Abstract
Reverse or inverted dipping (ie, the phenomenon characterized by higher nighttime compared with daytime blood pressure values) is an alteration of circadian blood pressure rhythm frequently documented in hypertension, type 2 diabetes mellitus, chronic kidney disease, and sleep apnea syndrome, and generally regarded as a harmful condition. Available literature on the clinical and prognostic implications of reverse dipping is scanty. The present article will review a number of relevant issues concerning reverse dipping, in particular: (1) its possible mechanisms; (2) prevalence and clinical correlates, (3) concomitant cardiac and extracardiac subclinical organ damage; (4) association with acute and chronic cardiovascular diseases; (5) prognostic value in predicting cardiovascular events and mortality; and (6) therapeutic interventions aimed at reverting this abnormal circadian blood pressure rhythm.
Keywords: cardiovascular disease, cardiovascular prognosis, hypertension, reverse dipping, target organ damage, treatment
1. INTRODUCTION
During the past 4 decades, combined office and out‐of‐office blood pressure (BP) measurements (self‐measured home BP or 24‐hour ambulatory BP monitoring) have been recognized to provide a more accurate assessment of BP status and are increasingly applied in clinical and research settings.1
Overall, combined office and home or 24‐hour ambulatory BP measurements define four different BP phenotypes: (1) sustained normotension (ie, normal office and out‐of‐office BP); (2) sustained hypertension (elevated in‐office and out‐of‐office BP); (3) white‐coat hypertension, alternatively termed isolated clinic hypertension (elevated office and normal out‐of‐office BP); and (4) masked hypertension (normal office and elevated out‐of‐office BP). These BP patterns have been reported to substantially differ in terms of prevalence, demographic and clinical characteristics, and burden of subclinical organ damage as well as risk of cardiovascular morbidity and mortality.2, 3, 4
Furthermore, by providing a much larger number of BP readings during 24 hours compared with office and home BP measurements, ambulatory BP monitoring offers the opportunity to evaluate day‐night BP variability. A circadian rhythm of BP has been documented in the majority of normotensive and hypertensive individuals, with nighttime BP values being 10% to 20% lower than daytime values due to the reduction in sympathetic tone and the parallel increase in vagal activity during the sleep period. Historically, a nocturnal BP drop lower than 10% has been largely accepted to define patients with abnormal circadian rhythm (nondippers).5, 6
A growing amount of evidence indicates that nondippers have more marked cardiac and extracardiac organ damage compared with patients with preserved nocturnal BP fall and, more importantly, an increased likelihood of cardiovascular events.7, 8, 9, 10 Reverse or inverted dipping (ie, the condition characterized by unchanged or even increased nighttime BP compared with daytime values) represents an extreme, not rare, alteration in circadian BP rhythm, usually regarded as a particularly harmful BP phenotype. Unfortunately, available literature on clinical and prognostic implications of reverse dipping (RD) is limited and a comprehensive report of key features of this BP pattern is lacking. Thus, the present article will review a number of issues concerning the paradoxical increase in nighttime BP with particular attention to: (1) possible mechanisms of altered circadian BP rhythm; (2) prevalence and clinical correlates; (3) the association with subclinical organ damage; (4) the association with overt cardiovascular disease; (5) prognostic significance; and (6) therapeutic interventions.
2. SEARCH METHOD
A systematic computerized literature search was performed using PubMed, Ovid, Embase, and Cochrane Library databases from January 1, 1978, to January 31, 2017. Studies were identified by crossing the following search terms: “ambulatory blood pressure,” “nocturnal blood pressure,” “reverse dipping,” “inverted dipping,” “riser pattern,” “altered circadian BP rhythm,” “hypertension,” “target organ damage,” “cardiac disease,” “cerebrovascular disease,” and “mortality.” Checks of the reference lists of selected papers and pertinent reviews complemented the electronic search. Data were extracted by two independent investigators (CC and CS).
2.1. Pathogenetic mechanisms
A blunted or absent BP reduction during the nighttime period is a multifactorial condition that is more frequently documented in patients with secondary hypertension, severe or refractory essential hypertension, preeclampsia, chronic renal disease, type 1 and 2 diabetes mellitus, sleep‐apnea syndrome, and autonomic dysfunction. General agreement exists on the direct relationship between the magnitude of nocturnal BP fall and the decrease in sympathetic activity, driving a reduction in cardiac output, peripheral resistance, and heart rate. Clinical studies have documented diurnal variations of circulating levels of norepinephrine and epinephrine that reaches a nadir during nighttime sleep.11
Our group investigated the association between nocturnal BP patterns and sympathetic drive, as assessed by direct microneurographic measurement of sympathetic activity at the peroneal muscle in patients with essential hypertension. A stepwise increase in muscle sympathetic nerve traffic occurred from normotensive controls to extreme dipper, dipper, nondipper, and RD hypertensives. Notably, day‐night BP difference in the whole study population was inversely related to sympathetic nerve traffic (r=−.76, P<.0001), suggesting that sympathetic activation in human hypertension may potently affect circadian BP variations.12 Also, in patients with obstructive sleep apnea, a condition frequently associated with nocturnal hypertension, increased sympathetic tone responsible for BP elevations during apneic episodes has been documented in microneurographic studies.13
Impaired renal capacity to excrete sodium has been shown to be a factor leading to a blunted nocturnal BP fall.14 This condition has been associated with reduced daytime sodium excretion, and the nocturnal BP increase via the pressure‐natriuresis mechanism is a likely compensatory mechanism to increase sodium excretion and preserve sodium balance.15 The role of sodium in regulating circadian BP rhythm is also supported by intervention studies showing that a dipping pattern is restored after salt restriction or administration of diuretics.16 Since preserved glomerular filtration function is a major determinant of sodium balance, deterioration in renal function is generally paralleled by a reduction of the physiologic fall in nighttime BP. Cystatin C is a more sensitive marker of glomerular filtration rate than serum creatinine, as its circulating levels are less affected by confounders such as age, sex, and muscle mass. Among patients with essential hypertension, this compound has been found to be significantly higher in patients with RD than in patients with dipping pattern.17
2.2. Prevalence and clinical aspects
Data on the prevalence of RD profile have been reported in population‐based samples as well as in several clinical settings including those with hypertension, chronic kidney disease (CKD), diabetes mellitus, and sleep apnea syndrome (Figure 1).
