Abstract
Asymmetric dimethylarginine (ADMA), which is the main endogenous inhibitor of nitric oxide synthase, plays a critical role in the process of endothelial dysfunction. The authors evaluated the association between high plasma ADMA levels in patients with hypertension and the presence of cardiovascular risk factors and the development of type 2 diabetes mellitus (DM) and cardiovascular outcomes, including death. The authors evaluated 191 patients with hypertension who were stratified into two groups according to the median value of basal ADMA: those with high levels of plasma ADMA (>0.55 μmol/L) and low levels of plasma ADMA (≤0.55 μmol/L) who were prospectively evaluated over 5.8 years. High ADMA levels were seen in patients with higher weight, body mass index, waist circumference, triglycerides, uric acid, and high‐sensitivity C‐reactive protein, and lower levels of high‐density lipoprotein cholesterol and in patients with type 2 DM. There was an association between high plasma ADMA levels and the occurrence of cardiovascular death. In a subgroup of patients with hypertension free from metabolic syndrome and DM at baseline, there was an association between high ADMA levels and the development of type 2 DM. This study confirms the association of high plasma ADMA levels and the presence of cardiovascular risk factors in patients with hypertension and suggests a positive predictive value of high plasma ADMA levels for cardiovascular death in patients with hypertension and also for the development of type 2 DM in a subgroup of patients with hypertension free from metabolic abnormalities.
Keywords: asymmetrical dimethylarginine, cardiovascular death, cardiovascular disease, cardiovascular risk factors, metabolic syndrome, type 2 diabetes mellitus
1. INTRODUCTION
Asymmetrical dimethylarginine (ADMA) is a naturally occurring amino acid that circulates in plasma. It inhibits the production of nitric oxide (NO), which is a potent vasodilator, from L‐arginine, and generates considerable cardiovascular biological effects1, 2, 3, 4 High plasma concentrations of ADMA have been associated with endothelial dysfunction, atherosclerosis, and cardiovascular disease.5 A growing number of studies also suggest that high ADMA concentrations are associated with the incidence and progression of cardiovascular outcomes and all‐cause mortality.6, 7 Altogether, ADMA and NO play a pivotal role in endothelial dysfunction, which is the essential first step in atherogenesis.8 However, the interpretation of these studies has been complicated because of the heterogeneity of study populations and the outcomes assessed.
There is evidence of the occurrence of endothelial dysfunction in patients with insulin resistance, suggesting that reduced NO bioavailability is a crucial factor for the vasodilator action of insulin.9 The activation of NO synthase augments blood flow to insulin‐sensitive tissues (ie, skeletal muscle, liver, and adipose tissue), and its activity is impaired in insulin resistance. Therefore, the inhibition of NO synthase reduces the microvascular delivery of nutrients and blunts insulin‐stimulated glucose uptake in skeletal muscles.10
To help clarify this evidence, we conducted a prospective study with 191 patients with hypertension. We had two principal aims: (1) to evaluate the association of circulating ADMA concentration with prevalent cardiovascular risk factors in patients with hypertension, and (2) to quantify the association between high plasma ADMA levels and the development of type 2 diabetes mellitus (DM) and cardiovascular outcomes, including death, both in patients with general hypertension and in metabolically healthy patients.
2. METHODS
The study was approved by the ethics and research committee of the Ministry of Health, Brazil (No. 116.001/97988). All patients provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and with the Brazilian National Ministry of Health Resolution CNS 196/96.
A total of 638 patients who were treated at the Integrated Center for Hypertension and Cardiovascular Metabolism of the Universidade Federal de São Paulo (UNIFESP, Federal University of São Paulo) were evaluated between 2004 and 2005 using anamnesis, physical examination, and laboratory tests, as previously described.11 The following inclusion criteria were used: age of at least 18 years and a washout period of 1 month from lipid‐modifying agents. Patients presenting with active infectious or inflammatory disease were excluded, as were pregnant/breastfeeding patients and patients with HIV. The use of the following medications was discontinued in the 4 weeks preceding inclusion in the study in 2004 and 2005: 3‐hydroxy‐3‐methyl‐glutariyl coenzyme A reductase inhibitors (statins); cholesterol absorption inhibitors such as ezetimibe, probucol, cholestyramine, niacin, and fibric acid derivatives (fibrates); and drugs used to treat obesity (orlistat and sibutramine). All medications were reintroduced after the enrollment as clinically indicated. Blood samples were stored at −80°C.
