Abstract
Reservoir pressure parameters [eg, reservoir pressure (RP) and excess pressure (XSP)] are biomarkers derived from blood pressure (BP) waveforms that have been shown to predict cardiovascular events independent of conventional cardiovascular risk markers. However, whether RP and XSP can be derived non‐invasively from operator‐independent cuff device measured brachial or central BP waveforms has never been examined. This study sought to achieve this by comparison of cuff reservoir pressure parameters with intra‐aortic reservoir pressure parameters. 162 participants (aged 61 ± 10 years, 72% male) undergoing coronary angiography had the simultaneous measurement of cuff BP waveforms (via SphygmoCor XCEL, AtCor Medical) and intra‐aortic BP waveforms (via fluid‐filled catheter). RP and XSP derived from cuff acquired brachial and central BP waveforms were compared with intra‐aortic measures. Concordance between brachial‐cuff and intra‐aortic measurement was moderate‐to‐good for RP peak (36 ± 11 vs 48 ± 14 mm Hg, P < 0.001; ICC 0.77, 95% CI: 0.71‐0.82), and poor‐to‐moderate for XSP peak (28 ± 10 vs 24 ± 9 mm Hg, P < 0.001; ICC 0.49, 95% CI: 0.35‐0.60). Concordance between central‐cuff and intra‐aortic measurement was moderate‐to‐good for RP peak (35 ± 9 vs 46 ± 14 mm Hg, P < 0.001; ICC 0.77, 95% CI: 0.70‐0.82), but poor for XSP peak (12 ± 3 vs 24 ± 9 mm Hg, P < 0.001; ICC 0.12, 95% CI: −0.13 to 0.31). In conclusion, both brachial‐cuff and central‐cuff methods can reasonably estimate intra‐aortic RP, whereas XSP can only be acceptably derived from brachial‐cuff BP waveforms. This should enable widespread application to determine the clinical significance, but there is significant room for refinement of the method.
Keywords: arterial blood pressure, hemodynamics, non‐invasive, oscillometry, reservoir
1. INTRODUCTION
High blood pressure (BP) is the leading contributor to the global burden of disease.1 Many investigators have proposed that useful clinical biomarkers may be derived from analysis of arterial BP waveforms.2 One such construct is the reservoir‐excess pressure model in which the arterial BP waveform is theorized to represent the sum of a reservoir pressure (RP, determined by global systemic compliance and resistance) and an excess pressure (XSP, related to local wave travel).3 Reservoir pressure parameters (RP, XSP, and the associated systolic rate constant) derived from non‐invasively acquired BP waveforms (eg, via carotid or radial tonometry) predict cardiovascular events independent of conventional cardiovascular risk factors.4, 5, 6 However, these modes of BP waveform acquisition are technically challenging, which limits widespread application of non‐invasively derived reservoir pressure parameters.
Technological advancements now allow recording of brachial BP waveforms and estimation of central BP using a standard operator‐independent, oscillometric BP cuff method that enables the analysis of brachial and central reservoir pressure parameters. Altogether, the cuff approach could be useful for more widespread measurement of reservoir pressure parameters, but it has not been tested before. Therefore, this study aimed to determine whether reservoir pressure parameters could be derived non‐invasively from cuff acquired brachial or central BP waveforms. We sought to achieve this by comparison of reservoir pressure parameters derived non‐invasively from cuff‐measured brachial and central BP waveforms with invasively recorded aortic reservoir pressure parameters.
2. METHODS
2.1. Participants
A total of 239 patients scheduled for diagnostic coronary angiography at the Royal Hobart Hospital (Hobart, Australia) were screened for participation in this study. Exclusion criteria included participants with atrial fibrillation, aortic stenosis, or waveform data of insufficient quality. Complete data from 162 participants were included for the analysis of brachial‐cuff measurement, and 151 participants for the analysis of central‐cuff measurement. The description of participant flow and quality control is provided in Figure 1. The study was approved by the University of Tasmania Human Research Ethics Committee, all participants provided written consent, and all research was carried out in accordance with the Declaration of Helsinki.
Figure 1.