Figure 1.
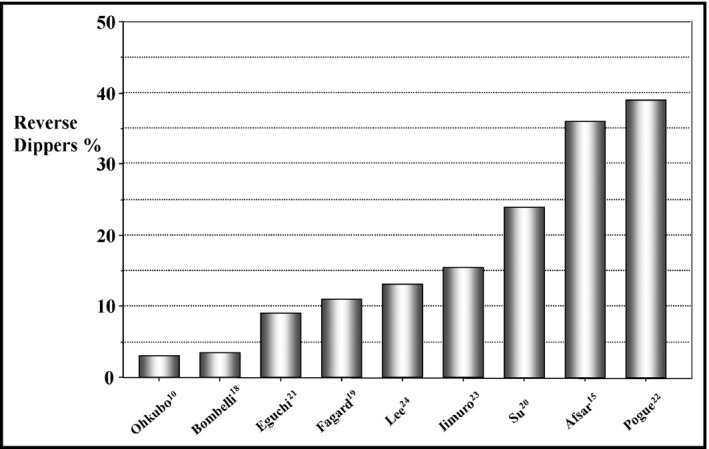
Prevalence rates of reverse dipping pattern in nine studies conducted in different clinical settings including general population samples 10, 18 and patients with untreated 20 and treated 15, 19 essential hypertension, diabetes mellitus, 21 sleep apnea syndrome, 24 and chronic kidney disease 22, 23
In a pioneering study published 2 decades ago that aimed to investigate the relationship between nocturnal BP decline and mortality in 1542 adult residents of a rural Japanese community during a mean follow‐up period of 5.1 years, Ohkubo and colleagues10 provided the first data on the prevalence of RD in a general population sample. Participants were classified into four groups according to percent fall in nocturnal BP: (1) extreme dippers (BP fall >20%; (2) dippers: BP fall >10% and <20%; (3) nondippers: BP fall >0% and <10%; and (4) inverted (reverse) dippers: nocturnal BP rise. Forty six of 1542 participants (3%) fulfilled the criteria of RD pattern. The majority of participants were dippers (67%), followed by extreme dippers (15%) and nondippers (13%). Compared with other groups, participants with RD were older, more frequently male, and current or previous smokers, and had a higher prevalence of history of cardiovascular disease.
Among participants in the Pressioni Arteriose Monitorate E Loro Associazioni (PAMELA) trial, an Italian epidemiologic study designed to determine normal values and prognostic significance of ambulatory and home BP in the population, the prevalence of RD was similar to that reported by Ohkubo and colleagues.18 Indeed, 71 of 2008 patients (3.5%) had a nighttime to daytime systolic BP ratio >1. A Belgian Ambulatory Blood Pressure Monitoring database including 3648 patients with hypertension from four European studies documented that patients with RD represented approximately 11% of the pooled population.19
A recent single‐center study performed in 708 untreated essential hypertensive patients (26% with type 2 diabetes mellitus) reported that average nocturnal systolic BP levels were higher than daytime values in about one fourth of patients (24%).20 Older age, type 2 diabetes mellitus, elevated low‐density lipoprotein cholesterol, and triglyceride concentrations were found to be associated with RD pattern. A remarkable prevalence of RD (36%) was reported by Afsar and colleagues15 among 210 treated patients with essential hypertension. Of note, more than a half of reverse dippers were classified as having isolated nocturnal hypertension, as their office and daytime BP values were within normal range.
In a sample of 300 asymptomatic normotensive and hypertensive (57%) patients with type 2 diabetes mellitus (mean age 68 years, 48% men) seen in a multicenter study conducted in Japan,21 RD prevalence was approximately 9%. In the African American Study of Kidney Disease and Hypertension (AASK) trial including 617 hypertensive participants aged 18 to 70 years, with a glomerular filtration rate between 20 and 65 mL/min per 1.73 m2, a blunted nocturnal BP fall was documented in the majority of patients, as up to 495 participants (80%) were nondippers (41%) or reverse dippers (39%).22 Participants in the RD category were older, more likely to be men and have a longer duration of hypertension, and taking a larger number of antihypertensive medications than dippers and nondippers.
The Chronic Kidney Disease Japan Cohort (CKDJC), an observational study aimed at investigating clinical correlates of ambulatory BP monitoring among patients with CKD showed that 167 (15.5%) of 1075 patients had a nocturnal BP rise.23 The prevalence of risers was higher among patients with CKD stage 4 and 5 compared with those with stage 3, among diabetic than nondiabetic patients, and among patients with nocturia. The association between day‐night BP behavior and white matter changes and its interaction with obstructive sleep apnea among 703 normotensive adults (mean age 59 years) has been investigated by Lee and coworkers.24 In this relatively older population, 13% were reverse dippers and exhibited a greater prevalence of moderate to severe obstructive sleep apnea than other groups (13.5% vs 9.0% in nondippers and 7.5% in dippers).
Racial differences in the magnitude of nocturnal BP fall and distribution of dipping/nondipping status have been reported by various authors. In particular, smaller reductions in mean BP at night have been found in black patients compared with other ethnic groups. Emerging evidence supports the view that day‐night BP variations are related to seasonal changes. Recently, Wong and colleagues25 reported a progressive decrease in prevalence of reverse dippers from summer to winter. This trend was accompanied by an increased number of dippers independent of ethnicity.
Finally, whether and to what extent the RD pattern represents a stable clinical trait over time remains undetermined and needs further investigation.
2.3. Association with subclinical organ damage
Asymptomatic target organ damage is regarded as an intermediate step in the continuum of cardiovascular disease and a powerful predictor of cardiovascular morbidity and mortality and all‐cause mortality. A consistent body of evidence supports the view that quantitative markers of organ damage (ie, increased QRS voltage/duration, left ventricular mass index, carotid intima‐media thickening, pulse wave velocity, urinary albumin excretion, and reduced glomerular filtration rate) are associated with a higher incidence of cardiovascular events independently from traditional risk factors in the general population and in different clinical settings.
