Abstract
The authors investigated the relationship of white‐coat hypertension (WCH) with subclinical organ damage and potential relevant mechanisms. A total of 386 untreated patients were enrolled and divided into 204 patients with WCH and 183 with normotension. Flow‐mediated dilation (FMD), pulse wave velocity (PWV), intima‐media thickness, left ventricular mass index (LVMI), and cystatin C levels were measured. All tests were two‐sided, and a P value <.05 was considered statistically significant. The WCH group exhibited higher LVMI and PWV values, decreased E/A ratio and FMD values, and increased prevalence for left ventricular hypertrophy compared with controls (P<.001 for all). Cystatin C was significantly higher in the WCH group compared with controls (P=.035) and was positively associated with LVMI (P<.05 for both). The presence of WCH is associated with more pronounced subclinical organ damage compared with normotension. Cystatin C may play a significant role and therefore warrants further investigation.
The white‐coat effect on blood pressure (BP) has long been recognized, referring to an office BP higher than the one measured out of the office.1 Although end organ damage and cardiovascular (CV) events are reportedly less common in patients with white‐coat hypertension (WCH) compared with sustained hypertension,2, 3, 4 it remains unclear whether WCH conveys an increased CV and organ damage risk in relation to normotension.
Several studies have shown that WCH is associated with subclinical organ damage as indicated by elevated left ventricular (LV) mass index (LVMI) and decreased E/a ratio,5 increased arterial stiffness on pulse wave analysis6 and intima‐media thickness (IMT)7, 8 and endothelial dysfunction as demonstrated by a decrease in flow‐mediated dilation (FMD).9 However, other studies have not yielded consistent results.10 Whether there is a potential role of underlying inflammation, which is reportedly associated with essential hypertension, also remains to be established. There are data suggesting increased levels of inflammatory markers, namely procalcitonin and high sensitivity C‐reactive protein (hsCRP), in patients with WCH compared with normotension,11, 12, 13 while other studies have failed to confirm this finding.14
Novel biomarkers such as cystatin C have only lately been acknowledged with regards to their correlation with CV risk.15, 16, 17 We have previously demonstrated that cystatin C levels correlate with higher LVMI values in untreated hypertensive individuals.18 Nonetheless, its role in WCH has not been fully elucidated.
In the present study we investigated the relation of WCH with subclinical organ damage as well as potential clinically relevant mechanisms, such as inflammatory response and cystatin C.
Methods and Population
Study population
The study population consisted of newly diagnosed untreated individuals who had been referred to the Hypertension Unit at Hippokrateion University Hospital, Athens, Greece, for hypertension workup and further management during a 48‐month period (March 2009–2011). All individuals underwent complete physical examination and routine blood and urine biochemical analyses, with the intention of assessing the presence and extent of target organ damage. Ambulatory BP was recorded during a working day using the automatic Spacelabs 90207 device (Redmond, WA). BP measurements were taken between 7 am and 11 pm (awake period) and between 11 pm and 7 am (asleep period) while the device was programmed to record BP every 20 minutes during the awake period and every 30 minutes during the asleep period. WCH was defined as an office BP ≥140/90 mm Hg on at least three occasions in the presence of a healthcare worker (particularly a physician), with normal 24‐hour (≤130/80 mm Hg) and day ABPM (≤135/85 mm Hg). Normotension was defined as systolic office BP <140 mm Hg and diastolic BP <90 mm Hg together with daytime ABPM ≤135/85 mm Hg with normal 24‐hour BP (≤130/80 mm Hg) according to the 2013 guidelines published by the European Society of Hypertension and the European Society of Cardiology.19 According to the above, 204 patients with WCH and 183 normotensive controls were identified. Patients with essential hypertension as well as 53 patients recently treated with any antihypertensive agent were excluded from the study.
Participants underwent anthropometric and BP measurements, standard 12‐lead electrocardiography, and laboratory determinations. Because cystatin C levels are affected by chronic liver disease, malignancy, and inflammatory conditions,20, 21, 22 patients with suspicion of the above were excluded. To avoid a confounding effect on assessment of WCH‐associated target organ damage, patients with suspicion of secondary hypertension, renal disease, chronic heart failure (New York Heart Association heart failure class III or IV) and diabetes mellitus were also excluded. The study was approved by the institutional ethics committees, and informed consent was given by all of the participants.
Biomarkers measurements
Blood samples were obtained after an 8‐ to 12‐hour overnight fast, with samples collected in sterile tubes, centrifuged at 3000 × g for 10 minutes at 48οC, and then stored at −80°C until assayed. Plasma was recovered by centrifugation, and biochemical parameters were determined by standard validated automatic methods using commercially available reagents. Levels of fibrinogen and hsCRP were measured by immunonephelometry (Dade Behring, Marburg, Germany). Circulating levels of intercellular adhesion molecule 1 (ICAM‐1), vascular cell adhesion molecule 1 (VCAM‐1), interleukin 6 (IL‐6), and tumor necrosis factor α (TNF‐α) were measured by enzyme‐linked immunosorbent assay (ELISA). Serum cystatin C levels were measured by BioVendor's Human Cystatin C ELISA kit (BioVendor R&D, Brno, Czech Republic).