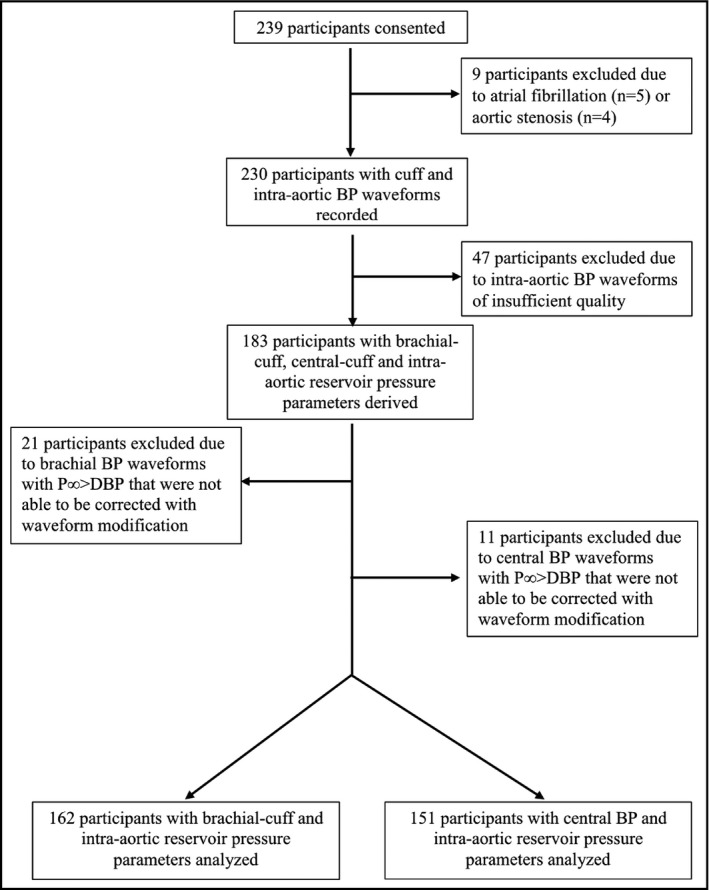
Participant flow diagram. The P∞ was found to be >DBP during derivation of reservoir pressure parameters among several cuff BP waveforms. This occurred due to a small upstroke at end diastole that was an artifact of ensemble averaging of the BP waveforms and is non‐physiological (see Protocol in Methods). The anomaly was corrected by removal of the small upslope occurring at end diastole and then re‐applying the algorithm to derive reservoir pressure parameters. This correction was not possible in brachial‐cuff BP waveforms from 21 participants or in central BP waveforms from 11 participants, and thus were excluded from analysis (representing 18% of available participants). BP, blood pressure; P∞, pressure infinite; DBP, diastolic blood pressure
2.2. Protocol
Patients were prepared for coronary angiography in accordance with standard clinical care. All study measurements were obtained in the supine position under stable hemodynamic conditions and prior to the clinical procedure. The brachial‐cuff waveforms were measured via an oscillometric BP device, simultaneously with intra‐aortic BP waveforms that were continuously recorded at the ascending aorta via a fluid‐filled catheter. The central BP waveforms were estimated from the cuff device measured brachial BP waveforms via a generalized transfer function (GTF), thus, central‐cuff BP waveforms were simultaneously acquired to the recording of intra‐aortic BP waveforms. The non‐invasive cuff and intra‐aortic BP waveform measurements were performed in duplicate on the majority of participants (ie, 73%), with the remaining only having one recording. The total time to complete each study was approximately three minutes. Reservoir pressure parameters were derived from the measured BP waveforms, and brachial‐cuff and central‐cuff reservoir pressure parameters were, respectively, compared with intra‐aortic reservoir pressure parameters. Quality control measures conducted on BP waveforms were as follows: (a) inconsistent intra‐aortic BP waveforms caused by the issues that arose during the procedure, such as participant or catheter was unexpectedly moved, were excluded; (b) non‐invasive cuff BP waveforms having P∞> diastolic BP were excluded as this is the result of an artifact of ensemble averaging BP waveforms without time gating, and is non‐physiological.
2.3. Cuff BP waveform measurement
Cuff BP waveforms were measured using a SphygmoCor Xcel device (Atcor Medical, Sydney, NSW, Australia) with an appropriately sized cuff positioned on the left upper arm level with the right atrium. The device first measures brachial BP using a validated oscillometric algorithm (Medical model 222, Sun Tech Medical Inc Morrisville, NC, USA),7, 8 and then re‐inflates to a sub‐diastolic BP (10 mm Hg below diastolic BP), at which point 5 s of brachial volume displacement waveforms were recorded simultaneously with intra‐aortic BP waveforms. The brachial‐cuff volumetric waveforms were ensemble averaged offline, with the peak and nadir calibrated to oscillometric brachial systolic and diastolic BP, respectively. The central‐cuff BP waveforms were automatically estimated from the ensemble averaged brachial‐cuff BP waveforms with an application of a built‐in GTF. These brachial‐cuff and central‐cuff BP waveforms were used to derive reservoir pressure parameters using a customized algorithm.
2.4. Intra‐aortic BP waveform measurement
Intra‐aortic BP waveforms were acquired using 5Fr and 6Fr fluid‐filled catheters inserted via the radial artery and positioned within the ascending aorta, approximately 5 cm distal to the aortic valve (position confirmed by fluoroscopy). The catheter system was flushed prior to continuous BP waveform acquisition. BP signals were recorded via an analog‐to‐digital signal converter (Labview, ADInstruments, Bella Vista, NSW, Australia) within LabChart 7 software (ADInstruments). Five seconds of consistent aortic BP signals (corresponding precisely to the time of brachial‐cuff BP waveform acquisition) were extracted and calibrated offline using a 2‐point method to convert units of measurement from Volts to mmHg as previously described.9 The calibrated BP waveforms were ensemble averaged to derive reservoir pressure parameters. The dynamic response (frequency and damping) of the fluid‐filled system was assessed by performing “pop” tests, and confirmed in the appropriate range as outlined by Gardner10 (frequency>18 Hz and damping coefficient>0.3).