In 2012, we successfully contacted 213 of these patients. We excluded 17 patients without a diagnosis of hypertension and five patients who died for reasons other than cardiovascular causes. Therefore, we included 191 patients with hypertension for the prospective evaluation by final anamnesis, physical examination, review of medical records, and laboratory tests.
During both visits, we recorded the weight, height, blood pressure, and waist circumference of all patients. Body mass index (BMI) was calculated by dividing weight in kilograms by height in meters squared (kg/m²). Blood pressure was obtained by a trained operator with the patient in the sitting position after 5 minutes of rest. A mercury sphygmomanometer was used according to a standard protocol, and blood pressure was calculated as the average of values after excluding the first of four measurements.12
Plasma concentrations of glucose, total cholesterol, and triglycerides were determined by automated enzymatic assays. High‐density lipoprotein cholesterol and low‐density lipoprotein cholesterol levels were determined following the manufacturer's instructions.
Renal function was assessed by plasma creatinine levels determined by the Jaffe method. High‐sensitivity C‐reactive protein (hs‐CRP) was determined by chemiluminescence immunoassay.
Estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula: 141 × min (Cr/k,1)α × max (Cr/k,1)−1.209 × 0.993age × 1.018 [if female] × 1.159 [if black], where Cr is plasma creatinine (mg/dL), k is sex‐specific knots at 0.7 mg/dL for women and 0.9 mg/dL for men, α is −0.329 for women and −0.411 for men, min is the minimum Cr/k or 1, and max is the maximum Cr/k or 1.13
Cardiovascular disease was defined as the presence of coronary heart disease (evidence of silent myocardial infarction or myocardial ischemia, history of unstable angina or stable angina pectoris, and history of coronary angioplasty or coronary artery surgery) or coronary heart disease risk equivalents (stroke, peripheral arterial disease, abdominal aortic aneurysm, carotid artery disease, renal artery disease), according to American Heart Association guidelines.14 To complete the data collection and review of medical records, we used computerized hospital registries to identify hospitalizations attributable to stroke, coronary heart disease, and atherosclerotic vascular events for all participants.
The diagnosis of DM was determined according to American Diabetes Association guidelines.15
Metabolic syndrome (MS) was defined according to the National Cholesterol Education Program (NCEP) by the presence of three of the following criteria: waist circumference >102 cm for men and >88 cm for women; blood pressure ≥ 130/85 mm Hg or specific treatment; plasma triglycerides ≥150 mg/dL or specific treatment; high‐density lipoprotein cholesterol <40 mg/dL for men and <50 mg/dL for women or specific treatment; and fasting plasma glucose ≥110 mg/dL or established diagnosis of type 2 DM.16
We stratified the population into two groups according to the median value of ADMA in the baseline evaluation: patients with high levels of plasma ADMA (>0.55 μmol/L) and patients with low levels of plasma ADMA (≤0.55 μmol/L). We also analyzed the evolution of a subgroup of 80 patients who were free from MS and DM at baseline.
2.1. Plasma ADMA level
The plasma ADMA level was measured by high‐performance liquid chromatography as described by Teerlink and colleagues.17 Briefly, samples were prepared as follows: 200 μL of plasma containing EDTA or heparin was transferred to an Eppendorf tube (1.5 mL), and 100 μL of internal standard solution (40 μmol/L monomethylarginine) was then added. Phosphate‐buffered saline was added to make a volume of 1 mL. This mixture was introduced into an OASYS extraction cartridge (Waters) coupled to a vacuum system previously equilibrated with 1 mL of methanol and 1 mL of deionized water. Next, the cartridge was rinsed with 1 mL of 100 mmol/L HCl, followed by 1 mL of methanol to elute neutral compounds and acids, and elution was performed with 1 mL of ammonia/water/methanol (10/40/50) solvent. The eluate recovered was dried at 60°C in a speed‐vacuum system, and the residue obtained was dissolved in 100 μL of water, followed by the addition of 100 μL of ortho‐phthalaldehyde. After 15 minutes of the reaction, the samples were transferred to the appropriate high‐performance liquid chromatography vials.