Left ventricular hypertrophy (LVH) detected by electrocardiography or by the more accurate echocardiographic examination is regarded as a cardinal marker of hypertensive heart disease. Patients with LVH have been reported to have a two‐ to eight‐fold higher risk of cardiovascular events than those without LVH, with the magnitude of risk depending on demographic and clinical characteristics of the samples. Furthermore, LVH regression (at variance from other markers of organ damage) has been largely documented to be associated to a clear‐cut reduction of cardiovascular risk.
The association between LVH and RD pattern has been assessed in a limited number of studies. In electrocardiographic studies performed in older hypertensive patients, recently diagnosed patients with essential hypertension, and CKD and heart failure patients, prevalence rates of LVH (defined by Sokolow‐Lyon or Romhilt‐Estes criteria and Cornell voltage product) have been reported to be not significantly higher in reverse dippers than in dippers, nondippers, or extreme dippers.26, 27
Findings from echocardiographic studies have offered more consistent evidence on the increased risk of LVH associated with the RD pattern. In a study including 375 untreated and treated middle‐aged hypertensive patients, Ivanovic and colleagues28 were able to demonstrate that LVH rates increased progressively from extreme dippers (5%) to dippers (9%), nondippers (17%), and reverse dippers (31%, P<.01 vs all groups). A multicenter Japanese study performed in 334 hospitalized patients with chronic heart failure documented that left ventricular mass indexed to body surface area was significantly higher in nondippers (163±48 g/m2) and reverse dippers (164±51 g/m2) than in dippers (150±51 g/m2).29 Similarly, Wang and coworkers30 examined a cohort of 540 patients with CKD and reported that left ventricular mass indexed to height2.7 was significantly greater in reverse dipper (58±19 g/h.2.7) than in nondipper (52±24 g/h2.7) and dipper (45±16 g/h2.7) participants.
In contrast to the above‐mentioned echocardiographic studies, the AASK investigators were able to find only marginal differences in LVH prevalence among various ambulatory day/night BP phenotypes22 (Table 1).
Table 1.
Summary of echocardiographic data on LVH and/or LVMI in extreme dipper, dipper, nondipper, and reverse dipper patients provided by four echocardiographic studies
| Author (reference) | Extreme dippers | Dippers | Nondippers | Reverse dippers | ||||
|---|---|---|---|---|---|---|---|---|
| LVH, % | LVMI, g/h2.7 | LVH, % | LVMI, g/h2.7 | LVH, % | LVMI, g/h2.7 | LVH, % | LVMI, g/h2.7 | |
| Pogue22 | NA | NA | 62 | NA | 69 | NA | 72 | NA |
| Ivanovic28 | 5 | 46±8 | 9 | 47±9 | 17 | 51±10 | 31a | 54±10a |
| Komori29 | NA | NA | NA | 150±51 g/m2 | NA | 163±51 g/m2 | NA | 164±48 g/m2 a |
| Wang30 | NA | NA | NA | 45±16 | NA | 52±24 | NA | 58±19a |
Abbreviations: LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; NA, not available.
P<.05 Reverse dippers vs nondippers, dippers, and extreme dippers.
P=.05 Reverse dippers vs dippers.
P<.05 Reverse dippers vs nondippers and dippers.
In the Coronary Artery Risk Development in Young Adults (CARDIA) study, young adults with less pronounced or absent nocturnal BP fall at the initial evaluation had the greatest risk of developing coronary calcification at 10 to 15 years of follow‐up.31
Both in the general population and in diabetic patients, increased urinary protein excretion constitutes a powerful predictor of future cardiovascular/renal events and death. The majority of findings targeting the association between proteinuria and microalbuminuria with day‐night BP variations have been obtained in the setting of CKD; only a few studies have specifically addressed this topic in essential hypertension.
In the AASK trial, nondippers and participants with RD exhibited a higher prevalence of proteinuria (35% and 30%, respectively) compared with dippers (20%).22 Similar results were reported in the Assessment of Blood Pressure Control and Target Organ Damage in Patients With Chronic Kidney Disease and Hypertension (APRODITE) study, a nationwide multicenter trial conducted in 1317 Korean patients.27 Reverse dippers exhibited the highest values of protein‐creatinine ratio (1165±1446 mg/g) compared with nondippers (1098±1382 mg/g), dippers (774±1001 mg/g), and extreme dippers (662±948 mg/g).
The strong relationship between RD and nondipping status with increased urinary protein excretion has been recently confirmed by Wang and colleagues32 in 588 Chinese patients (67% with chronic glomerulonephritis). Proteinuria in reverse dippers was 66% higher than in dippers and approximately four‐fold greater than in extreme dippers. Other studies, however, failed to find significant differences in proteinuria, expressed as continuous or binary variables in reverse dipper CKD patients compared with nondipper and dipper patients.
A couple of European studies performed in uncomplicated essential hypertensive patients documented a stepwise increase in microalbuminuria from extreme dippers, dippers, nondippers, and reverse dippers. The largest of these studies included 2800 untreated hypertensive patients and demonstrated that reverse dippers (10.2%) had the highest microalbuminuria values compared with other groups, with the intergroup differences being significant after adjusting for confounders.33
Subclinical vascular alterations are detectable by a variety of diagnostic tools such as carotid ultrasonography, fundoscopy, carotid‐femoral pulse wave velocity, and brain magnetic resonance imaging. Univocal information is not available on carotid intima‐media thickness in reverse dippers, likely because of the different clinical and demographic characteristics of patients included in the studies. Garcia‐Ortiz and colleagues26 documented that in patients with RD and nondipper hypertensive patients, common carotid intima‐media thickness was significantly higher (795±126 μm and 760±124 μm, respectively) than dippers and extreme dippers (741±108 μm and 719±96 μm, respectively). In a study including 524 hypertensive patients, RD pattern was the strongest independent variable in predicting the presence of mild carotid plaques.34 In contrast to previous findings, Wang and colleagues30 did not find an association between circadian BP patterns and carotid atherosclerosis, assessed either as intima‐media thickness or plaque prevalence.
