Recent large randomized clinical trials (RCT) have shown that inhibitors of sodium/glucose cotransporter 2 (SGLT2i) significantly reduce the rate of adverse cardiovascular outcomes (especially heart failure (HF)1) and preserve renal function in patients with type 2 diabetes at high cardiovascular risk.1, 2 The use of SGLT2i is well established in clinical practices around the world and likely to increase further. Several potential novel pathways associated with the cardiovascular effects of SGLT2i have been suggested by clinical and mechanistic studies.3, 4, 5 In addition to body weight reduction, risk factors such as glycemia, blood pressure (BP), insulinemia, and oxidative stress are reported to be improved by SGLT2 inhibition. However, the improvement of these factors does not completely explain the beneficial effects of SGLT2i.
We propose that control of nocturnal hypertension is a particularly efficient hemodynamic mechanism underlying the beneficial effect of SGLT2i in reducing heart failure in diabetic patients. Nocturnal hypertension and the nondipper/riser pattern of nocturnal BP increase the risk of cardiovascular events (including stroke, heart failure, and coronary artery disease) and organ damage (including chronic kidney disease, left ventricular hypertrophy, silent cerebral infarct, and brain atrophy) in both population‐based and clinical practice‐based samples.6, 7, 8 Nocturnal hypertension and the nondipper/riser pattern—which are partly determined by an increased circulating volume—are frequently observed in individuals with diabetes, chronic kidney disease, sleep apnea, and heart failure.6, 7, 8 Uncontrolled nocturnal hypertension poses an additional risk of cardiovascular disease in individuals with diabetes or heart failure with a preserved ejection fraction.9, 10 In addition, a recent study has suggested that impaired long‐term glycemic control and heightened inflammatory states may contribute partly to blunted BP dipping in middle‐aged and older adults with obesity‐related prediabetes.11
To illustrate our proposition, we detail the time course of the hemodynamic consequences of SGLT2 inhibition in a 41‐year‐old diabetic man with resistant hypertension, obstructive sleep apnea, and shortness of breath who was newly initiated on the SGLT2i, canagliflozin. He had been previously treated with an angiotensin receptor blocker, a calcium channel blocker, thiazides, and a dipeptidyl peptidase‐4 inhibitor (candesartan 8 mg, hydrochlorothiazide 6.25 mg, and sitagliptin 25 mg). Ambulatory BP monitoring (ABPM) disclosed that the patient's baseline 24‐hour BP and daytime and nocturnal BP levels were markedly elevated (Figure 1), and pulse oximetry simultaneously performed by ABPM during sleep disclosed mild/moderate sleep apnea, with an oxygen desaturation index (ODI) 12.7/h (Figure 1).
Figure 1.
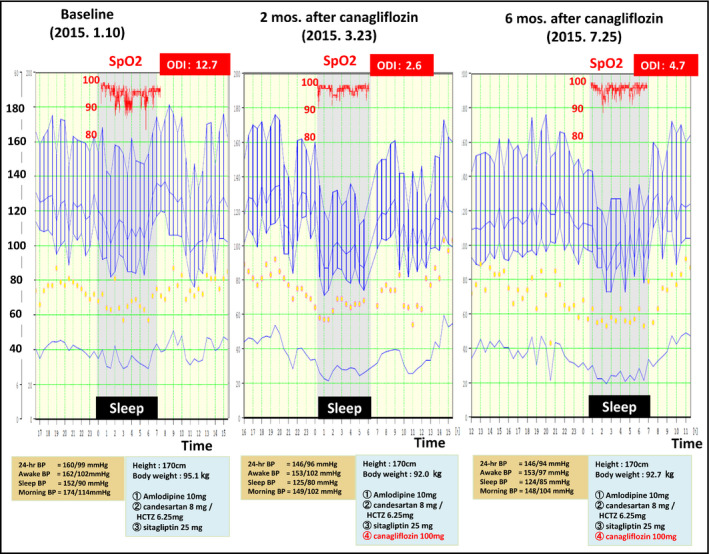
Change in the 24‐h ambulatory BP profile by daily treatment with 100‐mg canagliflozin in the patient, a 41‐y old diabetic man with resistant hypertension and obstructive sleep apnea. After the baseline evaluation (left), canagliflozin was started for 6 mo. ABPM was carried out at 2 mo (middle) and 6 mo (right) after the initiation of canagliflozin
After 2 months of daily 100‐mg canagliflozin treatment, the patient's 24‐hour BP level and particularly his nocturnal BP level were significantly reduced. As well, his ODI dropped sharply from 12.7/h to 2.6/h (Figure 1), the HbA1c declined from 7.3% to 6.5% and body weight decreased by 3.1 kg. Of note, awake mean BP (MBP) had decreased by 3% whereas sleep mean BP had decreased substantially more, by 14%. Six months following canagliflozin initiation, awake MBP was 5% lower than at baseline and sleep MBP was still 12% reduced as compared to baseline. Serial cardiac MRIs demonstrated that the patient's left‐ventricular (LV) end‐diastolic volume and stroke volume were decreased 2 months after initiation of canagliflozin but had returned to the baseline level at 6 months (Figure 2A). Despite the transient nature of these hemodynamic changes, LV mass was consistently and progressively reduced (by 5% and 15% at 2 and 6 months, respectively), along with an increased arterial distensibility of the ascending aorta (indexed as the percent increase of the cross‐sectional area, as well as the percent increase in area per 1 mm Hg of central pulse pressure as estimated by a SphygmoCor BP measurement device) (Figure 2B).