We used a Symmetry C18 column (3.9 × 150 mm; 4 μm) coupled to a precolumn equilibrated with the same stationary phase. The mobile phase A consisted of 50 mmol/L potassium phosphate buffer (pH 6.5) and mobile phase B (acetonitrile/water; 1/1, v/v). Samples (20 μL) were separated using a high‐performance liquid chromatography system with an automatic injector. Standard solutions containing arginine (25, 50, 75, 100, and 150 μmol/L) and ADMA (0.25, 0.50, 1.00, 2.5, 5.0 μmol/L) and 40 μmol/L of internal standard solution were extracted as described above to be injected before and after the injection of samples. The flow rate was 1.1 mL/min.
The time interval between each injection was 30 minutes, and fluorescence was measured at emission and excitation wavelengths of 340 and 455 nm, respectively.
2.2. Statistical analysis
All of the data were expressed as mean and standard deviation or the number and percentage. Comparisons of the baseline variables with respect to low or high ADMA values were analyzed using Student t test for paired samples for continuous variables. Categorical variables were analyzed using χ2 test. Comparison of mean ADMA values with respect to demographic and clinical conditions were analyzed using Student t test for independent samples.
The predictive value of high ADMA plasma levels at baseline for the occurrence of cardiovascular death and the incidence of DM and MS during follow‐up were calculated using χ2 test. We calculated the association of high plasma ADMA levels and the development of DM using binary logistic regression analysis corrected for age, sex, BMI, and statin use.
Analyses were performed using SPSS Statistics for Windows version 20.0 (IBM). For all tests, P < .05 was considered statistically significant.
3. RESULTS
The demographic and laboratory characteristics of the patients stratified by basal ADMA level are summarized in Table 1. A total of 191 patients were included in the baseline analysis, 100 of whom had low ADMA levels (≤0.55 μmol/L) and 91 had high ADMA levels (>0.55 μmol/L). Patients with high ADMA levels also had higher weight, BMI, waist circumference, triglycerides, uric acid, and hs‐CRP, and lower levels of high‐density lipoprotein cholesterol. The use of antihypertensive drugs, including angiotensin‐converting enzyme inhibitors, angiotensin II receptor blockers, β‐blockers, and diuretics, was the same in both groups of patients, as was the use of acid acetylsalicylic (AAS), statins, and fibrates.
Table 1.
Baseline characteristics according to plasma ADMA levels
| Low plasma ADMA (n = 100) | High plasma ADMA (n = 91) | P value | |
|---|---|---|---|
| Age, y | 61.0 ± 9.5 | 60.2 ± 8.4 | .559 |
| Men, No. (%) | 30 (30.0) | 23 (25.3) | .286 |
| Weight, kg | 71.3 ± 15.1 | 77.9 ± 15.7 | .004 |
| BMI, kg/m² | 28.6 ± 5.2 | 31.5 ± 6.1 | .001 |
| Waist circumference, cm | 94.2 ± 12.3 | 100.5 ± 12.7 | .001 |
| Obesity, No. (%) | 33 (33.0) | 50 (54.9) | .002 |
| SBP, mm Hg | 138.7 ± 17.8 | 136.6 ± 17.9 | .427 |
| DBP, mm Hg | 85.3 ± 8.8 | 85.1 ± 12.9 | .884 |