2.5. Derivation of reservoir pressure parameters
The customized Matlab program to derive reservoir pressure parameters has previously been described.9 RP was calculated using the pressure‐only approach as per Equation 1.9
Calculation of reservoir pressure
XSP was calculated by subtracting RP from total pressure. The systolic and diastolic rate constants of the system are A and B respectively, and represent the rate constants relating to the speed of upstroke and downstroke of the BP waveform.5 P is measured total pressure, is reservoir pressure, and P∞ is the arterial asymptotic pressure. Figure 2 represents a BP waveform with example reservoir pressure components.
Figure 2.
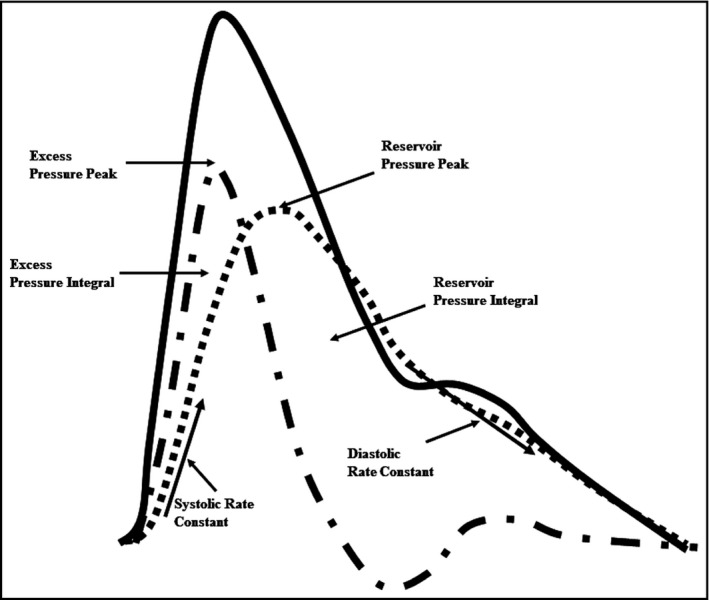
Blood pressure waveform ( ) with example reservoir pressure parameters. The reservoir pressure (
) with example reservoir pressure parameters. The reservoir pressure ( ) and excess pressures (
) and excess pressures ( ) are expressed as peak and integrals (area under the pressure curves)
) are expressed as peak and integrals (area under the pressure curves)
2.6. Statistical analysis
All data were analyzed using SPSS (version 22.0; SPSS Inc, Chicago, IL, USA). Concordance between non‐invasive cuff and intra‐aortic reservoir pressure parameters was assessed based on: (a) consistency determined by intra‐class correlation coefficients (ICC) using a single rater measurement, consistency, 2‐way mixed‐effects model; (b) mean difference tested by paired t test, and; (c) variability in mean differences examined by Bland‐Altman. The strength of consistency between measurements was defined from ICC and 95% confidence intervals (95% CI) as: <0.50 poor; 0.50 to 0.75 moderate; 0.75 to 0.90 good; and 0.90 to 1.0 excellent, according to Koo and Li.11 Systematic bias was assessed from within Bland‐Altman plots by Pearson correlation and the Z‐statistic. P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Clinical characteristics
Participants were predominantly male and middle‐to‐older aged, with a high prevalence of a history of high BP and currently taking antihypertensive medications (Table 1). Kidney function (as determined from estimated glomerular filtration rate) was slightly reduced on average and almost two thirds of participants had a significant stenosis in one or more coronary artery.
Table 1.
Clinical characteristics of study participants (n = 162)
| Variable | Mean (SD) or n (%) |
|---|---|
| Age (years) | 61 (10) |
| Sex (men %) | 116 (72) |
| Body mass index (kg/m2) | 28 (7) |
| History of high BP (≥140/90 mm Hg) n (%) | 151 (93) |
| eGFR (mL/min/1.73 m2) | 77 (26) |
| Diabetes n (%) | 38 (24) |
| Smoking n (%) | 35 (22) |
| Antihypertensive medication n (%) | 138 (86) |
| Lipid profile (mmol/L) | |
| High‐density lipoprotein cholesterol | 0.8 (0.4) |
| Low‐density lipoprotein cholesterol | 1.9 (0.8) |
| Triglycerides | 1.5 (0.7) |
| Angiographic findings n (%) | |
| No significant stenosis | 57 (36) |
| Single‐vessel disease | 33 (21) |
| Double‐vessel disease | 42 (27) |
| Multi‐vessel disease | 25 (16) |
A history of high blood pressure (BP) was determined from the participant's medical records. Significant stenosis was defined by ≥50% occlusion. eGFR estimated glomerular filtration rate.