Figure 2.
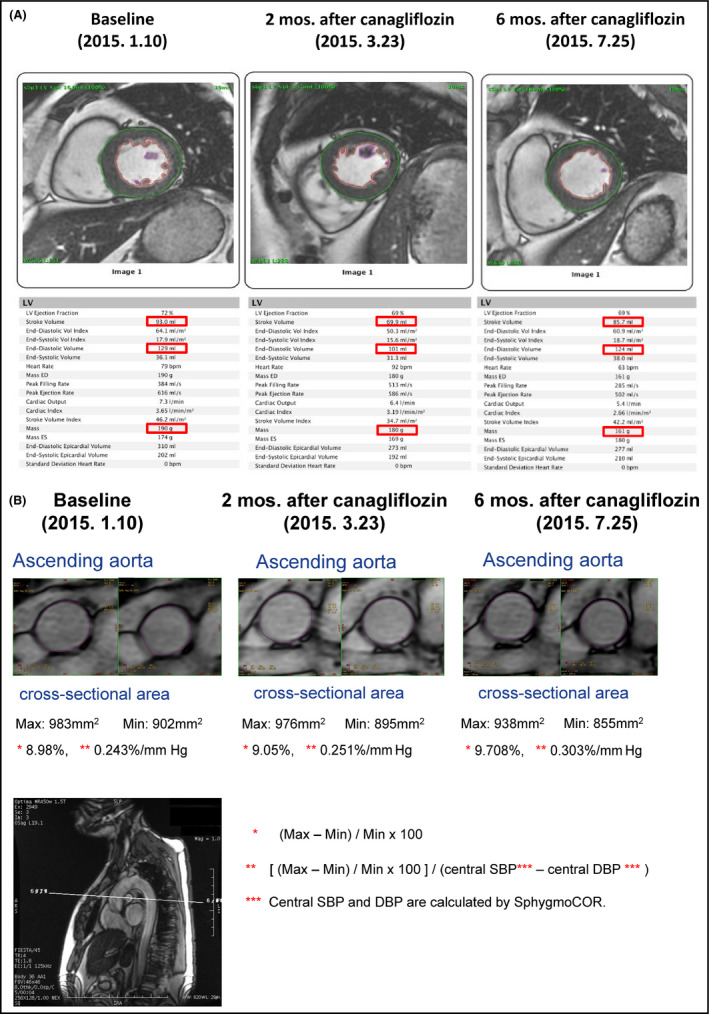
A, Cardiac MRI showing a significant reduction in stroke volume and end‐diastolic volume after 2 mo of daily 100‐mg canagliflozin treatment. At 6 mo after the initiation of treatment, these values had returned to the baseline levels. The LV mass was progressively reduced at 2 and 6 mo. B, Aortic MRI demonstrating that the percent change in the ascending aortic cross‐sectional area of 8.98% (0.243%/mm Hg, when divided by the central pulse pressure) at baseline was significantly increased to 9.05% at 2 mo and 9.78% at 6 mo after the initiation of treatment with 100‐mg canagliflozin
Figure 3 conceptualizes the impact of nocturnal hypertension on HF and posits the decrease in nocturnal LV strain as a possible synergistic mechanism of SGLT2i‐associated improvement. The initial effect of an SGLT2i is a reduction in LV preload caused by a reduction of the circulating volume (which resulted in a mean 3% increment in hematocrit sustained throughout the 3 years of treatment with empagliflozin in the EMPA‐REG OUTCOME trial1). In addition, a decrease of the LV mass with an amelioration of aortic stiffness reduces LV afterload. The impact of higher BP on wall stress is stronger during sleep, because the supine position increases the venous return from the lower body, LV wall stress being determined by both LV pressure and LV diameter (ie, Laplace law). Thus, the nocturnal BP‐lowering effect of SGLT2 inhibition would be synergistic with the reduction of the circulating volume. In addition, the fluid shift from the lower body to the upper body during sleep worsens obstructive sleep apnea by increasing intrathoracic pressure. The effect of SGLT2 inhibition to contract extracellular fluid volume reduces intrathoracic pressure.