Pulse wave velocity, a parameter of arterial stiffness of recognized prognostic value, was found by Jerrard‐Dunne and coworkers35 to be significantly increased in 28 untreated middle‐aged hypertensive patients with RD compared with 107 nondippers, 173 dippers, and 45 extreme dippers after adjustment for several confounders. In a magnetic resonance imaging study including 703 normotensive adults, Lee and colleagues24 demonstrated a significant association between RD and cerebral white matter changes; the association persisted after adjusting for age, sex, total cholesterol, body mass index, diabetes mellitus, smoking, and alcohol consumption.
2.4. Association with cardiovascular disease
A further line of research has investigated the link between RD and overt cardiovascular disease in a variety of clinical settings such as cognitive impairment/vascular dementia, stroke, coronary heart disease, and heart failure. In a study designed to delineate the relationships between circadian BP variations, lacunar infarcts, white matter lesions, and vascular dementia in 200 patients after first symptomatic transient ischemic attack or stroke, a strong independent association of cognitive impairment and vascular dementia was documented with advanced periventricular hyperintensities (hazard ratio [HR], 14.42; 95% confidence interval [CI], 5.62–36.98), followed by RD pattern (HR, 11.95; 95% CI, 1.27–112.11).36 In a cohort of 362 hypertensive patients studied by Yan and colleagues,37 RD pattern was found to be the most important predictor of lacunar infarction. Among 98 consecutive patients hospitalized within 24 hours after ischemic stroke onset, Castilla‐Guerra and associates38 found that nocturnal systolic BP decline was abolished in approximately 89% of the sample (49% nondippers and 39% reverse dippers) and the prevalence of RD pattern was significantly higher in patients with nonlacunar than in lacunar stroke. Known and colleagues39 consecutively recruited 162 hypertensive patients within 1 week after cerebral infarction to explore the relationship between nocturnal dipping and cerebral microbleeds, as assessed by magnetic resonance imaging. After adjusting for confounding factors, reverse dippers showed a greater likelihood of cerebral microbleeds (HR, 3.81; 95% CI, 1.36–10.65 [P=.01]) than dippers and nondippers. Data on circadian BP patterns and autonomic function among patients with transient ischemic attack or minor stroke recently published by Zhang and colleagues40 revealed that abnormal BP patterns were similarly distributed among patients and controls (ie, RD 17% and 19%, respectively) and no differences were present in a reliable marker of autonomic function such as heart rate variability. In a retrospective case‐control study including 189 hypertensive patients with spontaneous intracerebral hemorrhage and 182 hypertensive controls, Sun and colleagues41 documented that RD pattern was much more prevalent in cases (51%) than in controls (4%). The association between RD and coronary artery disease has been evaluated in a hypertensive cohort of 267 patients with and 461 patients without stable coronary artery disease.42 The authors reported that this abnormal BP phenotype was 1.6‐fold more prevalent in the former than in the latter group. In multivariate logistic regression analysis, RD (HR, 1.77; P=.03), age (HR, 1.06; P<.001), and total cholesterol (HR, 1.22; P=.035) were significantly related to coronary heart disease.
Among 508 hospitalized patients with chronic heart failure (46% with preserved ejection fraction), a paradoxical increase in BP during sleep was significantly more common in patients with preserved left ventricular ejection fraction (29%) compared with those with reduced systolic function (20%).43 RD pattern was shown to be an independent correlate of heart failure with preserved systolic function (HR, 1.73; 95% CI, 1.02–2.91 [P=.04]) after adjusting for several covariates.
2.5. Prognostic significance
The value of RD pattern in predicting cardiovascular events and all‐cause mortality has been addressed in population‐based samples and in patients with hypertension, type 2 diabetes mellitus, CKD, stroke, and heart failure (Table 2). The first report in this research area was published by Ohkubo and colleagues10 20 years ago and examined 1542 residents in the Ohasama district, Japan. The relationship between the decline in nocturnal BP and mortality was investigated by taking into account several potential confounders such as age, sex, smoking status, history of cardiovascular disease, 24‐hour systolic BP, and use of antihypertensive medication. The risk of total mortality in patients with RD was approximately two‐fold higher than in dippers (P=.02). Of note, the increased mortality risk documented in patients with RD was mainly attributable to cardiovascular causes. In a survey performed in a Belgium primary care practice including 374 elderly patients without major cardiovascular events or other comorbidities at baseline, RD had a prognostic significance in univariable analyses but lost its predictive value after adjustment for confounders, including 24‐hour BP.44 A pilot study involving 97 hypertensive diabetic patients at high risk of cardiovascular events revealed that RD status was independently related to higher rates of composite outcome (encompassing myocardial infarction, stroke, or cardiovascular death), compared with other patients with dipping or nondipping profile during a follow‐up period of 2.6 years.45 In the setting of CKD, two studies consisting of 1024 individuals evaluated the risk of end‐stage renal disease and nonfatal/fatal cardiovascular events associated with RD. Both reports consistently documented that patients with RD had an increased risk of renal death and cardiovascular events compared with patients with preserved circadian BP rhythm, independently of 24‐hour systolic BP levels.32, 46
Table 2.
Summary of studies reporting data on prognostic significance of reverse dipping pattern
| Author (reference) | Sample size, No. | Setting | Reverse dipping, % | Duration follow‐up, y | Outcome |
|---|---|---|---|---|---|
| Okhubo10 | 1542 | General population | 3 | 5.1 | Mortality |
| Fagard44 | 374 | Primary care | 13 | 11.0 | Cardiovascular mortality |
| Bouhanick45 | 97 | Type 2 diabetes mellitus | 15 | 5.5 | Nonfatal and fatal cardiovascular events |
| Minutolo46 | 436 | Chronic kidney disease | 14 | 4.2 | Nonfatal and fatal cardiovascular events |
| Shin47 | 118 | Heart failure | 29 | 4.1 | Hospitalization, mortality |
| Komori48 | 516 | Heart failure | 22 | 1.7 | Cardiovascular events mortality |
| Park49 | 426 | Cerebral infarction | 32 | 7.6 | Mortality |
| Kario50 | 575 | Hypertension | 9 | 5.8 | Stroke, cardiovascular mortality |
| Eguchi51 | 1268 | Hypertension | 8 | 4.1 | Nonfatal and fatal cardiovascular events |
| Bastos52 | 1200 | Hypertension | 7 | 8.2 | Nonfatal and fatal cardiovascular events |
| Fagard19 | 3468 | Hypertension | 12 | 6.6 | All‐cause mortality, cardiovascular mortality |
| Kim53 | 401 | Hypertension | 26 | 10.0 | Cardiovascular mortality |
Shin and colleagues47 tested the role of altered diurnal BP pattern in predicting adverse outcomes such as hospitalization attributable to heart failure exacerbation or death in 118 men with symptomatic left ventricular systolic dysfunction. The authors showed that adverse outcome rates were lowest in dippers and highest in reverse dippers (P=.05). Predictors of adverse outcomes were New York Heart Association functional class (HR, 1.96; 95% CI, 1.11–3.44), anemia (HR, 2.50; 95% CI, 1.23–5.08), and RD status (HR, 1.65; 95% CI, 1.08–2.50). Similar results were recently reported by Komori and colleagues48 in 516 hospitalized patients with heart failure and preserved ejection fraction. The RD subgroup had a significantly higher incidence of the composite outcome (all‐cause mortality and cardiovascular events) than the other subgroups.
