Abstract
We investigated the association between working memory (WM) impairment and blood pressure variability (BPV) in very elderly patients. Japanese outpatients ≥80 years who engaged in normal activities of daily living were the study cohort. WM function was evaluated by a simple visual WM test consisting of 3 figures. We considered the number of figures recalled by the patient his/her test score. We defined the patients with a score of 0 or 1 as those with WM impairment and those with scores of 2 or 3 as those without. To investigate the relative risk of WM impairment, we evaluated each patient's 24 hour ambulatory systolic blood pressure (SBP) and its weighted standard deviation (SDSBP), office SBP, and the visit‐to‐visit SDSBP during the 1 year period from the patient's enrollment. A total of 66 patients (mean 84 ± 3.6 years) showed WM impairment, and 431 patients (mean 83 ± 3.1 years) showed no WM impairment. There were no significant differences in 24 hour ambulatory SBP or office SBP between these two groups. However, the WM impairment patients showed significantly higher weighted SDSBP and visit‐to‐visit SDSBP values compared to the no‐impairment group even after adjusting for age. Among these ≥80‐year‐old patients, those with the highest quartile of both weighted SDSBP (≥21.4 mm Hg) and visit‐to‐visit SDSBP (≥14.5 mm Hg) showed the highest relative risk (odds ratio 3.52, 95% confidence interval 1.42‐8.72) for WM impairment. Exaggerated blood pressure variability parameters were significantly associated with working memory impairment in very elderly individuals.
Keywords: blood pressure, blood pressure variability, very elderly patients, working memory impairment
1. INTRODUCTION
Elevated blood pressure (BP) is a major negative factor in the development of cognitive dysfunction in middle age.1, 2, 3, 4, 5, 6 However, there is no consensus about the association between elevated BP and cognitive function in later life.7 The data on the association between antihypertensive treatment and altered cognitive function in elderly populations are limited.8 Antihypertensive treatment targeting lower BP levels in elderly patients (≥80 years) resulted in a non‐significant reduction of cognitive dysfunction.9 Therefore, other factor(s), not the BP level, might be associated with cognitive function in individuals ≥80 years.
Blood pressure variability (BPV) has been highlighted as a surrogate marker of target organ damage10, 11, 12, 13, 14, 15 and as a prognostic factor of future cardiovascular events.16, 17, 18, 19, 20, 21 Several studies have revealed that exaggerated short‐term BPV (ie, ambulatory BPV)22, 23 and exaggerated long‐term BPV (ie, visit‐to‐visit BPV)24, 25, 26, 27, 28 are significant indicators of global cognitive dysfunction. However, no study has investigated the association between both short‐term and long‐term exaggerated BPV and cognitive dysfunction in the same patient group. In addition, there is no information regarding the direct relationships between working memory (WM) impairment (which is a core feature of cognitive dysfunction)29, 30 and BPV parameters, especially in very elderly individuals. In the present study, we considered ≥80 years as “very elderly.”
In the present study, we therefore used data from the Japanese‐based study known as the SEARCH (search longevity in very elderly with ambulatory pressure in Tochigi) study, a prospective observational study of elderly patients (≥80 years), to test our hypothesis that the indices of BPV would be significantly associated with WM impairment in very elderly individuals. We also assessed whether the individuals with both exaggerated short‐term BPV and exaggerated long‐term BPV showed a high relative risk for WM impairment.
2. METHODS
2.1. Patients
The SEARCH study examined 525 elderly outpatients who were recruited between September 2008 and December 2013 and followed up through June 2015 by 26 doctors at 21 institutions (including two specialized university hospitals). Details of the study design and methods are described in the present study's Data S1.
The 3 inclusion criteria were: (1) age ≥80 years, (2) ability to attend a clinic or hospital without difficulty in walking, and (3) living without assistance. The exclusion criteria were: (1) prevalent cardiovascular disease or cerebrovascular disease, excluding transient ischemic attack, within 6 months; (2) current dialysis; (3) malignant disease at baseline; and (4) inability to provide informed consent due to severe cognitive dysfunction or dementia. All participants provided written informed consent, and the ethics committee of the Jichi Medical University School of Medicine approved the study.
2.2. BP and other measurements
The office BP of each patient was measured at each visit to the participating institution, with the use of a validated cuff oscillometric device in accord with the Japanese Society of Hypertension 2004 guidelines.31 BP was measured after the patient rested for at least 5 minutes in a seated position. Two consecutive BP measurements were taken at a 1‐2 minute intervals and the average of the measurements was used as the office BP value. We measured office BP at baseline and at each office visits during the 1‐year period from the patient's enrollment.