Echocardiography
A two‐dimensionally guided M‐mode echocardiogram was performed on each participant at the same visit by an expert sonographer. Accurate linear measurements of interventricular septal wall thickness, posterior wall thickness, and LV internal dimensions, including end‐diastolic/systolic diameters, were obtained. LVMI was calculated by Devereux's formula.23 The geometric classification is based on the combination of increased relative wall thickness (RWT) and LV hypertrophy (LVMI >115 g/m2 for men, >95 g/m2 for women) at end diastole. Left atrial volume (LAV) was also calculated by the ellipsoid model. Ejection fraction, mitral pulse‐wave Doppler E/A ratio (Em/Am), deceleration time, and isovolumic relaxation time were estimated with two‐dimensional echocardiography. Measurements of longitudinal plane tissue Doppler imaging myocardial diastolic velocities at five sites on the mitral annulus included peak early myocardial tissue velocity (E), peak late myocardial tissue velocity (A), and the mean ratio E/A.
Vascular measurements
Carotid‐femoral pulse wave velocity (cf‐PWV) measurements were obtained in participants in a controlled room temperature (22°C) using a validated noninvasive automatic device (Complior SP; Artech Medical, Pantin, France). This device allows online pulse wave recording and automatic calculation of cf‐PWV, an established index of aortic stiffness, by assessing the time delay between the rapid upstroke of the carotid and femoral artery pulse waves. Pressure waveforms were gated with simultaneous electrocardiographs and were used to calculate the PWV between these two sites (carotid‐femoral) with two pressure‐sensitive transducers. Augmentation index (AIx) was determined by applanation tonometry of the left radial artery by using the SphygmoCor device. AIx was calculated from the aortic pressure waveform obtained by applying a transfer function to the radial pressure waveform.
Flow‐mediated dilation (FMD) as a marker of endothelial dysfunction was assessed in the brachial artery.24 It was measured as the absolute and percentage change in vessel diameter after cuff release from rest to the maximal diameter (60s). In all patients, the ABI value was determined in our vascular laborator, while Doppler measurements of brachial and ankle pressures were assessed with 10‐cm wide sphygmomanometer cuffs, which were manually inflated and deflated.
Carotid arteries were evaluated with the Vivid 7 ultrasound system (General Electric, Fairfield, CT) using a 7.5‐MHz probe. IMT of the common carotid arteries was obtained 2 cm proximal of the bulb from anterior and posterior approaches and averaged to obtain the mean IMT. In addition, from multiple approaches, carotid plaques, as the presence of wall thickening >1.3 mm, were detected in both arteries. Of note, when detected, atheromatous plaques were not included in the measurements of IMT.
Furthermore, the presence, type, and extent of hypertensive retinopathy were investigated via direct ophthalmoscopy. Images were evaluated by the same ophthalmologist who was unaware of the patients’ clinical characteristics. Retinal features obtained were venule nipping, venule deviation, and light reflex change at all clearly visualized crossings, and patients who underwent fundoscopy were then distributed to four groups according to Scheie's classification system (0–3).
Statistical Analysis
Qualitative variables are presented as absolute frequencies. Continuous variables were tested for normal distribution by Kolmogorov‐Smirnov test. Differences in continuous variables between two groups were assessed by Student t test for parametric data. For variables found to deviate from normality, nonparametric tests (Kruskal‐Wallis/Mann‐Whitney) were applied. Relationships among variables were assessed using Pearson's correlation coefficient. Multiple logistic regression was applied to estimate the odds ratio (OR) of LV hypertrophy (LVH) in the hypertensive compared with the normotensive group. To evaluate the diagnostic performance of cystatin C, as a marker for differentiating between WCH and controls, receiver operator characteristics (ROC) analysis was performed for all significant differences between groups. ROC curves were generated by plotting the sensitivity against 1 − specificity, and the area under the curve (AUC) with 95% confidence intervals (CIs) was calculated. The optimum cutoff point based on the ROC analysis was established by selecting the value that provides the greatest sum of the sensitivity and specificity, ie, the point closest to the upper left point of the ROC plot. All tests were two‐sided, and a P value of <.05 was considered to indicate statistical significance. SPSS version 18.0 (SPSS, Chicago, IL) software was used for all statistical calculations.
Results
Demographics
The anthropometric data, demographic characteristics, and BP measurements of the population are shown in Tables 1 and 2. Ten‐year risk of cardiovascular morbidity and mortality according to the Framingham score was 6.8%, and there was no significant difference between the two study groups (P=.237). Because of variations in anthropometry between the BP groups, structural and functional variables of the left ventricle and the vasculature were adjusted for age, sex, and body mass index to analyze whether differences were related to BP groups independently of these potential confounders.
Table 1.