| Total cholesterol, mg/dL | 202.9 ± 40.7 | 196.2 ± 41.9 | .269 |
| HDL cholesterol, mg/dL | 57.3 ± 15.7 | 51.8 ± 14.6 | .012 |
| LDL cholesterol, mg/dL | 119.6 ± 34.9 | 109.6 ± 35.9 | .056 |
| Triglycerides, mg/dL | 129.8 ± 61.9 | 178.7 ± 97.8 | <.001 |
| Dyslipidemia, No. (%) | 13 (13.1) | 22 (23.9) | .041 |
| Fasting glucose, mg/dL | 100.6 ± 37.5 | 108.7 ± 37.4 | .139 |
| CVD, No. (%) | 10 (10) | 12 (13.2) | .322 |
| Metabolic syndrome, No. (%) | 28 (28) | 61 (67) | <.001 |
| Type 2 DM, No. (%) | 27 (27.0) | 42 (46.2) | .005 |
| Smoking, No. (%) | 7 (7.0) | 12 (13.2) | .118 |
| Family history of CVD, No. (%) | 19 (19.0) | 14 (15.4) | .321 |
| Uric acid, mg/dL | 5.4 ± 1.5 | 5.8 ± 1.4 | .041 |
| Creatinine, mg/dL | 0.9 ± 0.2 | 0.9 ± 0.2 | .419 |
| eGFR, mL/min per 1.732 | 75.7 ± 15.8 | 71.9 ± 15.4 | .097 |
| eGFR <60 mL/min per 1.732, No. (%) | 11 (11.2) | 19 (20.9) | .053 |
| Albuminuria, mg/g | 14.5 ± 47.9 | 22.8 ± 67.8 | .346 |
| Albuminuria >26 mg/g, No. (%) | 7 (7.4) | 12 (14.0) | .115 |
| Albuminuria >300 mg/g, No. (%) | 1 (1.1) | 1 (1.2) | .726 |
| CRP, mg/dL | 0.2 ± 0.3 | 0.9 ± 0.8 | <.001 |
| Framingham risk score, % | 8.6 ± 7.3 | 7.9 ± 6.1 | .652 |
ADMA, asymmetric dimethylarginine; BMI, body mass index; CRP, C‐reactive protein; CVD, cardiovascular disease; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SBP, systolic blood pressure. Data are expressed as mean ± standard deviation.
P value for Student t test of independent samples or χ2 test.
We also verified higher levels of ADMA in patients with obesity, dyslipidemia, MS, and type 2 DM, as shown in Table 2. The mean value of ADMA increased with the number of components of MS by NCEP, as shown in Figure 1.
Table 2.
Mean baseline plasma ADMA levels according to demographic and laboratory characteristics of the study patients
| ADMA | P value | |
|---|---|---|
| Women (n = 138) | 0.57 ± 0.10 | .976 |
| Men (n = 53) | 0.57 ± 0.09 | |
| Obese (n = 83) | 0.59 ± 0.09 | .010 |
| Nonobese (n = 108) | 0.55 ± 0.09 | |
| With dyslipidemia (n = 35) | 0.60 ± 0.08 | .020 |
| Without dyslipidemia (n = 156) | 0.56 ± 0.10 | |
| Smoking (n = 19) | 0.57 ± 0.05 | .980 |
| Nonsmoking (n = 172) | 0.57 ± 0.10 | |
| With MS (n = 89) | 0.60 ± 0.09 | <.001 |
| Without MS (n = 102) | 0.53 ± 0.09 | |
| With CVD (n = 22) | 0.59 ± 0.10 | .365 |
| Without CVD (n = 169) | 0.56 ± 0.09 | |
| With type 2 DM (n = 69) | 0.59 ± 0.09 | .004 |
| Without type 2 DM (n = 122) | 0.55 ± 0.10 | |
| eGFR ≥60 mL/min (n = 159) | 0.55 ± 0.09 | .006 |
| eGFR <60 mL/min (n = 30) | 0.62 ± 0.10 | |
| Albuminuria <26 mg/g creatinine (n = 162) | 0.56 ± 0.09 | .052 |
| Albuminuria >26 mg/g creatinine (n = 19) | 0.62 ± 0.12 | |
| Albuminuria <300 mg/g creatinine (n = 179) | 0.56 ± 0.09 | ‐ |
| Albuminuria >300 mg/g creatinine (n = 2) | 0.55 ± 0.00 |
ADMA, asymmetric dimethylarginine; DM, diabetes mellitus; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; MS, metabolic syndrome.
P value descriptive for Student t test for independent samples.
Figure 1.