Non‐invasive 24‐hour ambulatory BP monitoring (ABPM) was carried out at the baseline with a validated automatic device (TM‐2425 or TM‐2431) that recorded the patient's BP using an oscillometric method at 30‐minute intervals throughout the 24‐hour day. Morning BP was defined as the average of BP values during the first 2 hours of being awake. Nighttime BP was defined as the average BP value from those taken at bedtime and when the patient got out of bed in the morning. Daytime BP was defined as the average BP value for the rest of the day.
For the short‐term BPV parameters, we calculated the standard deviation (SD), coefficient of variation (CV), and the weighted SD of the 24‐hour ambulatory systolic BP (SBP) and diastolic BP (DBP) values. For the long‐term BPV parameters, we calculated the SD, CV, and maximum and minimum BP difference (MMD) of the visit‐to‐visit SBP and DBP values. The weighted SD was the average daytime and nighttime BP SD divided by the duration in hours of each time period. The MMD was calculated as the maximum BP minus the minimum BP in the follow‐up period.
Fasting blood and spot urine samples were collected in the morning at baseline. All samples were sent to a single laboratory (SRL, Tokyo) within 24 hours of collection. Questionnaires were used to collect demographic data and clinical and behavioral characteristics of patients (details are provided in the Data S1). Pre‐existing cardiovascular disease (CVD) was defined as pre‐existing angina pectoris, myocardial infarction, or stroke.
2.3. Working memory assessment
We used a simple visual WM test to evaluate the WM function of study patients. This test was a part of the mini‐mental state examination (MMSE), whose validity and reproducibility have been confirmed;32, 33 it was conducted by trained medical staff upon patient entry. Each patient was shown the same 3 figures (a spoon, a pen, and a watch; Figure 1) and asked to name each figure out loud and to memorize the names. All of the patients correctly named the figures when they saw them. After patients had memorized the 3 figures, and before they were asked to recall them, the medical staff collected the demographic data and clinical and behavioral characteristics of patients (see the Data S1). Then, at 5 minutes after presentation of the figures, patients were asked to recall the names of the figures. The number of figures that the patient was able to recall was counted as the patient's test score, with a larger score indicating better WM function. We defined the patients with 0 or 1 of the test score as those with WM impairment and the patients with the score 2 or 3 as those without WM impairment.
Figure 1.
The three figures used in the simple visual working memory test
2.4. Statistical analysis
Statistical analyses were performed with SPSS software ver. 24.0 (SPSS). We used a two‐sided unpaired t‐test to compare the clinical parameters in patients with versus without WM impairment. Clinical parameters that are evaluated as percentages were compared using chi‐squared statistics. We used Pearson's correlation coefficient for a bivariate analysis examining SDBP versus other BPV parameters. To assess the differences in BPV parameters between the “with” and “without” WM impairment groups, we conducted a logistic regression analysis with adjustment for age. A P‐value <.05 was considered significant.
3. RESULTS
3.1. Patient characteristics
Of the 525 participants for whom entry data were obtained, we excluded 1 participant who had no information on age, 5 participants who did not take the WM test, 5 participants who did not have ABPM, and 17 participants who visited their physician's offices only 1 time during the 1‐year follow‐up period after their inclusion in this study. The included participants (n = 497) had higher percentages of calcium‐channel blockers and angiotensin‐converting enzyme inhibitor use, and higher numbers of office visits than those excluded (n = 28); other variables were similar between the included and excluded patients (Table S1). The mean age of the 497 patients was 83.2 ± 3.2 years, and 55.7% were women. Pre‐existing CVD was observed in 24.9% (n = 124) of the patients (stroke, 50 patients; angina pectoris or myocardial infarction, 90 patients). The distribution of the simple visual WM test scores was as follows: 0 (n = 17, 3.4%), 1 (n = 49, 9.9%), 2 (n = 155, 31.2%), and 3 (n = 276, 55.5%).
Table 1 summarizes the differences in the demographic and clinical characteristics of the patients with and without WM impairment. The age of the patients with WM impairment (n = 66) was significantly higher than that of the patients without WM impairment (n = 431), and they tended to have lower body mass index values. There were no significant differences in the classes of antihypertensive medication use between the patients with and without WM impairment. The percentage of pre‐existing CVD was not significantly different between the two groups (21.2% vs 25.5%, respectively; P = .451); nor was the percentage of stroke (4.5% vs 10.9%, respectively; P = .110). The average number of office visits, which indicates the number of times that visit‐to‐visit BP was measured during the follow‐up period, was not significantly different between the groups (10.0 ± 2.9 vs 9.4 ± 3.1, respectively; P = .175).