Baseline Characteristics of the Study Population
| WCH | NT | P Value | |
|---|---|---|---|
| Patients, No. | 204 | 183 | |
| Age, y | 54.3±0.9 | 52.4±0.9 | .126 |
| Male sex, No. (%)a | 95 (47) | 72 (39) | .134 |
| Family history, No. (%)a | 143 (70) | 124 (67) | .704 |
| Smokers, No. (%)a | 76 (37.4) | 79 (42.9) | .228 |
| Dyslipidemia, No. (%)a | 83 (40.6) | 81 (43.5) | .843 |
| Body nass index, kg/m2 | 28.5±0.6 | 27.4±0.6 | .429 |
| Glucose, mg/dL | 99.4±1.1 | 96.6±0.9 | .060 |
| Creatinine, mg/dL | 0.91±0.02 | 0.88±0.01 | .075 |
| eGFR, mL/min/m2 | 99±2.5 | 102±2.4 | .330 |
| Cystatin C, ng/mL | 783±17 | 725±13 | .007 |
| Cystatin C–based eGFRb | 93±1.7 | 98±1.9 | .036 |
| Average of eGFR, creatinine/cystatin C | 96±2.1 | 100±2.2 | .062 |
| Uric acid, mg/dL | 5.2±0.2 | 5.3±0.2 | .542 |
| Total cholesterol, mg/dL | 216.4±3.1 | 212.5±3.6 | .399 |
| Ejection fraction, % | 63.5±0.4 | 63.2±0.5 | .563 |
| LVMI, g/m2 | 80.0±1.3 | 73.6±1.1 | <.0001 |
| LAV, mL/m2 | 41.7±1.4 | 40.3±2.0 | .571 |
| Em/Am | 1.06±0.02 | 1.19±0.03 | .001 |
| FMD, % | 5.23±0.27 | 6.79±0.29 | <.001 |
| c‐fPWV, m/s | 8.60±0.10 | 7.60±0.09 | <.001 |
| AIx | 26.5±1.4 | 25.2±1.3 | .179 |
| ABI | 1.11±0.008 | 1.13±0.009 | .686 |
| IMT, mm | 695±18 | 674±18 | .399 |
| Advanced retinal artery lesions,c % | 6.3 | 3.3 | .394 |
Abbreviations: ABI, ankle brachial index; AIx, augmentation index; BP, blood pressure; c‐fPWV, carotid‐femoral pulse wave velocity; DAP, diastolic arterial pressure; eGFR, estimated glomerular filtration rate; Em/Am, mitral pulse wave Doppler; FMD, flow‐mediated dilation; IMT, carotid intima media thickness; LAV, left atrial volume; LVMI, left ventricular mass index; NT, normotension; SAP, systolic arterial pressure; WCH, white‐coat hypertension. a P by chi‐square analysis. b76.6(cystatin C) − 1.16(mL/min/m2). cRetinal features of stages 3 and 4 according to Scheie's classification. Values are expressed as mean±standard error of the mean. Bold values indicate significance at P < .05.
Table 2.
BP Between Groups
| WCH | NT | P Value | |
|---|---|---|---|
| Office SAP, mm Hg | 152.9±1.0 | 131.3±1.0 | .001 |
| Office DAP, mm Hg | 92.9±0.9 | 83.1±0.9 | .001 |
| 24‐h systolic BP, mm Hg | 120.1±0.5 | 113.9±0.5 | .001 |
| 24‐h diastolic BP, mm Hg | 71.4±0.5 | 67.8±0.6 | .001 |
| Day systolic BP, mm Hg | 122.4±0.5 | 117.3±0.5 | .001 |
Abbreviations: BP, blood pressure; DAP, diastolic arterial pressure; NT, normotension; SAP, systolic arterial pressure; WCH, white‐coat hypertension. Values are expressed as mean±standard error of the mean. a P by chi‐square analysis.
Vascular indices of organ damage
We found a significant difference in PWV between WCH patients and controls in PWV (8.6±0.10 vs 7.6±0.09 m/s, P<.001) (Figure 1a), while ΑΙx values did not differ significantly between the two groups (26.5±1.4 vs 25.2±1.3, P=.179). A positive association was found between PWV and average 24‐hour systolic BP (r=.18, P<.010), but not with average 24‐hour diastolic BP (r=.021, P=.206). Multiple regression analysis of PWV with clinical parameters (age, sex, weight, height, body mass index, smoking, heart rate) and other biological parameters (total cholesterol, glucose, creatinine) showed that PWV was positively and independently associated with age and body mass index values (P<.001 for both). The group with WCH did not exhibit higher values of IMT compared with the normotensive group (695±18 vs 674±18 μm, P=.399). Moreover, a difference between the two groups was detected regarding endothelial function as estimated by FMD values (5.23±0.27 vs 6.79±0.29, P<.001) (Figure 1b). In addition, no significant effect was observed in ABI values (P=.818) or advanced retinal lesions (P=.394).
Figure 1.