In a prospective study performed in 426 patients with cerebral infarction followed‐up for an average period of 7.6 years to detect recurrent cerebral events or all‐cause mortality, RD pattern diagnosed within 2 weeks after hospitalization was associated with an increased rate of mortality.49 Age (HR, 1.11; 95% CI, 1.09–1.13 [P<.001]), elevated nighttime heart rate (HR, 1.02; 95% CI, 1.01–1.04 [P=.004]), and RD (HR, 1.68; 95% CI, 1.15–2.43 [P=.007]) were independently correlated with all‐cause mortality. Type 2 diabetes mellitus and elevated daytime systolic BP predicted cerebral infarction recurrence.
Findings on RD prognostic value are mainly derived from studies conducted in hypertensive patients in Europe and Asia.
To provide comprehensive evidence on this issue we performed a meta‐analysis (Comprehensive Meta‐Analysis, version 2; Biostat, Englewood, NJ, USA; for methodological details see references 2 and 9) of five publications providing data on incident nonfatal and fatal cardiovascular events among patients categorized as dipper/extreme dipper, nondipper, and reverse dipper.19, 50, 51, 52, 53 Of note, the largest of these studies19 reported pooled data from four previously published papers.
Overall, 6918 untreated and treated hypertensive patients (518 extreme dippers, 2900 dippers, 2722 nondippers, and 696 reverse dippers) of both sexes (mean age 62±4 years; 95% CI, 54–69 years) were included in the reports (sample size range, 401–3468 participants). A total of 890 cardiovascular events (12.9%) were recorded among follow‐up periods ranging from 41 to 120 months. Figure 2 shows event rates recorded in dipper, nondipper, and reverse dipper hypertensive patients in the five studies.
Figure 2.
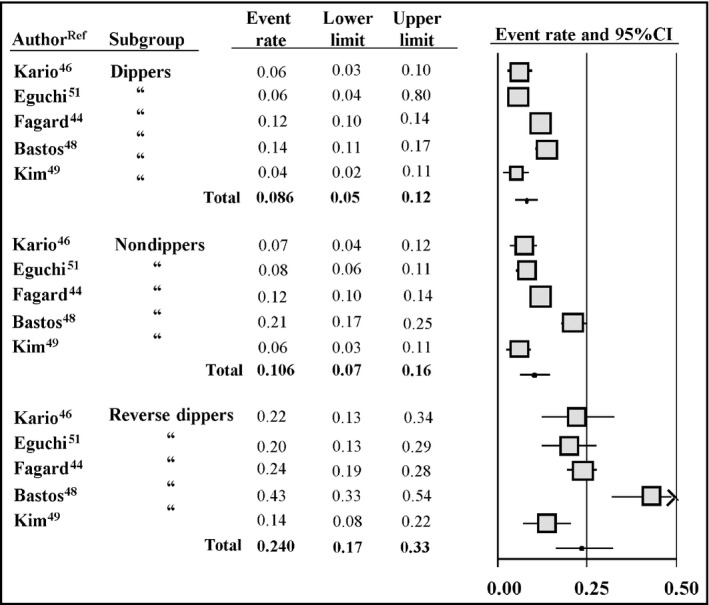
Cardiovascular event rates and 95% confidence intervals (CIs) in five studies conducted in the hypertensive setting according to the classification in dippers (n=2900), nondippers (n=2712), and reverse dippers (n=696)
A progressive increase in cardiovascular events occurred from dipper (10.2%) to nondipper (12.7%) and reverse dipper participants (24.1%). The incidence of cardiovascular events in extreme dippers (15.6%) was provided in only three of five studies.
In the pooled population, the risk of nonfatal and fatal cardiovascular events in patients with RD was 2.5‐fold greater than in dippers (95% CI, 2.11–2.96, P<.01; Figure 3) and persisted to be 2.1‐fold higher compared with nondippers (95% CI, 1.77–2.45, P<.001; Figure 4).
Figure 3.
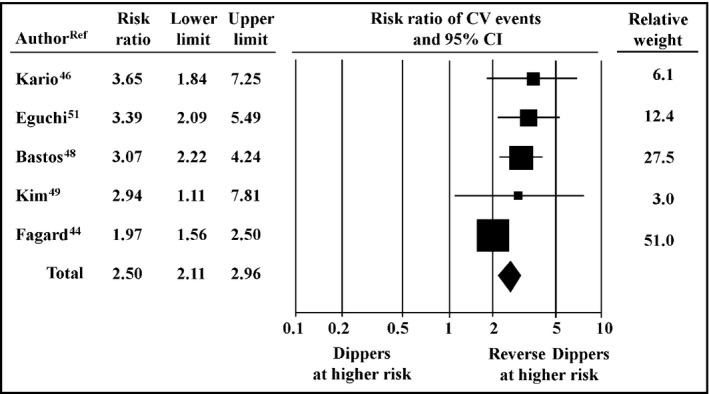
Relative risk of cardiovascular (CV) events, 95% confidence intervals (CIs), and relative weight in reverse dippers (n=696) vs dippers (n=2900)
Figure 4.