Table 1.
Baseline characteristics of the patients with or without working memory impairment (n = 497)
| Variable | With WM impairment (n = 66) | Without WM impairment (n = 431) | P‐value |
|---|---|---|---|
| Age, yrs | 84.0 ± 3.6 | 83.0 ± 3.1 | .015 |
| Male, n (%) | 24 (36.4) | 196 (45.5) | .165 |
| BMI, kg/m2 | 22.5 ± 3.9 | 23.4 ± 3.4 | .056 |
| Current smoking, n (%) | 4 (7.0) | 25 (7.7) | .859 |
| Daily drinker, n (%) | 14 (21.5) | 115 (26.7) | .378 |
| Antihypertensive medication | |||
| Calcium‐channel blockers, n (%) | 40 (60.6) | 288 (66.8) | .321 |
| ACE inhibitors, n (%) | 20 (30.3) | 102 (23.7) | .243 |
| Angiotensin receptor blockers, n (%) | 29 (43.9) | 210 (48.7) | .469 |
| Diuretics, n (%) | 13 (19.7) | 118 (27.4) | .187 |
| Alpha‐blockers, n (%) | 3 (4.5) | 38 (8.8) | .240 |
| Beta‐blockers, n (%) | 9 (13.6) | 82 (19.0) | .292 |
| Hypertension, n (%) | 55 (83.3) | 371 (86.1) | .553 |
| Dyslipidemia, n (%) | 25 (37.9) | 182 (42.2) | .505 |
| Diabetes mellitus, n (%) | 15 (22.7) | 111 (25.8) | .599 |
| Pre‐existing CVD, n (%) | 14 (21.2) | 110 (25.5) | .451 |
| Stroke, n (%) | 3 (4.5) | 47 (10.9) | .110 |
| Angina pectoris or myocardial infarction, n (%) | 12 (18.2) | 78 (18.1) | .987 |
| Fasting glucose, mg/dL | 103.2 ± 18.5 | 106.7 ± 27.0 | .301 |
| Total cholesterol, mg/dL | 192.5 ± 28.0 | 189.1 ± 29.5 | .379 |
| High‐density lipoprotein, mg/dL | 57.4 ± 15.2 | 56.6 ± 14.6 | .672 |
| No. of office visits | 10.0 ± 2.9 | 9.4 ± 3.1 | .175 |
ACE, angiotensin converting enzyme; CVD, cardiovascular disease; WM, working memory.
Data are mean ± SD or number (percentage). Pre‐existing CVD includes pre‐existing angina pectoris, myocardial infarction, or stroke. P‐values were obtained by unpaired t‐test or chi‐squared test.
3.2. Blood pressure and BPV parameters
Table 2 shows the mean 24‐hour ambulatory BP values and their short‐term BPV parameters. The mean systolic and diastolic BP levels (including 24‐hour ambulatory BP, morning BP, daytime BP, and nighttime BP) showed no significant differences between the patients with and without WM impairment. However, all of the short‐term BPV parameters were significantly higher in the patients with WM impairment compared to those without.
Table 2.
Twenty‐four ambulatory BP parameters of study cohort (n = 497)
| Variable | With WM impairment (n = 66) | Without WM impairment (n = 431) | P‐value |
|---|---|---|---|
| 24‐hr ambulatory SBP, mm Hg | 131.0 ± 15.0 | 130.3 ± 14.6 | .873 |
| 24‐hr ambulatory DBP, mm Hg | 71.5 ± 7.0 | 70.4 ± 7.3 | .198 |
| Morning SBP, mm Hg | 140.2 ± 22.4 | 142.1 ± 20.4 | .488 |
| Morning DBP, mm Hg | 79.7 ± 16.9 | 78.1 ± 13.0 | .370 |
| Daytime SBP, mm Hg | 135.5 ± 15.2 | 135.4 ± 15.3 | .014 |
| Daytime DBP, mm Hg | 74.3 ± 7.3 | 73.2 ± 7.7 | .257 |
| Nighttime SBP, mm Hg | 123.2 ± 18.7 | 121.4 ± 16.7 | .591 |
| Nighttime DBP, mm Hg | 66.6 ± 8.8 | 65.4 ± 8.5 | .244 |
| SDSBP, mm Hg | 22.6 ± 4.6 | 20.3 ± 5.6 | .003 |
| SDDBP, mm Hg | 13.4 ± 2.8 | 12.2 ± 3.3 | .005 |
| CVSBP, % | 17.3 ± 3.5 | 15.7 ± 4.2 | .003 |
| CVDBP, % | 18.8 ± 3.8 | 17.3 ± 4.7 | .022 |
| Weighted SDSBP, mm Hg | 20.3 ± 4.3 | 18.2 ± 4.9 | .003 |
| Weighted SDDBP, mm Hg | 12.2 ± 2.9 | 11.1 ± 3.1 | .007 |
BP, blood pressure; CV, coefficient of variation; DBP, diastolic blood pressure; SBP, systolic blood pressure; WM, working memory.