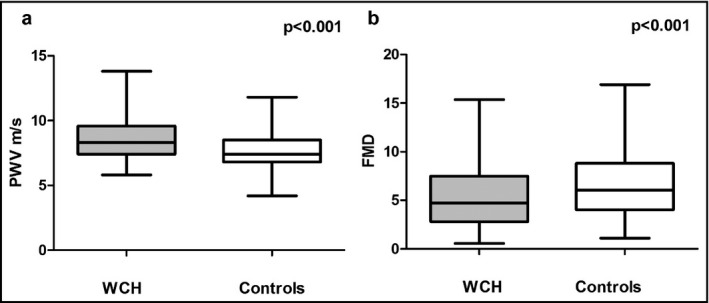
(a) Pulse wave velocity (PWV) between white‐coat hypertension (WCH) and controls. (b) Flow‐mediated dilation between white coat hypertension and controls.
Cardiac structural and functional indices
Our results showed significant differences in LV measurements and diastolic dysfunction parameters between WCH and normotension groups. WCH may have significant effects on indexes of early damage to the left ventricle as well as diastolic dysfunction parameters. More specifically, we found higher values of LVMI in the WCH group compared with the control group (80.0±1.3 vs 73.6±1.1 g/m2, P<.0001) (Figure 2). Accordingly, we tested the association between WCH and LVH after adjustments for confounders and an independent association was revealed (OR, 2.3; 95% CI, 1.41–3.81; P<.001). In line with this evidence, we observed a mean E/A ratio, which differed significantly between the two groups (1.06±0.02 vs 1.19±0.03, P=.001). However, there were no significant variations in LV diameter (46.8±0.3 vs 46.9±0.3 mm, P=.657) and LAV (41.7±1.4 vs 40.3±2.0 mm3, P=.571) between patients with WCH and the control group. It was also shown that LV diameter and cystatin C levels were independently associated with LVMI (P<.05 for both).
Figure 2.

The white‐coat hypertension (WCH) group exhibited higher left ventricular mass index (LVMI) values compared with controls.
Renal function
The two groups of patients showed no statistically significant differences with regards to creatinine levels (0.91±0.02 vs 0.88±0.01 mg/dL, P=.075) and estimated creatinine clearance (94±2 vs 99±2, P=.088). We further compared the two groups regarding cystatin C values as a more sensitive marker of renal function. Of note, the WCH group differed significantly compared with controls regarding cystatin C values (784±17 vs 737±14 ng/mL, P=.035) (Figure 3). Of note, cystatin C levels were positively associated with LVMI (r=+.19, P=.036) (Figure 4) and PWV (r=+.38, P<.001). To determine the efficiency of cystatin C in distinguishing WCH and controls, we used ROC analysis (Figure 5). A cystatin C cutoff value of 820 ng/mL yielded 60.1% sensitivity and 40.9% specificity with a P value of .052.
Figure 3.
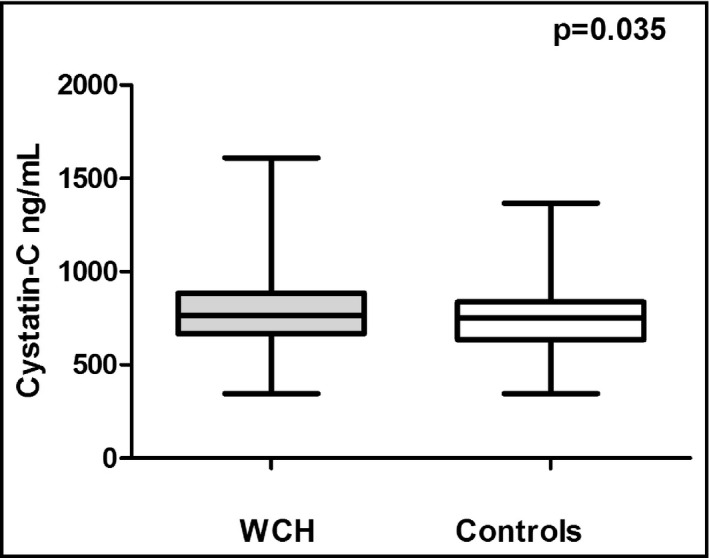
The white‐coat hypertension (WCH) group exhibited higher cystatin C values compared with controls.
Figure 4.
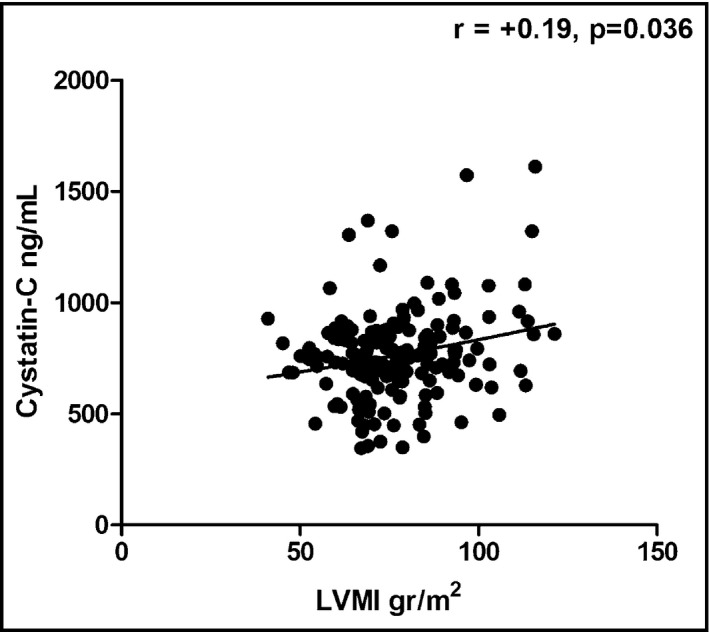
Cystatin C values were positively associated with left ventricular mass index (LVMI).