3.2. Comparison between brachial‐cuff and intra‐aortic reservoir pressure parameters
There was a small difference between brachial‐cuff and intra‐aortic systolic BP (128 ± 16 mm Hg vs 126 ± 19 mm Hg, P = 0.17), whereas brachial‐cuff diastolic BP was significantly higher than intra‐aortic diastolic BP (73 ± 9 mm Hg vs 65 ± 10 mm Hg, P < 0.001). Figure 3 shows example waveforms to illustrate the difference of reservoir pressure parameters derived from brachial‐cuff and intra‐aortic BP waveforms. Table 2A presents the comparisons between brachial‐cuff and intra‐aortic reservoir pressure parameters. There was moderate‐to‐good consistency for RP peak, but with significant mean difference and systematic bias indicating a trend for greater underestimation of intra‐aortic RP peak by brachial‐cuff measurement at higher RP peak (Figure 4A and 4B). Similarly, for the RP integral, there was moderate consistency, a significant mean difference, and systematic bias for greater underestimation with increasing values (r = −0.69, P < 0.001). For XSP peak, there was poor‐to‐moderate consistency and a significant overestimation without evidence of systemic bias (Figure 4C,D). There were similar findings for XSP integral. For the systolic and diastolic rate constants, there was poor‐to‐moderate and poor consistency, respectively. There was significant mean difference and evidence of systematic bias for both rate constants (systolic r = 0.51 and diastolic r = 0.54, P < 0.001 both).
Figure 3.
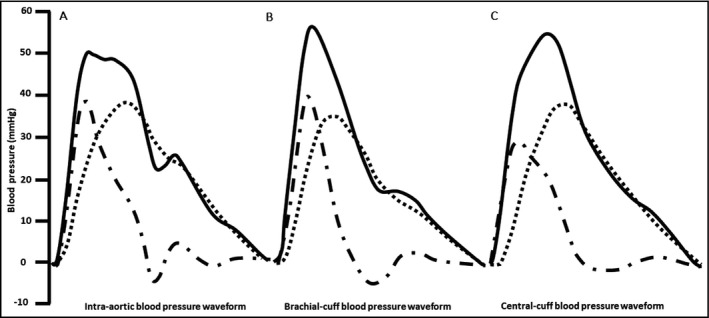
Ensemble averaged A) intra‐aortic B) brachial‐cuff and C) central‐cuff blood pressure waveforms ( ) separated into reservoir (
) separated into reservoir ( , RP) and excess pressure (
, RP) and excess pressure ( , XSP) components from a 63‐year‐old female participant. Waveforms have been rescaled so that diastolic blood pressure is equal to 0
, XSP) components from a 63‐year‐old female participant. Waveforms have been rescaled so that diastolic blood pressure is equal to 0
Table 2.
Comparison between cuff and intra‐aortic reservoir pressure parameters
| Parameters | Cuff mean (SD) | Intra‐aortic mean (SD) | Cuff‐aortic mean difference (SD) | p‐value | ICC (95% CI) | Regression equation (y) |
|---|---|---|---|---|---|---|
| A. Comparison between brachial‐cuff and intra‐aortic reservoir pressure parameters (n = 280) | ||||||
| RP peak, mm Hg | 36 (11) | 48 (14) | −12 (1) | <0.001 | 0.77 (0.71, 0.82) | −0.32x + 1.59 |
| RP integral, mm Hg/s | 10 (3) | 18 (6) | −8 (4) | <0.001 | 0.66 (0.57, 0.73) | −0.81x + 3.69 |
| XSP peak, mm Hg | 28 (10) | 24 (9) | 5 (1) | <0.001 | 0.49 (0.35, 0.60) | 0.16x + 0.77 |
| XSP integral, mm Hg/s | 5 (2) | 4 (2) | 1 (2) | 0.003 | 0.60 (0.49, 0.68) | 0.11x + 1.57 |
| Systolic rate constant, per seconds | 0.1537 (0.0948) | 0.1713 (0.0699) | −0.0176 (0.0070) | 0.013 | 0.39 (0.23, 0.52) | 0.51x − 0.11 |
| Diastolic rate constant, per seconds | 0.0385 (0.0355) | 0.0227 (0.0128) | 0.0157 (0.0023) | <0.001 | 0.03 (−0.22, 0.24) | 0.54x − 0.01 |
| B. Comparison between central‐cuff and intra‐aortic reservoir pressure parameters (n = 262) | ||||||
| RP peak, mm Hg | 35 (9) | 46 (14) | −11 (10) | <0.001 | 0.77 (0.70, 0.82) | −0.52x + 9.72 |
| RP integral, mm Hg/s | 11 (3) | 17 (6) | −6 (4) | <0.001 | 0.67 (0.58, 0.74) | −0.73x + 4.41 |
| XSP peak, mm Hg | 12 (3) | 24 (9) | −12 (9) | <0.001 | 0.12 (−0.13, 0.31) | −1.53x + 15.06 |
| XSP integral, mm Hg/s | 2 (1) | 4 (2) | −1 (2) | <0.001 | 0.23 (0.01, 0.39) | −1.45x + 2.89 |
| Systolic rate constant, per seconds | 0.2307 (0.0497) | 0.1655 (0.0677) | 0.0652 (0.0818) | <0.001 | 0.10 (−0.16, 0.29) | −0.57x + 0.18 |
| Diastolic rate constant, per seconds | 0.0377 (0.0117) | 0.0228 (0.0157) | 0.0109 (0.0184) | <0.001 | 0.21 (0.00, 0.39) | −0.51x + 0.03 |
CI, confidence interval; RP, reservoir pressure; SD, standard deviation; XSP, excess pressure.