Data are mean ± SD. P‐values were obtained by logistic regression analysis adjusted by age.
Table 3 provides the office BP values at baseline and their long‐term BPV parameters. The baseline office systolic and diastolic BP levels showed no significant differences between the groups with and without WM impairment. However, the SDSBP, CVSBP, and MMDSBP values were all significantly higher in the patients with WM impairment compared to those without. Figure S1 shows the office BP variation during the follow‐up period in patients with and those without WM impairment. The office BP levels remained similar in both groups during the follow‐up period.
Table 3.
Visit‐to‐visit BP parameters of study cohort (n = 497)
| Variable | With WM impairment (n = 66) | Without WM impairment (n = 431) | P‐value |
|---|---|---|---|
| Office SBP, mm Hg | 144.8 ± 22.8 | 139.6 ± 20.5 | .068 |
| Office DBP, mm Hg | 75.6 ± 13.7 | 73.4 ± 13.3 | .156 |
| SDSBP, mm Hg | 13.5 ± 5.5 | 11.8 ± 4.6 | .017 |
| SDDBP, mm Hg | 7.8 ± 2.8 | 7.0 ± 2.8 | .058 |
| CVSBP, % | 9.7 ± 4.2 | 8.5 ± 3.1 | .012 |
| CVDBP, % | 10.8 ± 4.2 | 10.0 ± 4.1 | .193 |
| MMDSBP, mm Hg | 40.3 ± 15.5 | 35.4 ± 14.4 | .032 |
| MMDDBP, mm Hg | 23.3 ± 8.4 | 20.9 ± 9.0 | .057 |
BP, blood pressure; CV, coefficient of variation; DBP, diastolic blood pressure; MMD, maximum and minimum blood pressure difference; SBP, systolic blood pressure; WM, working memory.
Data are mean ± SD. P‐values were obtained by logistic regression analysis adjusted by age.
3.3. Relative risk of WM impairment
To evaluate the relative risk of WM impairment, we used weighted SDSBP and visit‐to‐visit SDSBP as the short‐term and the long‐term BPV parameters, respectively, because the other respective parameters were significantly correlated with them (Table S2). The correlation between weighted SDSBP and visit‐to‐visit SDSBP was significant, but it was very weak (r = .104, P = .021). We divided the weighted SDSBP and visit‐to‐visit SDSBP values into quartiles, and used the references of weighted SDSBP < 14.8 mm Hg (the lowest quartile of weighted SDSBP) and visit‐to‐visit SDSBP < 8.6 mm Hg (the lowest quartile of visit‐to‐visit SDSBP), respectively.
Both the highest quartile of weighted SDSBP (≥21.4 mm Hg) and that of visit‐to‐visit SDSBP (≥14.5 mm Hg) presented a significantly high relative risk of WM impairment compared to the references; the odds ratio (OR) of the highest quartile of weighted SDSBP was 5.79, with the 95% confidence interval (CI) of 2.13‐15.74 (P = .001). The OR of the highest quartile of visit‐to‐visit SDSBP was 2.21, with the 95% CI of 1.02‐4.78 (P = .045; Figure 2).
Figure 2.
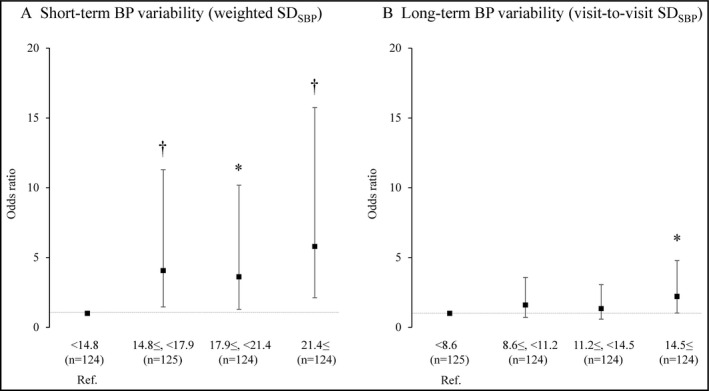
The relative risk of working memory impairment according to systolic BP variability quartiles, with OR and 95% CI values. Weighted SDSBP < 14.8 mm Hg and visit‐to‐visit SDSBP < 8.6 mm Hg were used as references (Ref.), respectively, and the bars represent ORs (95% CIs) with adjustment for age. A logistic regression analysis was used with adjustment for age. *P < .05, † P < .01
We next set the highest quartile of weighted SDSBP and that of visit‐to‐visit SDSBP as the high BPV group, and the other quartile of weighted SDSBP (<21.4 mm Hg) and that of visit‐to‐visit SDSBP (<14.5 mm Hg) as the low BPV group, respectively. The patients with both high weighted SDSBP and high visit‐to‐visit SDSBP showed the highest relative risk (OR 3.52, 95% CI 1.42‐8.72, P = .007) of WM impairment compared to those with both low weighted SDSBP and low visit‐to‐visit SDSBP (Figure 3).