Figure 3.
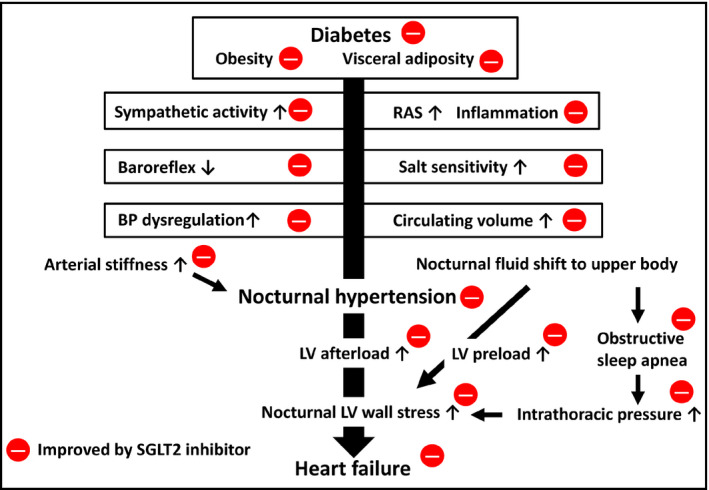
Synergistic decrease in nocturnal LV strain by the management of nocturnal hypertension with reduced fluid retention as a possible mechanism of SGLT2i‐associated cardiovascular protection in diabetes. SGLT2, sodium/glucose cotransporter 2; RAS, renin‐angiotensin system; BP, blood pressure; LV, left ventricle
In summary, better control of nocturnal hypertension acts synergistically with fluid offload to decrease nocturnal LV strain; in the longer term, this may lead to protection against both HF and renal failure, especially in diabetic patients with obstructive sleep apnea and/or individuals with salt‐sensitivity augmented by renin‐angiotensin system inhibitors.
In a recent randomized trial in high‐risk hypertensive patients, intensive BP control guided by the automated office BP < 120 mm Hg was superior to standard BP control < 140 mm Hg.12 In addition to strict BP control (ie, quantity of BP control), “perfect 24‐hour BP” control including BP variability and nocturnal hypertension (ie, quality of BP control) is recommended for hypertensive patients especially for the high‐risk group.6, 13, 14 Calcium channel blockers and angiotensin receptor blockers may be protective against stroke via BP variability, whereas drugs that reduce circulating volume—such as diuretics, sacubitril/valsartan, and SGLT2i—may be protective against HF via lowering of nocturnal BP and LV preload.14
A recent phase III study and a meta‐analysis have shown that SGLT2i significantly reduce 24‐hour ambulatory BP, with significant reductions in both daytime and nighttime BP.15, 16 Other recent studies have demonstrated that administration of the SGLT2i empagliflozin can change the circadian rhythm of BP from a nondipper to a dipper profile in salt‐treated, obese Otsuka Long Evans Tokushima Fatty (OLETF) rats17 and likewise with luseogliflozin administered to SHR/NDmcr‐cp (+/+) rats, a model of metabolic syndrome.18 Similarly, in human studies BP reduction by an SGLT2 inhibitor has been shown to be associated with the restoration of a disrupted circadian rhythm of BP from a nondipper to a dipper pattern in hypertensive subjects with impaired glucose metabolism and insulin resistance.19
In the recent 2017 AHA/ACC Clinical Practice Hypertension Guidelines,20 diagnostic threshold and target BP levels were lowered to 130/80 mm Hg for clinic, home, and daytime ambulatory BPs. In addition, values for nocturnal BP have been set at 110/65 mm Hg. These recommendations will affect many hypertensive patients and require more aggressive treatment of their office and 24‐hour blood pressures. In addition, for the hypertension specialist, it is recommended that nocturnal hypertension in high‐risk hypertensive patients, especially those with diabetes, chronic kidney disease, sleep apnea, and heart failure, be detected and treated.21 SGLT2i agents may be an effective approach for dealing with nocturnal hypertension in diabetic patients. Therefore, the global impact of SGLT2i therapy could be effective prevention of heart failure by reducing nocturnal BP as well as by reducing circulating volume, especially in diabetic hypertensive patients with increased salt sensitivity.