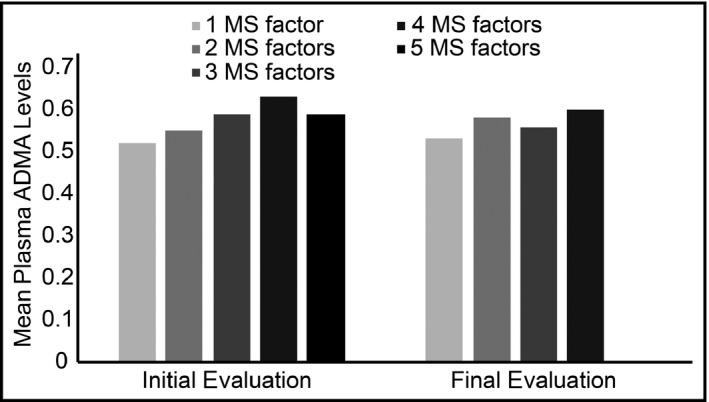
Mean plasma asymmetric dimethylarginine (ADMA) level according to the number of metabolic syndrome (MS) factors
Patients were followed for 5.8 ± 1.2 years (71.2 ± 15 months). In this period, there were seven cardiovascular deaths and seven cardiovascular events. There was an association between high plasma ADMA levels and cardiovascular death, although there was no association between ADMA values and cardiovascular events (Figure 2).
Figure 2.
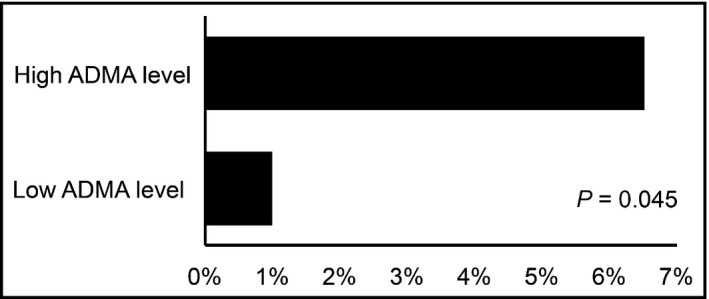
Cardiovascular death according to baseline plasma asymmetric dimethylarginine (ADMA) level
Eighty patients had hypertension and were free from MS and DM at baseline evaluation. Their characteristics are described in Table 3. Again, the use of antihypertensive drugs, AAS, statins, and fibrates was the same in both groups of patients. There were four deaths in this group, leaving 76 patients for analysis. Of these, 40 (52.6%) developed MS and 13 (17.1%) developed type 2 DM. There was no association between ADMA levels and the development of MS, but there was an association between high ADMA levels and the development of type 2 DM (Figure 3), even after adjusting for age, sex, BMI, and statin use (Table 4).
Table 3.
Baseline characteristics according to plasma ADMA levels in patients free from MS and DM
| Free from MS and DM n = 80 | MS and/or DM in baseline n = 111 | P value | |
|---|---|---|---|
| Age, y | 61.0 ± 9.7 | 60.3 ± 8.4 | .568 |
| Men, No. (%) | 22 (27.5) | 31 (27.9) | .541 |
| Weight, kg | 69.2 ± 15.0 | 78.2 ± 15.2 | <.001 |
| BMI, kg/m² | 28.0 ± 5.0 | 31.4 ± 5.9 | <.001 |
| Waist circumference, cm | 92.0 ± 11.6 | 100.8 ± 12.5 | <.001 |
| Obesity, No. (%) | 21 (26.3) | 62 (55.9) | <.001 |
| SBP, mm Hg | 138.3 ± 18.5 | 137.2 ± 17.4 | .670 |
| DBP, mm Hg | 86.2 ± 12.1 | 84.3 ± 9.8 | .254 |
| Total cholesterol, mg/dL | 204.6 ± 34.6 | 196.1 ± 45.2 | .142 |
| HDL cholesterol, mg/dL | 61.2 ± 15.3 | 49.9 ± 13.6 | .012 |
| LDL cholesterol, mg/dL | 118.9 ± 31.2 | 111.9 ± 38.4 | <.001 |
| Triglycerides, mg/dL | 122.5 ± 56.5 | 175.1 ± 94.1 | <.001 |
| Dyslipidemia, No. (%) | 4 (5.0) | 31 (27.9) | <.001 |
| Fasting glucose, mg/dL | 88.4 ± 10.4 | 115.9 ± 45.1 | <.001 |
| CVD, No. (%) | 6 (7.5) | 16 (14.4) | .105 |
| Smoking, No. (%) | 3 (3.8) | 16 (14.4) | .012 |
| Family history of CVD, No. (%) | 11 (13.8) | 22 (19.8) | .184 |
| Uric acid, mg/dL | 5.4 ± 1.4 | 5.8 ± 1.4 | .077 |
| Creatinine, mg/dL | 1.0 ± 0.2 | 0.9 ± 0.2 | .047 |
| eGFR, mL/min per 1.73² | 71.1 ± 14.4 | 75.8 ± 16.3 | .035 |
| Albuminuria, mg/g | 9.9 ± 21.0 | 24.5 ± 73.6 | .056 |
| CRP, mg/dL | 0.4 ± 0.6 | 0.7 ± 0.7 | .033 |
| Framingham risk score, % | 7.6 ± 6.7 | 8.8 ± 7.3 | .239 |
| Baseline ADMA | 0.52 ± 0.09 | 0.59 ± 0.09 | <.001 |
ADMA, asymmetric dimethylarginine; BMI, body mass index; CRP, C‐reactive protein; CVD, cardiovascular disease; DBP, diastolic blood pressure; DM diabetes mellitus; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MS, metabolic syndrome; SBP, systolic blood pressure.