Figure 3.
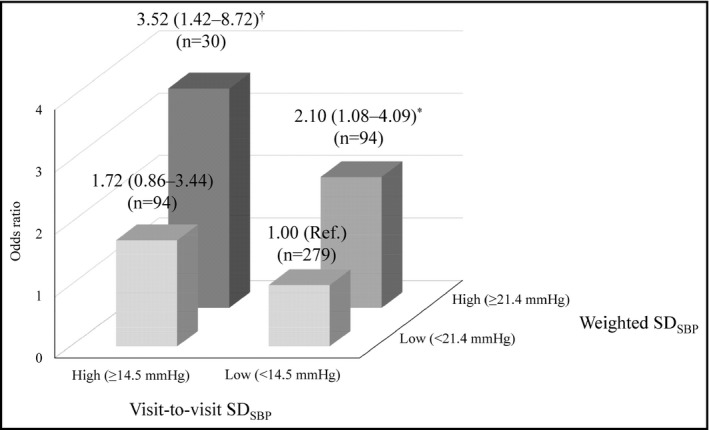
Relative risk of working memory impairment according to each systolic BP variability category group, with OR and 95% CI values. The findings for the group with both low weighted SDSBP (<21.4 mm Hg) and visit‐to‐visit SDSBP < 14.5 mm Hg were used as references (Ref.). A logistic regression analysis was used with adjustment for age. *P < .05, † P < .01
We calculated the parameters of diastolic BPV in the same way as those of systolic BPV. The correlation between weighted SDDBP and visit‐to‐visit SDDBP was not significant (r = .071, P = .117). The highest quartile of weighted SDDBP (≥13.0 mm Hg) presented a significantly high relative risk and the highest quartile of visit‐to‐visit SDDBP (≥8.8 mm Hg) showed a trend toward higher relative risk of WM impairment compared to the reference; the OR of the highest quartile of weighted SDDBP was 3.75, with a 95% CI of 1.53‐9.19 (P = .004). The OR of the highest quartile of visit‐to‐visit SDDBP was 1.95, with a 95% CI of 0.89‐4.26 (P = .096; Figure S2). The patients with both high weighted SDDBP and high visit‐to‐visit SDDBP showed the highest relative risk (OR 2.94, 95% CI 1.20‐7.20, P = .019) of WM impairment compared to those with both low weighted SDDBP (<13.0 mm Hg) and low visit‐to‐visit SDDBP (<8.8 mm Hg; Figure S3).
4. DISCUSSION
The main findings of this study of elderly patients (≥80 years) are as follows. First, both the short‐term and long‐term blood pressure variability (BPV) parameters, not BP levels, were significantly higher in the patients with working memory (WM) impairment compared to those without. Second, the patients with both high weighted SDSBP and visit‐to‐visit SDSBP showed the highest relative risk of WM impairment. These findings indicate that exaggerated BPV may be associated with WM impairment in very elderly patients (ie, those aged ≥80 years). The assessment of both short‐term and long‐term BPV parameters could thus be important for identifying patients with WM impairment.
The novel finding of the present study was that the patients with WM impairment showed exaggeration of both short‐term and long‐term BPV compared to those without WM impairment, despite the lack of difference in BP levels between the two groups. Our results indicate that the initial increase in BPV, not the BP level, could be associated with the progression of WM impairment. The contribution of exaggerated BPV leading to cognitive dysfunction in the elderly has been reported. Two studies of Japanese hypertensive patients showed that exaggerated ambulatory BPV (SD) was related to cognitive dysfunction,22, 23 and Nagai et al24 also showed that exaggerated visit‐to‐visit BPV (CV and MMD) were significantly associated with cognitive impairment independently of average BP levels in the elderly (mean age 80 years). Sabayan et al25 reported that higher visit‐to‐visit BPV (SD) was associated with worse performances regarding selective attention, reaction time, general cognitive speed, and immediate and delayed memory, independently of average BP levels in the elderly (mean age 75 years) in a longitudinal study with 3.2 years of follow‐up.
