, because of the abovementioned limitations of our study, more research is required before making recommendations on the initial high dose of antihypertensive drugs in future hypertension guidelines.
CONFLICT OF INTEREST
Dr Wang reports receiving research grants from Simcere Pharmaceutical and lecture and consulting fees from Simcere Pharmaceutical and other pharmaceutical companies. The other authors declared no conflicts of interest.
ACKNOWLEDGMENT
The authors gratefully acknowledge the study participants and the investigators from 22 participating hospitals, which are listed in descending order of the number of randomized patients with the name of the principal investigator and the number of randomized patients in parentheses. The Second Xiangya Hospital of Central South University, Changsha, Hunan Province (Shui‐Ping Zhao, n=108); West China Hospital, Sichuan University, Chengdu, Sichuan Province (Hong‐De Hu, n=108); People's Hospital of Liaoning, Shenyang, Liaoning Province (Ying Liu, n=72); The First Affiliated Hospital of Nanchang University, NanchangJiangxi Province (Ze‐Qi Zheng, n=72); The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui Province (Yan Xu, n=72); Xuan Wu Hospital, Capital Medical University, Beijing (Qi Hua, n=72); Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai (Ning‐Yuan Fang, n=54); Xuzhou Medical University Affiliated Hospital, Xuzhou, Jiangsu Province (Zhi‐Rong Wang, n=48); The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province (Luo‐Sha Zhao, n=39); Chao Yang Hospital, Capital Medical University, Beijing (Ming‐Ming Gao, n=36); Daping Hospital, The Third Military Medical University, Chongqing (Jie Chen, n=36); Peking University People's Hospital, Beijing (Da‐Yi Hu, n=36); Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai (Ji‐Guang Wang, n=36); The First Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian Province (Jin‐Xiu Lin, n=36); The First Affiliated Hospital of Jilin University, Changchun, Jilin Province (Yang Zheng, n=36); The First Hospital of Nanjing, Nanjing, Jiangsu Province (Yu‐Ling Ma, n=36); Xijing Hospital, the Fourth Military Medical University, Xi'an, Shanxi Province (Hai‐Chang Wang, n=36); PLA Nanjing General Hospital, Nanjing, Jiangsu Province (Shi‐Sen Jiang, n=33); Tongji Hospital, Huazhong University of Science and Technology, Wuhan, Hubei Province (Jiang‐Tao Yan, n=29); An Zhen Hospital, Capital Medical University, Beijing (Chang‐Sheng Ma, n=27); Jiangsu Province Hospital of Chinese Medicine, Nanjing, Jiangsu Province (Xiao‐Hu Chen, n=21); and The Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong Province (Long‐Gen Xiong, n=8).
Chen Q, Huang QF, Kang YY, et al. Efficacy and tolerability of initial high vs low doses of S‐(‐)‐amlodipine in hypertension. J Clin Hypertens. 2017; 19:973–982. 10.1111/jch.13022
Funding information
The study was financially supported by Simcere Pharmaceutical (Nanjing, Jiangsu Province, China)
Footnotes
Abbreviations: HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
REFERENCES
- 1. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507‐520. [DOI] [PubMed] [Google Scholar]
- 2. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281‐1357. [DOI] [PubMed] [Google Scholar]
- 3. European Society of Hypertension‐European Society of Cardiology Guidelines Committee . European Society of Hypertension‐European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;2003:1011‐1053. [DOI] [PubMed] [Google Scholar]
- 4. Chow CK, Teo KK, Rangarajan S, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high‐, middle‐, and low‐income countries. JAMA. 2013;310:959‐968. [DOI] [PubMed] [Google Scholar]
- 5. Sheng CS, Liu M, Kang YY, et al. Prevalence, awareness, treatment and control of hypertension in elderly Chinese. Hypertens Res. 2013;36:824‐828. [DOI] [PubMed] [Google Scholar]
- 6. Wang JG, Pimenta E, Chwallek F. Comparative review of the blood pressure lowering and cardiovascular benefits of telmisartan and perindopril. Vasc Health Risk Manag. 2014;10:189‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pedrinelli R, Dell'Omo G, Melillo E, Mariani M. Amlodipine, enalapril, and dependent leg edema in essential hypertension. Hypertension. 2000;35:621‐625. [DOI] [PubMed] [Google Scholar]