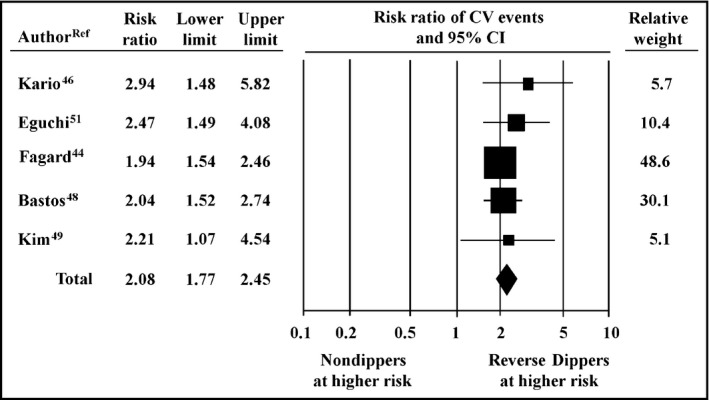
Relative risk of cardiovascular (CV) events, 95% confidence intervals (CIs) and relative weight, in reverse dippers (n=696) vs nondippers (n=2712)
The risk associated with RD pattern remained significantly increased when compared with extreme dippers (data not shown). Finally, a funnel plot excluded the presence of publication bias, and sensitivity analysis showed that the final result was not substantially affected by a single study effect.
2.6. Therapeutic interventions
Although reversal of altered circadian BP profiles is considered a therapeutic target for improving cardiovascular prognosis in hypertensive patients, no studies have addressed this topic in the setting of RD. Nonetheless, a brief summary of data concerning therapeutic interventions in patients with less extreme alterations in BP circadian rhythm (ie, nondippers) may be useful. More than 2 decades ago, Uzu and colleagues16 investigated the effect of short‐term diuretic treatment on circadian BP variations in 21 hypertensive patients (10 dippers and 11 nondippers). The magnitude of nocturnal BP drop was unaffected by treatment in dippers; on the contrary, it was markedly increased in nondippers, in whom a normal circadian rhythm was mostly restored. Available evidence on bedtime administration of antihypertensive drugs aimed to normalize nocturnal BP supports the view that this approach may be protective against cardiovascular risk associated with a disrupted BP rhythm. Likewise, emerging data suggest that drugs with a long‐lasting antihypertensive effect and elevated smoothness index exert a sustained reduction of nocturnal BP. In the Hypertension and Lipid Trial (HALT), the effect of bedtime administration of doxazosin on nighttime BP was assessed in 118 hypertensive patients with different nocturnal BP patterns (18 extreme dippers, 46 dippers, 48 nondippers, and 6 reverse dippers).54 The hypotensive effect of doxazosin on nocturnal systolic BP was greatest in reverse dippers (−18 mm Hg), intermediate in nondippers (−12 mm Hg), and lowest in dippers (−1 mm Hg) and extreme dippers (+4 mm Hg). Hermida and colleagues,55 comparing the effect of valsartan (160 mg/d) administered either in the morning or at bedtime in 148 nondipper hypertensive patients, observed a significant increase in diurnal/nocturnal BP ratio only when valsartan was administered at bedtime. As a result, 75% of patients randomized to bedtime therapy became dippers compared with 24% of patients treated with the morning dose. A Japanese trial examined the effect of cilnidipine on nocturnal BP in 615 hypertensive patients classified as extreme dippers, dippers, nondippers, and reverse dippers.56 BP reductions induced by cilnidipine were more pronounced at nighttime than daytime in patients with RD, similar at nighttime as daytime in nondippers, and more pronounced at daytime than nighttime in dippers. In a retrospective analysis including 1279 patients, telmisartan (a long‐acting angiotensin II receptor antagonist with a high smoothness index) normalized circadian BP pattern to a dipper profile in a larger proportion of patients compared with ramipril and reduced early‐morning systolic BP surge.57
The benefit of ameliorating nighttime BP control has recently been investigated by the MAPEC study.58 The investigators evaluated whether bedtime therapy with one or more hypertensive drugs was able to control BP and reduce cardiovascular risk more effectively than conventional therapy administration (ie, all antihypertensive drugs taken in the morning hours). After a follow‐up of 5.6 years, participants taking one or more BP‐lowering medications at bedtime had a lower nighttime BP as well as a reduced prevalence of nondipping/RD status than patients taking all drugs upon awakening. Importantly, the tighter control in nocturnal BP was associated with a substantial decrease (−60%) in relative risk of cardiovascular events.
It should be noted, however, that information on treatments aimed at restoring an abnormal circadian BP pattern remains scanty and has been provided by only a few research groups.
3. CONCLUSIONS
A paradoxical BP increase during sleep has been documented during 24‐hour BP monitoring. This impaired circadian BP rhythm, although of marginal prevalence in general population samples (<5%), has been reported to be up to 10 times more prevalent in patients with hypertension, type 2 diabetes mellitus, CKD, and sleep apnea syndrome.
Studies targeting the association of this condition with subclinical organ damage at the cardiac and extracardiac levels failed to provide univocal findings, although they support the view that RD is a risk factor for LVH, carotid abnormalities, proteinuria, and microalbuminuria.
A stronger body of evidence on the adverse clinical significance of RD pattern comes from studies addressing its relationship with incident cardiovascular events, where RD turned out to be a powerful marker of adverse cardiovascular prognosis, after adjusting for traditional risk factors.
Because of the high cardiovascular risk associated with RD, an appropriate antihypertensive chronotherapeutic approach to this condition should be performed to improve cardiovascular prevention. Further studies to assess the beneficial effects on cardiovascular prognosis of therapeutic interventions aimed to normalize circadian BP pattern are needed.
DISCLOSURE
The authors report no conflicts of interest.