The preferential effects of SGLT2i in the control of nocturnal hypertension and for organ protection should be tested in future clinical studies. As a first step, an RCT of SGLT2i therapy on nocturnal hypertension would be of great interest in diabetic hypertensive patients.
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:424‐428. [DOI] [PubMed] [Google Scholar]
- 2. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657 [DOI] [PubMed] [Google Scholar]
- 3. Inzucchi SE, Zinman B, Wanner C, et al. SGLT‐2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12:90‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA‐REG OUTCOME Trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39:1108‐1114. [DOI] [PubMed] [Google Scholar]
- 5. Ferrannini E. Sodium‐glucose co‐transporters and their inhibition: clinical physiology. Cell Metab. 2017;26:27‐38. [DOI] [PubMed] [Google Scholar]
- 6. Kario K. Essential Manual of 24‐hour Blood Pressure Management from Morning to Nocturnal Hypertension. London: Wiley‐Blackwell; 2015:1‐138. [Google Scholar]
- 7. Kario K, Hamasaki H. Nocturnal blood pressure surge behind morning surge in obstructive sleep apnea syndrome: another phenotype of systemic hemodynamic atherothrombotic syndrome. J Clin Hypertens. 2015;17:682‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kario K. Evidence and perspectives on the 24‐hour management of hypertension: hemodynamic biomarker‐initiated “anticipation medicine” for zero cardiovascular event. Prog Cardiovasc Dis. 2016;59:262‐281. [DOI] [PubMed] [Google Scholar]
- 9. Eguchi K, Pickering TG, Hoshide S, et al. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am J Hypertens. 2008;21:443‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Komori T, Eguchi K, Saito T, Hoshide S, Kario K. Riser pattern is a novel predictor of adverse events in heart failure patients with preserved ejection fraction. Circ J. 2017;81:220‐226. [DOI] [PubMed] [Google Scholar]
- 11. Lane‐Cordova AD, Kalil GZ, Wagner CJ, et al. Hemoglobin A1c and C‐reactive protein are independently associated with blunted nocturnal blood pressure dipping in obesity‐related prediabetes. Hypertens Res. 2018;41:33‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. SPRINT Research Group , Wright JT Jr, Williamson JD, et al. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kario K, Tomitani N, Kanegae H, et al. Development of a new ICT‐based multisensor blood pressure monitoring system for use in hemodynamic biomarker‐initiated anticipation medicine for cardiovascular disease: the National IMPACT Program Project. Prog Cardiovasc Dis. 2017. (in press). pii: S0033‐0620(17)30142‐1. [DOI] [PubMed] [Google Scholar]
- 14. Kario K. Hypertension: benefits of strict blood‐pressure lowering in hypertension. Nat Rev Cardiol. 2016;13:125‐126. [DOI] [PubMed] [Google Scholar]
- 15. Weber MA, Mansfield TA, Cain VA, Iqbal N, Parikh S, Ptaszynska A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double‐blind, placebo‐controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016;4:211‐220. [DOI] [PubMed] [Google Scholar]
- 16. Baker WL, Buckley LF, Kelly MS, et al. Effects of sodium‐glucose cotransporter 2 inhibitors on 24‐hour ambulatory blood pressure: a systematic review and meta‐analysis. J Am Heart Assoc. 2017;6:e005686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takeshige Y, Fujisawa Y, Rahman A, et al. A sodium‐glucose co‐transporter 2 inhibitor empagliflozin prevents abnormality of circadian rhythm of blood pressure in salt‐treated obese rats. Hypertens Res. 2016;39:415‐422. [DOI] [PubMed] [Google Scholar]
- 18. Rahman A, Kittikulsuth W, Fujisawa Y, et al. Effects of diuretics on sodium‐dependent glucose cotransporter 2 inhibitor‐induced changes in blood pressure in obese rats suffering from the metabolic syndrome. J Hypertens. 2016;34:893‐906. [DOI] [PubMed] [Google Scholar]
- 19. Rahman A, Hitomi H, Nishiyama A. Cardioprotective effects of SGLT2 inhibitors are possibly associated with normalization of the circadian rhythm of blood pressure. Hypertens Res. 2017;40:535‐540. 10.1038/hr.2016.193. Epub 2017 Jan 19. [DOI] [PubMed] [Google Scholar]
- 20. Whelton PK, Carey RM, Aronow WS, et al. 2017ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017. (in press). [Google Scholar]
- 21. Kario K. Global impact of 2017 AHA/ACC Hypertension Guidelines: a perspective from Japan. Circulation. 2018. (accepted). [DOI] [PubMed] [Google Scholar]