P value is for the comparison between cuff and intra‐aortic reservoir pressure parameters. ICC, interclass correlations with a single rater measurement, consistency, 2‐way mixed‐effects model.
Regression equation is the trend of systemic bias in the Bland‐Altman analysis.
Figure 4.
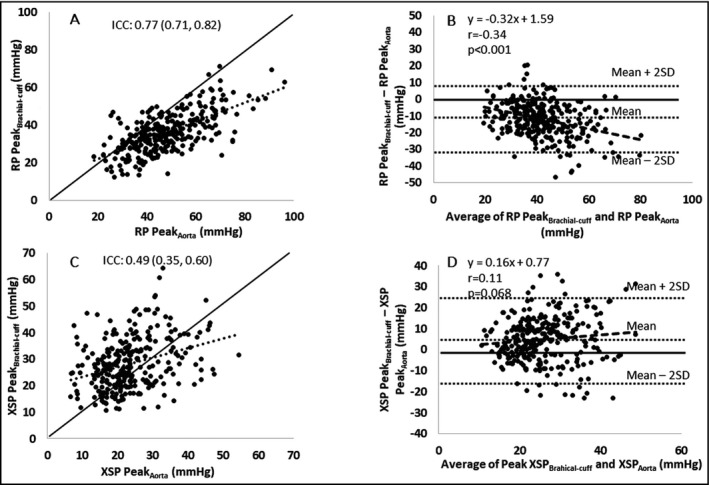
Comparisons of brachial‐cuff and intra‐aortic blood pressure waveform derived reservoir pressure (RP) and excess pressure (XSP) (n = 280).  is the line of identity,
is the line of identity,  is the trend line,
is the trend line,  is the systematic bias line within Bland‐Altman analysis. Intra‐class correlation coefficients and Bland‐Altman plots of the reliability between central‐cuff and intra‐aortic RP peak (A and B), and between central‐cuff and intra‐aortic XSP peak (C and D), respectively. Abbreviation: ICC: intra‐class correlation; SD: standard deviation. r: Pearson correlation. p value is for the comparison of systematic bias with identity line within Bland‐Altman plots
is the systematic bias line within Bland‐Altman analysis. Intra‐class correlation coefficients and Bland‐Altman plots of the reliability between central‐cuff and intra‐aortic RP peak (A and B), and between central‐cuff and intra‐aortic XSP peak (C and D), respectively. Abbreviation: ICC: intra‐class correlation; SD: standard deviation. r: Pearson correlation. p value is for the comparison of systematic bias with identity line within Bland‐Altman plots
3.3. Comparison between central‐cuff and intra‐aortic reservoir pressure parameters
Central‐cuff systolic BP was significantly lower than intra‐aortic systolic BP (116 ± 14 mm Hg vs 125 ± 18 mm Hg, P < 0.001). Conversely, central‐cuff diastolic BP was higher than intra‐aortic diastolic BP (74 ± 10 mm Hg vs 65 ± 10 mm Hg, P < 0.001). Figure 3 shows example waveforms to illustrate the difference of reservoir pressure parameters derived from central‐cuff and intra‐aortic BP waveforms. Table 2B presents the comparisons between central‐cuff and intra‐aortic reservoir pressure parameters. There was moderate‐to‐good consistency for RP peak, but with significant mean difference and systematic bias indicating a trend for greater underestimation of intra‐aortic RP peak by central‐cuff measurement at higher RP peak (Figure 5A,B). Similarly, for the RP integral, there was moderate consistency and a significant mean difference with systematic bias for greater underestimation as RP integral increases (r = −0.64, P < 0.001). However, for XSP peak, XSP integral, systolic rate constant, and diastolic rate constant, the consistencies were poor, and mean differences were significant with evidences of systemic bias (r = −0.81 for XSP peak, Figure 5C and 5D; r = −0.82 for XSP integral; r = −0.30 for systolic rate constant; and r = −0.29 for diastolic rate constant, respectively and all P < 0.001).
Figure 5.