Cuspidi C, Sala C, Tadic M, et al. Clinical and prognostic significance of a reverse dipping pattern on ambulatory monitoring: An updated review. J Clin Hypertens. 2017;19:713–721. 10.1111/jch.13023
REFERENCES
- 1. Mancia G, Verdecchia P. Clinical value of ambulatory blood pressure, evidence and limits. Circ Res. 2015;116:1034‐1045. [DOI] [PubMed] [Google Scholar]
- 2. Cuspidi C, Rescaldani M, Tadic M, et al. White‐coat hypertension, as defined by ambulatory blood pressure monitoring, and subclinical cardiac organ damage: a meta‐analysis. J Hypertens. 2015;33:24‐32. [DOI] [PubMed] [Google Scholar]
- 3. Tadic M, Cuspidi C, Radojkovic J, et al. Masked hypertension and left atrial dysfunction: a hidden association. J Clin Hypertens (Greenwich). 2017;19:305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mancia G, Facchetti R, Bombelli M, et al. Long‐term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension. 2006;47:846‐853. [DOI] [PubMed] [Google Scholar]
- 5. O'Brien E, Sheridan J, O'Malley K. Dippers and non‐dippers. Lancet. 1988;397:2. [DOI] [PubMed] [Google Scholar]
- 6. Pickering TG. The clinical significance of diurnal blood pressure variations. Dippers and nondippers. Circulation. 1990;81:700‐702. [DOI] [PubMed] [Google Scholar]
- 7. Verdecchia P, Schillaci G, Guerrieri M, et al. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation. 1990;81:528‐536. [DOI] [PubMed] [Google Scholar]
- 8. Mancia G, Parati G. Ambulatory blood pressure monitoring and organ damage. Hypertension. 2000;36:894‐900. [DOI] [PubMed] [Google Scholar]
- 9. Cuspidi C, Sala C, Tadic M, et al. Nondipping pattern and carotid atherosclerosis: a systematic review and meta‐analysis. J Hypertens. 2016;34:385‐391. [DOI] [PubMed] [Google Scholar]
- 10. Ohkubo T, Imai Y, Tsuji I, et al. Relation between nocturnal decline in blood pressure and mortality. Am J Hypertens. 1997;10:1201‐1207. [DOI] [PubMed] [Google Scholar]
- 11. Sherwood A, Steffen PR, Blumenthal JA, et al. Nighttime blood pressure dipping: the role of the sympathetic nervous system. Am J Hypertens. 2002;15:111‐118. [DOI] [PubMed] [Google Scholar]
- 12. Grassi G, Seravalle G, Quarti‐Trevano F, et al. Adrenergic, metabolic, and reflex abnormalities in reverse and extreme dipper hypertensives. Hypertension. 2008;52:925‐932. [DOI] [PubMed] [Google Scholar]
- 13. Henderson LA, Macefield VG. Obstructive sleep apnoea and hypertension: the role of the central nervous system. Curr Hypertens Rep. 2016;18:59. [DOI] [PubMed] [Google Scholar]
- 14. Kimura G. Kidney and circadian blood pressure rhythm. Hypertension. 2008;51:827‐828. [DOI] [PubMed] [Google Scholar]
- 15. Afsar B, Elsurer R, Kirkpantur A, Kanbay M. Urinary sodium excretion and ambulatory blood pressure findings in patients with hypertension. J Clin Hypertens (Greenwich). 2015;17:200‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uzu T, Kimura G. Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1999;100:1635‐1638. [DOI] [PubMed] [Google Scholar]
- 17. Han J, Gao Y, Guo Q, et al. Cross‐sectional study on the relationship between the level of serum cystatin C and blood pressure reverse dipping in hypertensive patients. BMJ Open. 2016;6:e011166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bombelli M, Toso E, Peronio M, et al. The Pamela study: main findings and perspectives. Curr Hypertens Rep. 2013;15:238‐243. [DOI] [PubMed] [Google Scholar]
- 19. Fagard RH, Thijs L, Staessen JA, et al. Night‐day blood pressure ratio and dipping pattern as predictors of death and cardiovascular events in hypertension. J Hum Hypertens. 2009;23:645‐653. [DOI] [PubMed] [Google Scholar]
- 20. Su D, Guo Q, Gao Y, et al. The relationship between red blood cell distribution width and blood pressure abnormal dipping in patients with essential hypertension: a cross‐sectional study. BMJ Open. 2016;6:e010456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eguchi K, Ishikawa J, Hoshide S, et al. Nighttime blood pressure variability is a strong predictor for cardiovascular events in patients with type 2 diabetes. Am J Hypertens. 2009;22:46‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pogue V, Rahman M, Lipkowitz M, et al. Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension. 2009;53:20‐27. [DOI] [PubMed] [Google Scholar]
- 23. Iimuro S, Imai E, Watanabe T, et al. Clinical correlates of ambulatory BP monitoring among patients with CKD. Clin J Am Soc Nephrol. 2013;8:721‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee S, Thomas RJ, Kim H, et al. Association between high nocturnal blood pressure and white matter change and its interaction by obstructive sleep apnoea among normotensive adults. J Hypertens. 2014;32:2005‐2012. [DOI] [PubMed] [Google Scholar]
- 25. Wong LH, Huang E, Kong RT. Racial differences affecting night time blood pressure dipping groups in hypertensive patients. J Hypertens (Los Angel). 2016;5:1. [PMC free article] [PubMed] [Google Scholar]
- 26. Garcıa‐Ortiz L, Gomez‐Marcos MA, Martın‐Moreiras J, et al. Pulse pressure and nocturnal fall in blood pressure are predictors of vascular, cardiac and renal target organ damage in hypertensive patients (LOD‐RISK study). Blood Press Monit. 2009;14:145‐151. [DOI] [PubMed] [Google Scholar]
- 27. Cha R, Kim S, Yoon SA, et al. Association between blood pressure and target organ damage in patients with chronic kidney disease and hypertension: results of the APrODiTe study. Hypertens Res. 2014;37:172‐178. [DOI] [PubMed] [Google Scholar]
- 28. Ivanovic BA, Tadic MV, Celic VP. To dip or not to dip? The unique relationship between different blood pressure patterns and cardiac function and structure. J Hum Hypertens. 2013;27:62‐70. [DOI] [PubMed] [Google Scholar]
- 29. Komori T, Eguchi K, Saito T, et al. Riser blood pressure pattern is associated with mild cognitive impairment in heart failure patients. Am J Hypertens. 2015;29:194‐201. [DOI] [PubMed] [Google Scholar]