The present study is the first to reveal that individuals ≥80 years, with both exaggerated short‐term and long‐term BPV parameters, showed the highest relative risk of WM impairment. The underlying mechanisms differ between short‐term and long‐term BPV. Short‐term BPV is affected by various types of intrinsic factors such as increased central sympathetic drive and reduced arterial and cardiopulmonary reflexes,34 increased arterial stiffness,35, 36 humoral,37 and genetic factors.21 In contrast, long‐term BPV was reported to be influenced by extrinsic factors such as compliance with and the improper administration of antihypertensive drugs, the type of antihypertensive drugs,38 errors in office BP measurements, and seasonal BP changes.37 In light of the very high ages of our study's patients, increases in both intrinsic and extrinsic factors for BPV might have more strongly affected the significant association with WM impairment compared to individuals <80 years.
In addition, the reports that both short‐term and long‐term BPVs are associated with each other, which leads to the progression of cerebral, cardiac, renal and vascular damage independently of mean BP levels37, 39 might support our results.
Ambulatory BP monitoring can provide a significant amount of BP information (including the mean BP level and its variability), which cannot be estimated by office BP monitoring. However, ABPM cannot be used routinely to assess BPV. Various types of BP monitoring should thus be performed, and the evaluation of the combination of different types of BPV parameters would be effective to detect WM impairment at an early stage.
An association between exaggerated BPV and cognitive dysfunction has been reported,22, 23, 24, 25 but whether exaggerated BPV may be causally related to cognitive dysfunction or simply a result of cognitive dysfunction remains unclear. Some studies have suggested that exaggerated BPV may contribute to cognitive dysfunction, since it has been demonstrated to have detrimental effects on the cerebral perfusion and cerebral hemodynamic40, 41 as well as alter the neurovascular coupling.42 On the other hand, there is also evidence that the autonomic dysregulation or neurodegeneration, both of which cause cognitive dysfunction, may lead to exaggerated BPV.43, 44, 45, 46 We were unable to establish causality based on our findings, but we found that exaggerated BPV and cognitive dysfunction were closely related to each other from the early stage of cognitive decline, and we considered that these factors might form a “vicious cycle.”39 To suppress this cycle at the early stage, various types of BPV should be evaluated in detail.
In this study, we evaluated the patients’ WM function by using a simple visual WM test. There are various types of screening tests to evaluate WM function, but some are very difficult to use for screening in general practice. The simple visual WM test used in this study easily evaluated WM function even in elderly patients ≥80 years. As we also reported regarding this test (for which the association between the decrease in cognitive function and mortality was established), we observed that cognitive dysfunction assessed by this same simple visual WM test was an independent risk factor for total death and cardiovascular death in elderly ≥80 years,47 which indicates that this simple test would be an effective method for evaluating both the cognitive function and mortality risk in the very elderly. Further studies are needed to validate the clinical implication of this simple test.
The major strength of this study includes the large number of patients ≥80 years in a general practice population. In addition, the patients had maintained their general intellect and activities of daily living without any signs of severe cognitive dysfunction or dementia. However, there are study limitations. First, we did not evaluate the patients’ global cognitive function. Second, we used an extremely simple test for evaluating WM function. Further studies are needed to investigate the association between exaggerated BPV and WM impairment assessed by other tests, such as the California Verbal Learning Test,48 Wechsler Memory Scale,49 and Gollin Figures Test,50 which have been confirmed to be valid and are widely used to assess cognitive function. Third, it is possible that the results of this study should not be extrapolated to individuals <80 years. Fourth, patients who had a pre‐existing stroke event were included in this study. Finally, we did not assess the changes of antihypertensive medications during the follow‐up period.
5. CONCLUSIONS
In very elderly patients (≥80 years), both short‐term and long‐term BP variability parameters were significantly associated with working memory impairment, and the patients with both exaggerated short‐term BP variability and exaggerated long‐term BP variability showed the highest relative risk of working memory impairment. The BP variability parameters could be a significant indicator of working memory impairment. In very elderly patients, we should evaluate not only BP levels but also their variability for the detection of working memory impairment at an early stage.
CONFLICTS OF INTEREST
None.