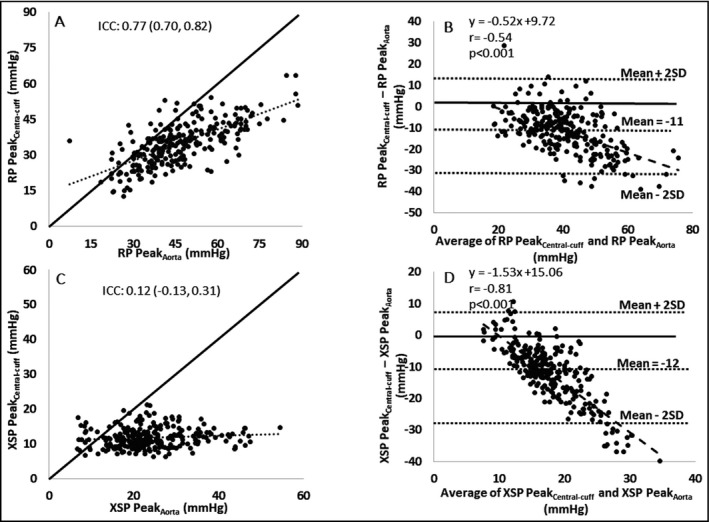
Comparisons of central‐cuff and intra‐aortic blood pressure waveform derived reservoir pressure (RP) and excess pressure (XSP) (n = 262).  is the line of identity,
is the line of identity,  is the trend line,
is the trend line,  is the systematic bias line within Bland‐Altman analysis. Intra‐class correlation coefficients and Bland‐Altman plots of the reliability between central‐cuff and intra‐aortic RP peak (A and B), and between central‐cuff and intra‐aortic XSP peak (C and D), respectively. Abbreviation: ICC: intra‐class correlation; SD: standard deviation. r: Pearson correlation. p value is for the comparison of systematic bias with identity line within Bland‐Altman plots
is the systematic bias line within Bland‐Altman analysis. Intra‐class correlation coefficients and Bland‐Altman plots of the reliability between central‐cuff and intra‐aortic RP peak (A and B), and between central‐cuff and intra‐aortic XSP peak (C and D), respectively. Abbreviation: ICC: intra‐class correlation; SD: standard deviation. r: Pearson correlation. p value is for the comparison of systematic bias with identity line within Bland‐Altman plots
4. DISCUSSION
In this study, we demonstrate that it is practically feasible to derive some reservoir pressure parameters from non‐invasively acquired cuff BP waveforms, albeit with variable reliability when compared with intra‐aortic reservoir pressure parameters. Intra‐aortic RP was reasonably measured from the cuff‐based device measured brachial and central BP waveforms, whereas the brachial‐cuff method more reliably estimated intra‐aortic XSP than the central‐cuff method. Neither of the two cuff waveforms were acceptable in terms of generating accurate estimation of the systolic and diastolic rate constants. These findings imply that the brachial‐cuff method may be more applicable in future work to determine the clinical importance of RP and XSP when compared with the central‐cuff method, but also indicate the need for further refinement of the cuff technique.
The reservoir‐excess pressure model of arterial hemodynamics was first applied to invasive BP waveforms in animal models and was conceived to circumvent conceptual limitations with wave only models of the arterial system.2, 12, 13 More importantly, the approach has been applied to clinical populations on BP waveforms captured non‐invasively outside of the aorta (including the carotid and radial arteries) via tonometry, and consistently shown that reservoir pressure parameters (eg, RP, XSP, and systolic rate constant) have prognostic value beyond standard BP and other cardiovascular risk factors.4, 5, 6, 14 The value of this current study is the demonstration that it is technically feasible to use a cuff‐based method to derive reservoir pressure parameters. The cuff technique is user‐friendly and non‐operator dependent, thus should have improved ease of use (compared with tonometry or invasive methods) and has the possibility for assessment over 24 hours. However, significant improvement in the estimation of reservoir pressure parameters using the cuff device is needed as waveform data from 18% of available participants were excluded due to the non‐physiological P∞> diastolic BP (and this was experienced under resting conditions, let alone whilst ambulatory where greater errors would be expected). Furthermore, from the available data, the rate constants of reservoir pressure parameters could not be accurately reproduced using the non‐invasive cuff methods applied in this study. This is likely to have arisen from the recording of the brachial‐cuff volumetric waveforms at sub‐diastolic BP, which dampens waveform features but is a problem that might be resolvable with waveform capture at higher inflation pressures.15 Issues of systematic bias (Figures 4 and 5) also need to be corrected so that the method has accuracy and applicability across a broad range of BP. Importantly, it is still yet to be determined if cuff‐derived measurements of reservoir pressure parameters have clinical value in the assessment of cardiovascular risk compared to BP methods already available. Accordingly, the next steps will be to determine the independent association of cuff‐derived reservoir pressure parameters with clinical indicators of arterial disease.
We expected good concordance between non‐invasive cuff and intra‐aortic RP because RP is relatively constant from central to peripheral human large arteries.16, 17 In fact, we observed moderate‐to‐good concordance of non‐invasive cuff RP with intra‐aortic RP (both brachial‐cuff and central‐cuff measurements, and both RP peak and RP integral assessments), but with cuff underestimation. A major factor likely contributing to this variation between non‐invasive cuff and intra‐aortic RP values is the volumetric technique related to measurement of the cuff BP waveform, rather than internal inconsistencies with the reservoir‐excess pressure model itself. Volume displacement waveforms captured in the lower pressure range (10 mm Hg lower than the diastolic BP) provide a relatively featureless signal by comparison to intra‐aortic BP waveforms. The observed RP underestimation is also likely attributable to the calibration of brachial volumetric waveforms, which probably introduced an error of underestimated systolic BP, but overestimated diastolic BP.18 Moreover, we found a trend toward greater underestimation of intra‐aortic RP at higher RP values in both brachial‐cuff and central‐cuff measurements. This trend might be related to greater underestimation of brachial systolic BP as systolic BP increases using the XCEL device,19 which is common for oscillometric devices.20, 21
On the other hand, compared with intra‐aortic XSP, brachial‐cuff XSP was higher, but central‐cuff XSP was lower. The higher brachial‐cuff XSP and lower central‐cuff XSP are concordant with the findings of our recent invasive study that XSP is amplified in peripheral arteries compared with the aorta.16, 17 We think there are two major reasons for the brachial‐cuff overestimation. Firstly, even though inaccurate calibration by cuff oscillometry (mentioned above) would reduce the overall amplitude of the brachial‐cuff BP waveform compared with invasive BP waveform, maintenance of higher XSP values (both peak and integral) suggests that the shape of the systolic portion of the waveform was reasonably well maintained, as XSP is predominantly determined by wave travel in systole.2 Secondly, we have previously demonstrated that XSP undergoes significant amplification from the aorta to the brachial (and radial) artery in parallel with the increase in systolic BP.17 Therefore, even though the reference (invasive) brachial XSP would have been underestimated by the cuff waveform approach, it was reasonably concordant with the intra‐aortic XSP because this variable is significantly lower than intra‐brachial XSP. These observations may help to explain the strong associations between XSP derived from the radial artery and target organ damage,4, 22, 23, 24 that is, because this brachial‐cuff approach is a reasonable estimate of the aortic XSP.