- 8. Jia T, Zhang LJ, Zhan YQ, Yu JM, Hu DY. The effectiveness and safety of L‐amlodipine besylate for blood pressure control in patients with mild to moderate essential hypertension. Chin J Cardiol. 2013;41:301‐303. [PubMed] [Google Scholar]
- 9. Park JY, Kim KA, Park PW, et al. Pharmacokinetic and pharmacodynamic characteristics of a new S‐amlodipine formulation in healthy Korean male subjects: a randomized, open‐label, two‐period, comparative, crossover study. Clin Ther. 2006;28:1837‐1847. [DOI] [PubMed] [Google Scholar]
- 10. O'Brien E, Asmar R, Beilin L, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821‐848. [DOI] [PubMed] [Google Scholar]
- 11. O'Brien E, Parati G, Stergiou G, , et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731‐1768. [DOI] [PubMed] [Google Scholar]
- 12. Parati G, Stergiou G, O'Brien E, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359‐1386. [DOI] [PubMed] [Google Scholar]
- 13. Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of the day versus night ambulatory blood pressure revisited in 7458 randomly recruited subjects. Lancet. 2007;370:1219‐1229. [DOI] [PubMed] [Google Scholar]
- 14. White WB, Littlejohn TW, Majul CR, et al. Effects of telmisartan and amlodipine in combination on ambulatory blood pressure in stages 1‐2 hypertension. Blood Press Monit. 2010;15:205‐212. [DOI] [PubMed] [Google Scholar]
- 15. Littlejohn TW, Majul CR, Olvera R, et al. Results of treatment with telmisartan‐amlodipine in hypertensive patients. J Clin Hypertens (Greenwich). 2009;11:207‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Palatini P, Dorigatti F, Mugellini A, et al. Ambulatory versus clinic blood pressure for the assessment of anti‐hypertensive efficacy in clinical trials: insights from the Val‐Syst Study. Clin Ther. 2004;26:1436‐1445. [DOI] [PubMed] [Google Scholar]
- 17. Mancia G, Parati G. Office compared with ambulatory blood pressure in assessing response to antihypertensive treatment: a meta‐analysis. J Hypertens. 2004;22:435‐445. [DOI] [PubMed] [Google Scholar]
- 18. Staessen JA, Byttebier G, Buntinx F, Celis H, O'Brien ET, Fagard R. Antihypertensive treatment based on conventional or ambulatory blood pressure measurement. A randomized controlled trial. Ambulatory Blood Pressure Monitoring and Treatment of Hypertension Investigators. JAMA. 1997;278:1065‐1072. [PubMed] [Google Scholar]
- 19. Fagard RH, Staessen JA, Thijs L, et al. Response to antihypertensive therapy in older patients with sustained and nonsustained systolic hypertension. Systolic Hypertension in Europe (Syst‐Eur) Trial Investigators. Circulation. 2000;102:1139‐1144. [DOI] [PubMed] [Google Scholar]
- 20. Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic value of ambulatory blood‐pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407‐2415. [DOI] [PubMed] [Google Scholar]
- 21. Verdecchia P, Angeli F, Gattobigio R. Clinical usefulness of ambulatory blood pressure monitoring. J Am Soc Nephrol. 2004;15(suppl 1):S30‐S33. [DOI] [PubMed] [Google Scholar]
- 22. Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin outcome study. Hypertension. 2005;46:156‐161. [DOI] [PubMed] [Google Scholar]
- 23. ABC‐H Investigators , Roush GC, Fagard RH, et al. Prognostic impact from clinic, daytime, and night‐time systolic blood pressure in nine cohorts of 13,844 patients with hypertension. J Hypertens. 2014;32:2332‐2340; discussion 2340. [DOI] [PubMed] [Google Scholar]
- 24. Kuramoto K, Ichikawa S, Hirai A, Kanada S, Nakachi T, Ogihara T. Azelnidipine and amlodipine: a comparison of their pharmacokinetics and effects on ambulatory blood pressure. Hypertens Res. 2003;26:201‐208. [DOI] [PubMed] [Google Scholar]
- 25. Kario K, Hoshide S. Age‐related difference in the sleep pressure‐lowering effect between an angiotensin II receptor blocker and a calcium channel blocker in Asian hypertensives: the ACS1 Study. Hypertension. 2015;65:729‐735. [DOI] [PubMed] [Google Scholar]
- 26. Yasuda G, Ando D, Hirawa N, Umemura S, Tochikubo O. Effects of losartan and amlodipine on urinary albumin excretion and ambulatory blood pressure in hypertensive type 2 diabetic patients with overt nephropathy. Diabetes Care. 2005;28:1862‐1868. [DOI] [PubMed] [Google Scholar]