Data are expressed as mean ± standard deviation. P value for Student t test for independent samples or the χ2 test.
Figure 3.
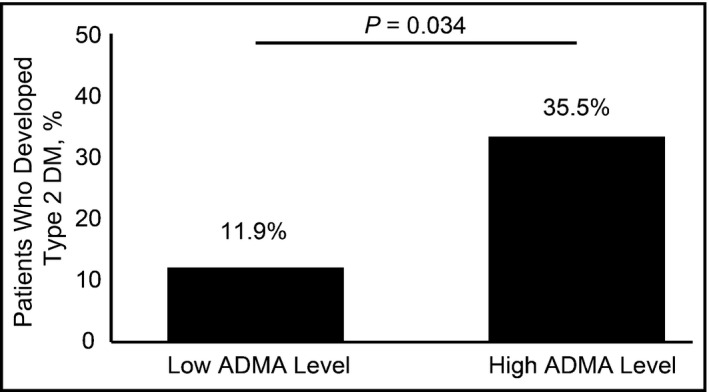
Development of type 2 diabetes mellitus (DM) in patients free from metabolic syndrome (MS) and DM at baseline
Table 4.
Association of high ADMA and the development of DM after adjustment
| Odds ratio | 95% CI | P value | |
|---|---|---|---|
| Age | 1.01 | 0.94–1.08 | .737 |
| Sex (male) | 0.40 | 0.07–2.21 | .295 |
| BMI | 1.04 | 0.92–1.18 | .476 |
| Statin use | 0.99 | 0.25–3.84 | .988 |
| High plasma ADMA level | 4.51 | 1.10–18.40 | .035 |
ADMA, asymmetric dimethylarginine; BMI, body mass index; CI, confidence interval; DM, diabetes mellitus.
P value for binary logistic regression analysis.
The median hs‐CRP was 0.29. Unlike ADMA, hs‐CRP was not associated with death in the entire sample or with DM in patients free from MS and DM.
4. DISCUSSION
The present study confirms the association between high circulating concentrations of ADMA and the presence of cardiovascular risk factors in patients with hypertension. It has been previously demonstrated that patients with hypertension have higher ADMA concentrations than controls with normotension and that ADMA is inversely related to endothelial function in these patients.18
We found, in a well‐characterized hypertension population, that individuals above the median value of ADMA concentration were at higher risk for cardiovascular death. Similarly, a recent systematic review described ADMA as an independent risk marker for all‐cause mortality (relative risk, 1.52; 95% confidence interval, 1.37–1.68) and cardiovascular disease (relative risk, 1.33; 95% confidence interval, 1.22–1.45) comparing high vs low ADMA concentrations.19 Zobel and colleagues20 also demonstrated in a population of patients with type 2 DM with microalbuminuria and without symptoms of coronary artery disease the association of ADMA with all‐cause mortality. 20
In a subgroup of patients with hypertension free from MS and DM, we found an association between higher‐than‐median circulating levels of ADMA and the development of type 2 DM. This finding suggests that endothelial dysfunction plays a role in the genesis of insulin resistance by reducing insulin‐induced vasodilation, increasing vascular resistance, and reducing blood flow, thus impairing the transport of insulin and glucose to the sensitive tissues. A case‐control study of 40 patients with early‐stage type 2 DM and 40 healthy adult volunteers matched for age, sex, and BMI showed that patients with DM had significantly higher ADMA values than healthy controls. Age‐ and sex‐adjusted ADMA values were significantly correlated with hs‐CRP (r = .279) and homeostatic model assessment of insulin resistance (r = .288) in patients with DM and were not significant in healthy controls. The association between ADMA and homeostatic model assessment of insulin resistance in patients with DM remained significant (r = .255; P < .005) after adjusting for BMI, waist circumference, serum lipids, and hs‐CRP, which suggests that in patients with early‐stage type 2 DM, ADMA is an independent