On the contrary, central‐cuff XSP significantly underestimated the intra‐aortic XSP and trended toward greater underestimation with increasing XSP values. This is likely from inaccurate calibration of brachial‐cuff BP waveforms and use of a GTF. Calibration with cuff oscillometry shrinks the amplitude of the brachial‐cuff BP waveform, which imputes an underestimated magnitude of the true aortic BP waveform into the central‐cuff BP measurement. In fact, we found that central‐cuff method underestimated intra‐aortic systolic BP (−9 ± 11 mmHg) and overestimated intra‐aortic diastolic BP (9 ± 7 mmHg). This result has been similarly reported by Shoji and colleagues,19 who found 5 ± 10 mmHg central‐cuff systolic BP underestimation and 13 ± 6 mmHg central‐cuff diastolic BP overestimation among 36 people.
Study strengths include the large sample size for an invasive study and employment of high‐grade standardized intra‐arterial procedures designed to minimize potential sources of error.25 However, study participants were undergoing diagnostic coronary angiography and most had at least one comorbidity or evidence of coronary artery disease, thus, results may not be generalizable to healthy populations. Secondly, even though we followed guideline best practice for intra‐aortic BP waveform recordings, it would have been optimal to use solid state catheters rather than the fluid‐filled catheter system. Another possible limitation was derivation of reservoir pressure parameters based on the pressure‐only equation, which does not take into account variations in local blood flow. Nevertheless, the pressure‐only approach demonstrates equivalence to the pressure‐flow method.26
We conclude that RP can be derived non‐invasively from the brachial and central BP waveforms measured using the clinically convenient cuff device with reasonable concordance to intra‐aortic measures, whereas XSP can only be acceptably derived from the brachial BP waveforms. There are some methodological considerations relating to the quality of BP waveform acquisition that limit the accuracy of non‐invasive cuff measured reservoir pressure parameters by comparison to intra‐aortic measures, and this is an area for future refinement of the method. The cuff‐based approach to measuring reservoir pressure parameters is user‐friendly and operator‐independent, which should enable more widespread application of brachial‐cuff RP and XSP to determine the clinical significance of reservoir pressure parameters.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
AUTHOR CONTRIBUTIONS
XP: performed experiments, analysis and interpretation of data, and drafted manuscript; MGS: project conception and study design, interpretation of data, and critical revision of manuscript; DSP, ND, JAB, and PR: performed experiments and critical revision of manuscript; JES: project conception and study design, interpretation of data, and critical revision of manuscript.
ACKNOWLEDGMENT
The work was supported (in part) by a starter project grant from the Royal Hobart Hospital Research Foundation. M.G.S. is supported by a National Health and Medical Research Council Australian Early Career Fellowship (reference 1104731). The authors thank all staff from the Royal Hobart Hospital cardiac catheterization laboratory for their assistance in facilitating this study. We are also grateful to Dr. Xun Yang, HeFei University of Technology, China for providing some technical assistance.
Peng X, Schultz MG, Picone DS, et al. Non‐invasive measurement of reservoir pressure parameters from brachial‐cuff blood pressure waveforms. J Clin Hypertens. 2018;20:1703–1711. 10.1111/jch.13411
Peng and Schultz are Joint first authors.