- 30. Wang C, Zhang J, Liu X, et al. Reversed dipper blood pressure pattern is closely related to severe renal and cardiovascular damage in patients with chronic kidney disease. PLoS One. 2013;8:e55419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Viera AJ, Lin FC, Hinderliter AL, et al. Nighttime bllod pressure dipping in young adults and coronary artery calcium 10‐15 years later: the CARDIA study. Hypertension. 2012;59:1157‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang C, Ye Z, Li Y, et al. Prognostic value of reverse dipper blood pressure pattern in chronic kidney disease patients not undergoing dialysis: prospective cohort study. Sci Rep. 2016;6:34962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Marinakis AG, Vyssoulis GP, Michaelides AP, et al. Impact of abnormal nocturnal blood pressure fall on vascular function. Am J Hypertens. 2003;16:209‐213. [DOI] [PubMed] [Google Scholar]
- 34. Yan B, Peng L, Han D, et al. Blood pressure reverse‐dipping is associated with early formation of carotid plaque in senior hypertensive patients. Medicine. 2015;94:e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jerrard‐Dunne P, Mahmud A, Feely J. Circadian blood pressure variation: relationship between dipper status and measures of arterial stiffness. J Hypertens. 2007;25:1233‐1239. [DOI] [PubMed] [Google Scholar]
- 36. Yamamoto Y, Akiguchi I, Oiwa K, et al. The relationship between 24‐hour blood pressure readings, subcortical ischemic lesions and vascular dementia. Cerebrovasc Dis. 2005;19:302‐308. [DOI] [PubMed] [Google Scholar]
- 37. Yan B, Peng L, Dong Q. Reverse‐dipper pattern of blood pressure may predict lacunar infarction in patients with essential hypertension. Eur J Neurol. 2015;22:1022‐1025. [DOI] [PubMed] [Google Scholar]
- 38. Castilla‐Guerra L, Espino‐Montoro A, Fernandez‐Moreno MC, Lopez‐Chozas JM. Abnormal blood pressure circadian rhythm in acute ischaemic stroke: are lacunar strokes really different? Int J Stroke. 2009;4:257‐261. [DOI] [PubMed] [Google Scholar]
- 39. Kwon HM, Lim JS, Kim YS, et al. Cerebral microbleeds are associated with nocturnal reverse dipping in hypertensive patients with ischemic stroke. BMC Neurol. 2014;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang WW, Cadilhac DA, Churilov L, et al. Does abnormal circadian blood pressure pattern really matter in patients with transient ischemic attack or minor stroke? Stroke. 2014;45:865‐867. [DOI] [PubMed] [Google Scholar]
- 41. Sun J, Yang W, Zhu Y, et al. The relationship between nocturnal blood pressure and hemorrhagic stroke in Chinese hypertensive patients. J Clin Hypertens (Greenwich). 2014;16:652‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yan B, Sun L, Gao Y, et al. Blood pressure reverse dipping may associate with stable coronary artery disease in patients with essential hypertension: a cross sectional study. Sci Rep. 2016;6:25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Komori T, Eguchi K, Saito T, et al. Riser pattern: another determinant of heart failure with preserved ejection fraction. J Clin Hypertens (Greenwich). 2016;18:994‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fagard RH, De Cort P. Orthostatic hypotension is a more robust predictor of cardiovascular events than nighttime reverse dipping in elderly. Hypertension. 2010;56:56‐61. [DOI] [PubMed] [Google Scholar]
- 45. Bouhanick B, Bongard V, Amar J, et al. Prognostic value of nocturnal blood pressure and reverse‐dipping status on the occurrence of cardiovascular events in hypertensive diabetic patients. Diabetes Metab. 2008;34:560‐567. [DOI] [PubMed] [Google Scholar]
- 46. Minutolo R, Agarwal R, Borrelli S, et al. Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Arch Intern Med. 2011;171:1090‐1098. [DOI] [PubMed] [Google Scholar]
- 47. Shin J, Kline S, Moore M, et al. Association of diurnal blood pressure pattern with risk for hospitalization or death in men with heart failure. J Card Fail. 2007;13:656‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Komori T, Eguchi K, Saito T. Riser pattern is a novel predictor of adverse events in heart failure patients with preserved ejection fraction. Circ J. 2017;81:220‐226. [DOI] [PubMed] [Google Scholar]
- 49. Park JH, Lee HS, Kim JH, et al. Reverse dipper and high night‐time heart rate in acute stage of cerebral infarction are associated with increased mortality. J Stroke Cerebrovasc Dis. 2014;23:1171‐1176. [DOI] [PubMed] [Google Scholar]
- 50. Kario K, Pickering TG, Matsuo T, et al. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852‐857. [DOI] [PubMed] [Google Scholar]
- 51. Eguchi K, Pickering TG, Hoshide S, et al. Ambulatory blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with/without type 2 diabetes. Am J Hypertens. 2008;21:443‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bastos JM, Bertoquini S, Polonia J. Prognostic value of subdivisions of nighttime blood pressure fall in hypertensives followed up for 8.2 years. Does nondipping classification need to be redefined? J Clin Hypertens (Greenwich). 2010;12:508‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim BK, Kim YM, Lee Y, et al. A reverse dipping pattern predicts cardiovascular mortality in a clinical cohort. J Korean Med Sci. 2013;28:1468‐1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kario K, Schwartz JE, Pickering TG. Changes of nocturnal blood pressure dipping status in hypertensives by nighttime dosing of alfa‐adrenergic blocker, doxazosin: results from the HALT study. Hypertension. 2000;35:787‐794. [DOI] [PubMed] [Google Scholar]
- 55. Hermida RC, Calvo C, Ayala DE, et al. Treatment of non‐dipper hypertension with bedtime administration of valsartan. J Hypertens. 2005;23:1913‐1922. [DOI] [PubMed] [Google Scholar]
- 56. Kario K, Nariyama J, Kido H, et al. Effect of a novel calcium channel blocker on abnormal nocturnal blood pressure in hypertensive patients. J Clin Hypertens (Greenwich). 2013;15:465‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gosse P, Schumacher H. Effect of telmisartan vs. ramipril on ‘dipping’ status and blood pressure variability: pooled analysis of the PRISMA studies. Hypertens Res. 2014;37:151‐157. [DOI] [PubMed] [Google Scholar]
- 58. Hermida RC, Ayala DE, Mojón A, Fernández JR. Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol Int. 2010;27:1629‐1651. [DOI] [PubMed] [Google Scholar]