Figure 5.
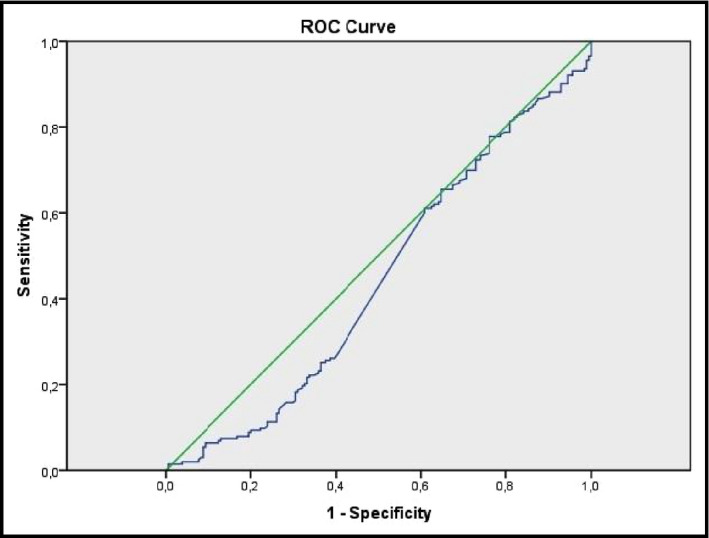
Receiver operating characteristic (ROC) analysis curves of cystatin C for differentiating between the two populations.
Biomarkers of inflammation and thrombosis
The two groups of patients were also examined for possible differences in markers of inflammation and thrombosis. In particular, WCH was characterized by similar values of hsCRP, VCAM‐1, and TNF‐α compared with controls (2.05±0.22 vs 2.11±0.23 mg/L [P=.815], 861±21 vs 831±15 ng/mL [P=.467], 5.05±0.30 vs 4.57±0.22 ng/mL [P=.289], respectively). We observed significantly different values in IL‐6 (P=.045) and fibrinogen (P=.034), in contrast to homocysteine levels (P=.243). Inflammatory biomarkers were not independently associated with the aforementioned indices of subclinical organ damage (P=not significant for all).
Discussion
In the present study we examined the possible involvement of WCH in subclinical organ damage and other associations that may be clinically relevant such as inflammatory process and cystatin C. In untreated WCH patients and normotensive controls we evaluated several indices of subclinical organ damage. At the same time, several inflammatory biomarkers as well as cystatin C levels, a novel cardiovascular disease biomarker that also reflects the underlying renal function, were also estimated in both groups. We observed that the WCH group presented with worse endothelial function, more pronounced arterial stiffness, and LVH. We found significantly different values of inflammatory biomarkers; however, it was of particular interest that cystatin C levels were not only significantly elevated in the WCH group but were also associated with arterial stiffness and LVH.
The impact of WCH on LVMI and diastolic function
It has been well established that WCH is not a benign condition, since it may induce alterations in cardiac structure and function. The HARVEST trial was one of the first studies to address this issue and concluded that all echocardiographic dimensions of the left ventricle were increased in patients with WCH compared with those measured in normotensive adults.25 These findings were consistent with the results derived from a number of studies and cross‐sectional surveys that demonstrated an increased prevalence of LVH in the WCH population.26, 27, 28 In agreement with the above, Cuspidi and colleagues,5 in their recent meta‐analysis that included 25 studies and a total of 7382 patients, reported that WCH patients presented with an elevated LVMI and a reduced mitral E/A ratio compared with normotensive patients. It seems, however, that WCH constitutes an intermediate condition between normotension and essential hypertension, since it causes a rise in LVMI with concomitant drop in mitral E/A.29, 30, 31 Nevertheless, other studies have failed to demonstrate a significant difference in the LVMI between WCH patients and normotensive controls.32, 33
The findings of the present association study favor a link between WCH and cardiac remodeling, as indicated by elevated LVMI in WCH individuals in consistency with the higher LVH prevalence in this group as well as a significant decrease in the E/A ratio. Conflicting results of previous studies may reflect several methodological differences. For example, the use of higher upper limits of normal for ambulatory BP resulted in a higher prevalence of WCH in hypertensive populations, thereby including more individuals who may be truly hypertensive and have target organ damage.32 WCH may therefore be associated with subclinical cardiac organ damage in a similar way as that in sustained hypertension, a hypothesis that needs to be tested in longitudinal studies with clinical end points.