REFERENCES
- 1. Collaborators GBDRF . Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Segers P, O'Rourke MF, Parker K, et al. Towards a consensus on the understanding and analysis of the pulse waveform: Results from the 2016 Workshop on Arterial Hemodynamics: Past, present and future. Artery Res. 2017;18:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang JJ, O'Brien AB, Shrive NG, et al. Time‐domain representation of ventricular‐arterial coupling as a windkessel and wave system. Am J Physiol Heart Circ Physiol. 2003;284(4):H1358–H1368. [DOI] [PubMed] [Google Scholar]
- 4. Davies JE, Lacy P, Tillin T, et al. Excess pressure integral predicts cardiovascular events independent of other risk factors in the conduit artery functional evaluation substudy of Anglo‐Scandinavian Cardiac Outcomes Trial. Hypertens. 2014;64(1):60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng HM, Chuang SY, Wang JJ, et al. Prognostic significance of mechanical biomarkers derived from pulse wave analysis for predicting long‐term cardiovascular mortality in two population‐based cohorts. Int J Cardiol. 2016;215:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang WT, Sung SH, Wang JJ, et al. Excess Pressure Integral Predicts Long‐Term All‐Cause Mortality in Stable Heart Failure Patients. Am J Hypertens. 2017;30(3):271–278. [DOI] [PubMed] [Google Scholar]
- 7. Jones SC, Bilous M, Winship S, et al. Validation of the OSCAR 2 oscillometric 24‐hour ambulatory blood pressure monitor according to the International Protocol for the validation of blood pressure measuring devices. Blood Press Monit. 2004;9(4):219–223. [DOI] [PubMed] [Google Scholar]
- 8. Goodwin J, Bilous M, Winship S, et al. Validation of the Oscar 2 oscillometric 24‐h ambulatory blood pressure monitor according to the British Hypertension Society protocol. Blood Press Monit. 2007;12(2):113–117. [DOI] [PubMed] [Google Scholar]
- 9. Aguado‐Sierra J, Alastruey J, Wang J, et al. Separation of the reservoir and wave pressure and velocity from measurements at an arbitrary location in arteries. Proc Inst Mech Eng H. 2008;222(4):403–416. [DOI] [PubMed] [Google Scholar]
- 10. Gardner RM. Direct blood pressure—measurement dynamic response requirements. Anesthesiology. 1981;54(3):227–236. [DOI] [PubMed] [Google Scholar]
- 11. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tyberg JV, Bouwmeester JC, Shrive NG, et al. CrossTalk opposing view: Forward and backward pressure waves in the arterial system do not represent reality. J Physiol Paris. 2013;591(5):1171–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tyberg JV, Davies JE, Wang Z, et al. Wave intensity analysis and the development of the reservoir‐wave approach. Med Biol Eng Comput. 2009;47(2):221–232. [DOI] [PubMed] [Google Scholar]
- 14. Hametner B, Wassertheurer S, Hughes AD, et al. Reservoir and excess pressures predict cardiovascular events in high‐risk patients. Int J Cardiol. 2014;171(1):31–36. [DOI] [PubMed] [Google Scholar]
- 15. Millasseau S, Agnoletti D. Non‐invasive estimation of aortic blood pressures: A close look at current devices and methods. Curr Pharm Des. 2015;21(6):709–718. [DOI] [PubMed] [Google Scholar]
- 16. Narayan O, Parker KH, Davies JE, et al. Reservoir pressure analysis of aortic blood pressure: an in‐vivo study at five locations in humans. J Hypertens. 2017;35(10):2025–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peng X, Schultz MG, Picone DS, et al. Arterial reservoir characteristics and central‐to‐peripheral blood pressure amplification in the human upper limb. J Hypertens. 2017;35(9):1825–1831. [DOI] [PubMed] [Google Scholar]
- 18. Picone DS, Schultz MG, Otahal P, et al. Accuracy of Cuff‐Measured Blood Pressure: Systematic Reviews and Meta‐Analyses. J Am Coll Cardiol. 2017;70(5):572–586. [DOI] [PubMed] [Google Scholar]
- 19. Shoji T, Nakagomi A, Okada S, et al. Invasive validation of a novel brachial cuff‐based oscillometric device (SphygmoCor XCEL) for measuring central blood pressure. J Hypertens. 2017;35(1):69–75. [DOI] [PubMed] [Google Scholar]
- 20. Groppelli A, Omboni S, Parati G, et al. Evaluation of noninvasive blood pressure monitoring devices Spacelabs 90202 and 90207 versus resting and ambulatory 24‐hour intra‐arterial blood pressure. Hypertens. 1992;20(2):227–232. [DOI] [PubMed] [Google Scholar]
- 21. Omboni S, Parati G, Groppelli A, et al. Performance of the AM‐5600 blood pressure monitor: comparison with ambulatory intra‐arterial pressure. J Appl Physiol. 1997;82(2):698–703. [DOI] [PubMed] [Google Scholar]
- 22. Climie RE, Srikanth V, Beare R, et al. Aortic reservoir characteristics and brain structure in people with type 2 diabetes mellitus; a cross sectional study. Cardiovasc Diabetol. 2014;13:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Climie R, Picone DS, Sharman JE. Longitudinal Changes in Excess Pressure Independently Predict Declining Renal Function Among Healthy Individuals‐A Pilot Study. Am J Hypertens. 2017;30(8):772–775. [DOI] [PubMed] [Google Scholar]
- 24. Climie RE, Srikanth V, Keith LJ, et al. Exercise excess pressure and exercise‐induced albuminuria in patients with type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2015;308(9):H1136–H1142. [DOI] [PubMed] [Google Scholar]
- 25. Sharman JE, Avolio AP, Baulmann J, et al. Validation of non‐invasive central blood pressure devices: ARTERY Society task force consensus statement on protocol standardization. Eur Heart J. 2017;38(37):2805–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davies JE, Hadjiloizou N, Leibovich D, et al. Importance of the aortic reservoir in determining the shape of the arterial pressure waveform–the forgotten lessons of Frank. Artery Res. 2007;1(2):40–45. [Google Scholar]