Supporting information
Fujiwara T, Hoshide S, Kanegae H, Eguchi K, Kario K. Exaggerated blood pressure variability is associated with memory impairment in very elderly patients. J Clin Hypertens. 2018;20:637‐644. 10.1111/jch.13231
REFERENCES
- 1. Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late‐life cognitive function. JAMA. 1995;274:1846‐1851. [PubMed] [Google Scholar]
- 2. Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment: a 20‐year follow‐up of 999 men. Hypertension. 1998;31:780‐786. [DOI] [PubMed] [Google Scholar]
- 3. Kivipelto M, Helkala E, Laakso MP, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. 2001;322:1447‐1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277‐281. [DOI] [PubMed] [Google Scholar]
- 5. Alonso A, Jacobs DR Jr, Menotti A, et al. Cardiovascular risk factors and dementia mortality: 40 years of follow‐up in the Seven Countries Study. J Neurol Sci. 2009;280:79‐83. [DOI] [PubMed] [Google Scholar]
- 6. Ninomiya T, Ohara T, Hirakawa Y, et al. Midlife and late‐life blood pressure and dementia in Japanese elderly: the Hisayama study. Hypertension. 2011;58:22‐28. [DOI] [PubMed] [Google Scholar]
- 7. Iadecola C, Yaffe K, Biller J, et al. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension. 2016;68:e67‐e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anderson C, Teo K, Gao P, et al. Renin‐angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol. 2011;10:43‐53. [DOI] [PubMed] [Google Scholar]
- 9. Peters R, Beckett N, Forette F, et al. Incident dementia and blood pressure lowering in the hypertension in the very elderly trial cognitive function assessment (HYVET‐COG): a double‐blind, placebo controlled trial. Lancet Neurol. 2008;7:683‐689. [DOI] [PubMed] [Google Scholar]
- 10. Sander D, Kukla C, Klingelhöfer J, Winbeck K, Conrad B. Relationship between circadian blood pressure patterns and progression of early carotid atherosclerosis: A 3‐year follow‐up study. Circulation. 2000;102:1536‐1541. [DOI] [PubMed] [Google Scholar]
- 11. Filomena J, Riba‐Llena I, Vinyoles E, et al. Short‐term blood pressure variability relates to the presence of subclinical brain small vessel disease in primary hypertension. Hypertension. 2015;66:634‐640; discussion 445. [DOI] [PubMed] [Google Scholar]
- 12. Pucci G, Battista F, Anastasio F, Schillaci G. Morning pressor surge, blood pressure variability, and arterial stiffness in essential hypertension. J Hypertens. 2017;35:272‐278. [DOI] [PubMed] [Google Scholar]
- 13. Satoh M, Hosaka M, Asayama K, et al. Association between N‐terminal pro B‐type natriuretic peptide and day‐to‐day blood pressure and heart rate variability in a general population: the Ohasama study. J Hypertens. 2015;33:1536‐1541. [DOI] [PubMed] [Google Scholar]
- 14. Yamaguchi Y, Wada M, Sato H, et al. Impact of ambulatory blood pressure variability on cerebral small vessel disease progression and cognitive decline in community‐based elderly Japanese. Am J Hypertens. 2014;27:1257‐1267. [DOI] [PubMed] [Google Scholar]
- 15. Kario K. Evidence and perspectives on the 24‐hour management of hypertension: hemodynamic biomarker‐initiated ‘anticipation medicine’ for zero cardiovascular event. Prog Cardiovasc Dis. 2016;59:262‐281. [DOI] [PubMed] [Google Scholar]
- 16. Kikuya M, Hozawa A, Ohokubo T, et al. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension. 2000;36:901‐906. [DOI] [PubMed] [Google Scholar]
- 17. Diaz KM, Tanner RM, Falzon L, et al. Visit‐to‐visit variability of blood pressure and cardiovascular disease and all‐cause mortality: a systematic review and meta‐analysis. Hypertension. 2014;64:965‐982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yano Y, Fujimoto S, Kramer H, et al. Long‐term blood pressure variability, new‐onset diabetes mellitus, and new‐onset chronic kidney disease in the Japanese general population. Hypertension. 2015;66:30‐36. [DOI] [PubMed] [Google Scholar]
- 19. Eto M, Toba K, Akishita M, Kozaki K, Watanabe T, Kim S, et al. Impact of blood pressure variability on cardiovascular events in elderly patients with hypertension. Hyperten Res. 2005;28:1‐7. [DOI] [PubMed] [Google Scholar]
- 20. Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta‐analysis. BMJ. 2016;354:i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gosmanova EO, Mikkelsen MK, Molnar MZ, et al. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. J Am Coll Cardiol. 2016;68:1375‐1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanemaru A, Kanemura K, Kuwajima I. The effects of short‐term blood pressure variability and nighttime blood pressure levels on cognitive function. Hypertens Res. 2001;24:19‐24. [DOI] [PubMed] [Google Scholar]