predictor of insulin resistance.21 On the contrary, it has been shown that high values of the insulin resistance index homeostatic model assessment are predictive of high ADMA values in patients with rheumatoid arthritis22 but the authors stated that the cross‐sectional design of the study does not allow assumption on causality or directionality of the association described. The longitudinal design of our study, however, allows us to assert that high ADMA levels predict the development of insulin resistance since, in patients with hypertension free from metabolic abnormalities and presumably low degrees of insulin resistance, in a follow‐up of almost 6 years, high levels of ADMA were associated with the development of type 2 DM. In these patients, high levels of ADMA reflect subjacent endothelial dysfunction that promotes insulin resistance by reducing insulin‐induced vasodilation, thus impairing the transport of insulin and glucose to the sensitive tissues. In addition, hs‐CRP, a known biomarker for cardiovascular risk, was not associated with death or development of DM in our population, suggesting that ADMA may be a better biomarker to identify patients with hypertension at higher risk of metabolic abnormalities or death.
Previous investigations have shown in rats23 that captopril, but not enalapril, may improve low‐density lipoprotein cholesterol–induced endothelial dysfunction, concomitantly with an increase in the activity of dimethylarginine dimethylaminohydrolase and a decrease in levels of ADMA. Kawata and colleagues24 also described that temocapril reduces ADMA concentration in patients with type 2 DM, improving coronary circulation. In our population of patients with hypertension, the frequency of use of angiotensin‐converting enzyme inhibitors was the same between the analyzed groups; therefore, we do not believe there was an influence of the treatment in the outcomes.
5. STUDY LIMITATIONS AND STRENGTHS
Our study is limited by the small sample of patients and few cardiovascular events and deaths, therefore large‐scale studies are needed to confirm our findings. We also did not determine the exact lipid‐lowering agents used by our patients for the entire study period. This is important because recent studies have shown that statin treatment, particularly at high doses, increases the risk of DM.25, 26 However, in our study, there was no association between the use of statins and DM development.
The strengths of our study merit consideration. We present the first prospective study to use circulating ADMA as a biomarker for the development of type 2 DM and cardiovascular death in patients with hypertension.
6. CONCLUSIONS
There is association of high plasma ADMA levels and the presence of cardiovascular risk factors in hypertensive patients. We suggest a positive predictive value of high plasma ADMA levels for cardiovascular death in hypertensive patients and also for the development of type 2 DM in a subgroup of hypertensive patients free from metabolic abnormalities.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
Endocrinology and Nephrology Division – Universidade Federal de São Paulo/UNIFESP.