The impact of WCH on arterial stiffness
Several studies have demonstrated that WCH can affect PWV, FMD, and IMT values, which are of subclinical target organ damage. Wimmer and associates6 showed that WCH patients presented with elevated central aortic systolic pressures compared with normotensive controls as assessed by PWV. In fact, it was recently demonstrated that the increase of PWV and IMT associated with WCH renders the latter more hazardous in terms of CV mortality than prehypertension.34 In addition, young individuals with WCH exhibited similar PWV values as patients with known hypertension.35 In accordance, Gómez‐Cerezo and colleagues9 showed that WCH and essential hypertension decrease FMD to a similar extent. Nonetheless, the existing evidence still remains controversial. Polonia and coworkers25 demonstrated that central aortic pressures and aortic stiffness in WCH population resemble closely to the ones obtained from the healthy population compared with those obtained from hypertensive patients, while Pierdomenico and colleagues36 reported no difference in the endothelium‐dependent dilatation between WCH patients and normotensive controls.
In the present study we have demonstrated that PWV is statistically significantly increased in WCH patients, whereas FMD is decreased, which is in unanimity with the results of the majority of existing studies so far. However, we reported no statistically significant difference in IMT values between the two groups. It seems the difference between office and ambulatory BP, which is an estimate of the white‐coat effect, is strongly associated with increased arterial stiffness and more impaired endothelial dysfunction using currently established noninvasive techniques. These findings imply that even though conduit arteries may be normal, in WCH, peripheral resistance and minimal forearm vascular resistance, a possible measure of small‐artery structural changes, may be affected. On the other hand, the prevalence of discrete carotid atherosclerosis was similar in the WCH and normotensive groups, suggesting that sustained rather than episodic BP elevation is important in producing preclinical disease of the arterial tree.
The impact of WCH on serum biomarkers: the emerging role of cystatin C
Inflammation is a key feature in the initiation, progression, and clinical implications of cardiovascular disorders, including essential hypertension.37 The association of elevated levels of inflammatory markers with WCH has been studied in previous studies; nevertheless, data are not adequate and results are controversial.11, 12, 13 Guven and colleagues14 recently failed to show a significant rise in CRP levels in WCH compared with healthy individuals. However, procalcitonin and CRP levels were significantly higher in the WCH group than in the normotension group according to a small, recent observational study.11 To the best of our knowledge, IL‐6 has not been found in a WCH population. We found different values of IL‐6 and fibrinogen levels even though the two groups exhibited similar values in terms of all other inflammatory biomarkers. Hypertension may induce proinflammatory and procoagulatory responses. In that state, tissue and systemic inflammatory mediators are increased, and it has been suggested that inflammatory markers are not just markers but rather mediators implicated in the pathogenesis of hypertension and vascular organ damage.38 Therefore, it seems reasonable that inflammatory molecules could be used as diagnostic objectives and might discriminate patients at high risk for subclinical organ damage in the state of WCH. Larger, well‐organized studies are required to investigate this theory.
Chronic kidney disease is an independent risk factor for cardiovascular disease morbidity and mortality associated with WHC.39 In a Japanese population, WCH was related to an increased risk of chronic kidney disease (OR, 1.67; P=.037) compared with normotension.40 Of note, a large, multiethnic, probability‐based population cohort, which was only recently published, investigated hypertensive target organ damage and adverse cardiovascular outcomes associated with WCH, masked hypertension, and sustained hypertension. Both WCH and masked hypertension were independently associated with increased aortic pulsed cystatin C and urinary albumin/creatinine ratio.41
We have previously demonstrated that cystatin C levels are associated with higher LVMI values in untreated hypertensive patients.18 In this context, we sought to investigate for the first time to our knowledge, the possible link between cystatin C and WCH. Cystatin C is a protein that is freely filtrated through the glomerulous and is considered a more sensitive marker of kidney dysfunction than creatinine, especially at higher estimated glomerular filtration rate levels.42, 43
In the present study, we showed that individuals with WCH present with significantly elevated cystatin C values compared with controls. In addition, cystatin C levels were positively associated with indexes of subclinical organ damage such as LVMI and PWV, highlighting the role of cystatin C as a possible prognostic marker in WCH for subclinical target organ damage. As for potential explanations of the aforementioned findings, cystatin C, as a better estimate of renal function, could be a stronger predictor of preclinical changes in vascular structure. In addition, PWV has been reported as a known parameter of arterial stiffness, which is likely associated with renal insufficiency.44 In addition, cystatin C could even directly participate in the development of arterial stiffness and LV hypertrophy geometry as a result of a potential imbalance between cysteine proteases and cystatin C in arterial wall and cardiac tissue.45
Conclusions
In the present study, we demonstrated that the presence of WCH in patients is accompanied by more pronounced subclinical organ damage compared with controls. Of particular interest was that cystatin C levels were significantly elevated in the WCH group, as well as arterial stiffness and LVH. Moreover, it may play a significant role as an early biomarker of renal function or, potentially, as a direct contributor to cardiac and vascular abnormalities. Therefore, the information provided by ambulatory BP monitoring may provide us with a more comprehensive picture of WCH burden and some of the currently investigated biomarkers may have a role in risk assessment. In this context, the role of cystatin C as a risk marker in patients with WCH warrants further consideration. Future prospective clinical studies evaluating the predictive role of cystatin C for cardiovascular disease in this subgroup of patients would be of great value.
Conflicts of interest
None declared.
J Clin Hypertens (Greenwich). 2017;19:190–197. DOI: 10.1111/jch.12882. © 2016 Wiley Periodicals, Inc.
References
- 1. Parati G, Ulian L, Santucciu C, et al. Difference between clinic and daytime blood pressure is not a measure of the white coat effect. Hypertension. 1998;31:1185–1189. [DOI] [PubMed] [Google Scholar]
- 2. Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white‐coat, masked and sustained hypertension versus true normotension: a meta‐analysis. J Hypertens. 2007;25:2193–2198. [DOI] [PubMed] [Google Scholar]
- 3. Mancia G, Facchetti R, Bombelli M, et al. Long‐term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension. 2006;47:846–853. [DOI] [PubMed] [Google Scholar]
- 4. Pierdomenico SD, Cuccurullo F. Prognostic value of white‐coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta‐analysis. Am J Hypertens. 2011;24:52–58. [DOI] [PubMed] [Google Scholar]
- 5. Cuspidi C, Rescaldani M, Tadic M, et al. White‐coat hypertension, as defined by ambulatory blood pressure monitoring, and subclinical cardiac organ damage: a meta‐analysis. J Hypertens. 2015;33:24–32. [DOI] [PubMed] [Google Scholar]
- 6. Wimmer NJ, Sathi K, Chen TL, et al. Comparison of pulse wave analysis between persons with white coat hypertension and normotensive persons. J Clin Hypertens. 2007;9:513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cuspidi C, Sala C, Tadic M, et al. Is white‐coat hypertension a risk factor for carotid atherosclerosis? A review and meta‐analysis Blood Press Monit. 2015;20:57–63. [DOI] [PubMed] [Google Scholar]
- 8. Fukuhara M, Arima H, Ninomiya T, et al. White‐coat and masked hypertension are associated with carotid atherosclerosis in a general population: the Hisayama study. Stroke. 2013;44:1512–1517. [DOI] [PubMed] [Google Scholar]
- 9. Gomez‐Cerezo J, Rios Blanco JJ, Suarez Garcia I, et al. Noninvasive study of endothelial function in white coat hypertension. Hypertension. 2002;40:304–309. [DOI] [PubMed] [Google Scholar]
- 10. Hanninen MR, Niiranen TJ, Puukka PJ, et al. Target organ damage and masked hypertension in the general population: the Finn‐Home study. J Hypertens. 2013;31:1136–1143. [DOI] [PubMed] [Google Scholar]
- 11. Yavuzer H, Cengiz M, Yavuzer S, et al. Procalcitonin and Pentraxin‐3: Current biomarkers in inflammation in white coat hypertension. J Hum Hypertens. 2016;30:424–429. [DOI] [PubMed] [Google Scholar]
- 12. Andrikou I, Tsioufis C, Dimitriadis K, et al. Similar levels of low‐grade inflammation and arterial stiffness in masked and white‐coat hypertension: comparisons with sustained hypertension and normotension. Blood Press Monit. 2011;16:218–223. [DOI] [PubMed] [Google Scholar]
- 13. Ozdogan M, Bozcuk H, Coban E. Low‐grade inflammation in white‐coat hypertension. Med Sci Monit. 2007;13:CR570–CR573. [PubMed] [Google Scholar]
- 14. Guven A, Tolun F, Caliskan M, et al. C‐reactive protein and nitric oxide level in patients with white coat hypertension. Blood Press. 2012;21:281–285. [DOI] [PubMed] [Google Scholar]
- 15. Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 2005;352:2049–2060. [DOI] [PubMed] [Google Scholar]
- 16. Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koenig W, Twardella D, Brenner H, Rothenbacher D. Plasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: more than simply a marker of glomerular filtration rate. Clin Chem. 2005;51:321–327. [DOI] [PubMed] [Google Scholar]
- 18. Tousoulis D, Androulakis E, Papageorgiou N, et al. Genetic predisposition to left ventricular hypertrophy and the potential involvement of cystatin‐C in untreated hypertension. Am J Hypertens. 2013;26:683–690. [DOI] [PubMed] [Google Scholar]
- 19. Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 20. Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. [DOI] [PubMed] [Google Scholar]
- 21. Nakai K, Kikuchi M, Fujimoto K, et al. Serum levels of cystatin C in patients with malignancy. Clin Exp Nephrol. 2008;12:132–139. [DOI] [PubMed] [Google Scholar]
- 22. Takeuchi M, Fukuda Y, Nakano I, et al. Elevation of serum cystatin C concentrations in patients with chronic liver disease. Eur J Gastro Hepatol. 2001;13:951–955. [DOI] [PubMed] [Google Scholar]
- 23. Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man. Anatomic validation of the method. Circulation. 1977;55:613–618. [DOI] [PubMed] [Google Scholar]