Triches CB, Mayer S, Quinto BMR, Batista MC, Zanella MT. Association of endothelial dysfunction with cardiovascular risk factors and new‐onset diabetes mellitus in patients with hypertension. J Clin Hypertens. 2018;20:935–941. 10.1111/jch.13269
REFERENCES
- 1. Willeit P, Freitag DF, Laukkanen JA, et al. Asymmetric dimethylarginine and cardiovascular risk: systematic review and meta‐analysis of 22 prospective studies. J Am Heart Assoc. 2015;4:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest. 1997;100:2153‐2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium‐derived relaxing factor. Nature. 1987;327:524‐526. [DOI] [PubMed] [Google Scholar]
- 4. Teerlink T, Luo Z, Palm F, Wilcox CS. Cellular ADMA: regulation and action. Pharmacol Res. 2009;60:448‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vallance P, Leone A, Calver A, Moncada S, Collier J. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572‐575. [DOI] [PubMed] [Google Scholar]
- 6. Boger RH, Sullivan LM, Schwedhelm E, et al. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119:1592‐1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xuan C, Liu ZF, Wang Q, et al. Increased serum concentrations of asymmetric dimethylarginine (ADMA) in patients with early‐onset coronary artery disease. Clin Chim Acta. 2016;464:195‐199. [DOI] [PubMed] [Google Scholar]
- 8. Boger R. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc Res. 2003;59:824‐833. [DOI] [PubMed] [Google Scholar]
- 9. Sydow K, Mondon CE, Cooke JP. Insulin resistance: potential role of the endogenous nitric oxide synthase inhibitor ADMA. Vasc Med. 2005;10:S35‐S43. [DOI] [PubMed] [Google Scholar]
- 10. Duplain H, Burcelin R, Sartori C, et al. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001;104:342‐345. [DOI] [PubMed] [Google Scholar]
- 11. Hirota AH, Rodrigues CJ, Borges RL, Ribeiro AB, Zanella MT, Batista MC. Performance of two metabolic syndrome definitions in the estimation of cardiovascular disease among hypertensive patients. J Clin Hypertens (Greenwich). 2010;12:588‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460‐2470. [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Stevens L, Schmid C. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. National Cholesterol Education Program NH, Lung and Blood Institute, National Institutes of Health . Third report of the National cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143. [PubMed] [Google Scholar]
- 15. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27:S11‐S14. [DOI] [PubMed] [Google Scholar]
- 16. Expert Panel on Detection, E, Treatment of High Blood Cholesterol in A . Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486‐2497. [DOI] [PubMed] [Google Scholar]
- 17. Teerlink T, Nijveldt RJ, de Jong S, van Leeuwen PAM. Determination of arginine, asymmetric dimethylarginine, and symmetric dimethylarginine in human plasma and other biological samples by high‐performance liquid chromatography. Anal Biochem. 2002;303:131‐137. [DOI] [PubMed] [Google Scholar]
- 18. Perticone F, Sciacqua A, Maio R, et al. Asymmetric dimethylarginine, L‐arginine, and endothelial dysfunction in essential hypertension. J Am Coll Cardiol. 2005;46:518‐523. [DOI] [PubMed] [Google Scholar]
- 19. Schlesinger S, Sonntag SR, Lieb W, Maas R. Asymmetric and symmetric dimethylarginine as markers for total mortality abd cardiovascular outcomes: a systematic review and meta‐analysis of prospective studies. PLoS One. 2016;11:e0165811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zobel EH, Scholten BJ, Reinhard H, et al. Symmetric and asymmetric dimethylarginine as risk markers of cardiovascular disease, all‐cause mortality and deterioration in kidney function in persons with type 2 diabetes and microalbuminuria. Cardiovasc Diabetol. 2017;16:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakhjavani M, Karimi‐Jafari H, Esteghamati A, Khalilzadeh O, Asgarani F, Ghadiri‐Anari A. ADMA is a correlate of insulin resistance in early‐stage diabetes independent of hs‐CRP and body adiposity. Ann Endocrinol. 2010;71:303‐308. [DOI] [PubMed] [Google Scholar]
- 22. Dimitroulas T, Sandoo A, Veldhuijzen van Zanten JJ, et al. Predictors of asymmetric dimethylarginine levels in patients with rheumatoid arthritis: the role of insulin resistance. Scand J Rheumatol. 2013;42:176‐181. [DOI] [PubMed] [Google Scholar]
- 23. Jiang JL, Zhu HQ, Chen Z, Xu HY, Li YJ. Angiotensin‐converting enzyme inhibitors prevent LDL‐induced endothelial dysfunction by reduction of asymmetric dimethylarginine level. Int J Cardiol. 2005;101:153‐155. [DOI] [PubMed] [Google Scholar]
- 24. Kawata T, Daimon M, Hasegawa R, et al. Effect of angiotensin‐converting enzyme inhibitor on serum asymmetric dimethylarginine and coronary circulation in patients with type 2 diabetes mellitus. 2009;132:286‐288. [DOI] [PubMed] [Google Scholar]
- 25. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta‐analysis of randomised statin trials. Lancet. 2010;375:735‐742. [DOI] [PubMed] [Google Scholar]
- 26. Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive‐dose compared with moderate‐dose statin therapy: a meta‐analysis. JAMA. 2011;305:2556‐2564. [DOI] [PubMed] [Google Scholar]