- 24. Tousoulis D, Antoniades C, Stefanadis C. Evaluating endothelial function in humans: a guide to invasive and non‐invasive techniques. Heart. 2005;91:553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palatini P, Mormino P, Santonastaso M, et al. Target‐organ damage in stage I hypertensive subjects with white coat and sustained hypertension: results from the HARVEST study. Hypertension. 1994;23:211–216. [DOI] [PubMed] [Google Scholar]
- 26. Polonia J, Carvalho N, Barbosa L, Silva JA. Blood pressure on arising, morning blood pressure surge and blood pressure variability in white coat hypertensives and in matched normotensives and sustained hypertensives. Rev Port Cardiol. 2006;8:693–704. [PubMed] [Google Scholar]
- 27. Sega R, Trocino G, Lanzarotti A, et al. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study). Circulation. 2001;104:1385–1392. [DOI] [PubMed] [Google Scholar]
- 28. Muscholl MW, Hense HW, Brockel U, et al. Changes in left ventricular structure and function in patients with white coat hypertension: cross sectional survey. BMJ. 1998;317:565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cardillo C, De Felice F, Campia U, Folli G. Psychophysiological reactivity and cardiac end‐organ changes in white coat hypertension. Hypertension. 1993;1:836–844. [DOI] [PubMed] [Google Scholar]
- 30. Karter Y, Curgunlu A, Altinisik S, et al. Target organ damage and changes in arterial compliance in white coat hypertension. Is white coat innocent?. Blood Press. 2003;6:307–313. [DOI] [PubMed] [Google Scholar]
- 31. Ihm SH, Youn HJ, Park CS, et al. Target organ status in white‐coat hypertensives: usefulness of serum procollagen type I propeptide in the respect of left ventricular diastolic dysfunction. Circ J. 2009;73:100–105. [DOI] [PubMed] [Google Scholar]
- 32. Cavallini MC, Roman MJ, Pickering TG, et al. Is white coat hypertension associated with arterial disease or left ventricular hypertrophy? Hypertension. 1995;26:413–419. [DOI] [PubMed] [Google Scholar]
- 33. Kotsis V, Stabouli S, Toumanidis S, et al. Target organ damage in “white coat hypertension” and “masked hypertension”. Am J Hypertens. 2008;21:393–399. [DOI] [PubMed] [Google Scholar]
- 34. Sung SH, Cheng HM, Wang KL, et al. White coat hypertension is more risky than prehypertension: important role of arterial wave reflections. Hypertension. 2013;61:1346–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Faria RA, Brandao AA, Pozzan R, et al. 3c.08: 24 h central blood pressure and pulse wave velocity monitoring in normotensive, hypertensive, white coat hypertension and masked hypertension young adults. J Hypertens. 2015;33(suppl 1):e39. [Google Scholar]
- 36. Pierdomenico SD, Cipollone F, Lapenna D, et al. Endothelial function in sustained and white coat hypertension. Am J Hypertens. 2002;15:946–952. [DOI] [PubMed] [Google Scholar]
- 37. Androulakis ES, Tousoulis D, Papageorgiou N, et al. Essential hypertension: is there a role for inflammatory mechanisms? Cardiol Rev. 2009;17:216–221. [DOI] [PubMed] [Google Scholar]
- 38. Stefanadi E, Tousoulis D, Androulakis ES, et al. Inflammatory markers in essential hypertension: potential clinical implications. Curr Vasc Pharmacol. 2010;8:509–516. [DOI] [PubMed] [Google Scholar]
- 39. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 40. Kanno A, Metoki H, Kikuya M, et al. Usefulness of assessing masked and white‐coat hypertension by ambulatory blood pressure monitoring for determining prevalent risk of chronic kidney disease: the Ohasama study. Hypertens Res. 2010;33:1192–1198. [DOI] [PubMed] [Google Scholar]
- 41. Tientcheu D, Ayers C, Das SR, et al. Target organ complications and cardiovascular events associated with masked hypertension and white‐coat hypertension: analysis from the dallas heart study. J Am Coll Cardiol. 2015;66:2159–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mussap M, Plebani M. Biochemistry and clinical role of human cystatin C. Crit Rev Clin Lab Sci. 2004;6:467–550. [DOI] [PubMed] [Google Scholar]
- 43. Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta‐analysis. Am J Kidney Dis. 2002;40:221–226. [DOI] [PubMed] [Google Scholar]
- 44. Peralta CA, Jacobs DR Jr, Katz R, et al. Association of pulse pressure, arterial elasticity, and endothelial function with kidney function decline among adults with estimated GFR >60 mL/min/1.73 m(2): the Multi‐Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2012;59:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tanaka A, Suemaru K, Araki H. A new approach for evaluating renal function and its practical application. J Pharmacol Sci. 2007;105:1–5. [DOI] [PubMed] [Google Scholar]