- 23. Sakakura K, Ishikawa J, Okuno M, Shimada K, Kario K. Exaggerated ambulatory blood pressure variability is associated with cognitive dysfunction in the very elderly and quality of life in the younger elderly. Am J Hypertens. 2007;20:720‐727. [DOI] [PubMed] [Google Scholar]
- 24. Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K. Visit‐to‐visit blood pressure variations: New independent determinants for cognitive function in the elderly at high risk of cardiovascular disease. J Hypertens. 2012;30:1556‐1563. [DOI] [PubMed] [Google Scholar]
- 25. Sabayan B, Wijsman LW, Foster‐Dingley JC, et al. Association of visit‐to‐visit variability in blood pressure with cognitive function in old age: prospective cohort study. BMJ. 2013;347:f4600. [DOI] [PubMed] [Google Scholar]
- 26. Yano Y, Ning H, Allen N, et al. Long‐term blood pressure variability throughout young adulthood and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Hypertension. 2014;64:983‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nagai M, Hoshide S, Nishikawa M, Masahisa S, Kario K. Visit‐to‐visit blood pressure variability in the elderly: associations with cognitive impairment and carotid artery remodeling. Atherosclerosis. 2014;233:19‐26. [DOI] [PubMed] [Google Scholar]
- 28. Böhm M, Schumacher H, Leong D, et al. Systolic blood pressure variation and mean heart rate is associated with cognitive dysfunction in patients with high cardiovascular risk. Hypertension. 2015;65:651‐661. [DOI] [PubMed] [Google Scholar]
- 29. Cowan N. What are the differences between long‐term, short‐term, and working memory? Prog Brain Res. 2008;169:323‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Japanese Society of Hypertension . Japanese Society of Hypertension guideline for the management of hypertension (JSH2004). Hypertens Res. 2006;29(Suppl):S1‐S105. [DOI] [PubMed] [Google Scholar]
- 32. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. [DOI] [PubMed] [Google Scholar]
- 33. Tombaugh TN, Mclntyre NJ. The mini‐mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40:922‐935. [DOI] [PubMed] [Google Scholar]
- 34. Parati G, Saul JP, Di Rienzo M, Mancia G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension. 1995;25:1276‐1286. [DOI] [PubMed] [Google Scholar]
- 35. Kotsis V, Stabouli S, Karafillis I, et al. Arterial stiffness and 24 h ambulatory blood pressure monitoring in young healthy volunteers: the early vascular ageing Aristotle University Thessaloniki Study (EVA‐ARIS Study). Atherosclerosis. 2011;219:194‐199. [DOI] [PubMed] [Google Scholar]
- 36. Gómez‐Marcos MA, Recio‐Rodríguez JI, Patino‐Alonso MC, et al. Ambulatory arterial stiffness indices and target organ damage in hypertension. BMC Cardiovasc Disord. 2012;12:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parati G, Ochoa JE, Lombardi V, Bilo G. Assessment and management of blood‐ressure variability. Nat Rev Cardiol. 2013;10:143‐155. [DOI] [PubMed] [Google Scholar]
- 38. Webb AJ, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive‐drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta‐analysis. Lancet. 2010;375:906‐915. [DOI] [PubMed] [Google Scholar]
- 39. Kario K. Orthostatic hypertension‐a new haemodynamic cardiovascular risk factor. Nat Rev Nephrol. 2013;9:726‐738. [DOI] [PubMed] [Google Scholar]
- 40. Buratti L, Cagnetti C, Balucani C, et al. Blood pressure variability and stroke outcome in patients with internal carotid artery occlusion. J Neurol Sci. 2014;339:164‐168. [DOI] [PubMed] [Google Scholar]
- 41. Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Blood pressure variability and clinical outcome in patients with acute intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2015;24:1493‐1499. [DOI] [PubMed] [Google Scholar]
- 42. Alrawi YA, Panerai RB, Myint PK, Potter JF. Pharmacological blood pressure lowering in the older hypertensive patients may lead to cognitive impairment by altering neurovascular coupling. Med Hypotheses. 2013;80:303‐307. [DOI] [PubMed] [Google Scholar]
- 43. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672‐2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kalaria RN. Cerebrovascular disease and mechanisms of cognitive impairment: evidence from clinicopathological studies in humans. Stroke. 2012;43:2526‐2534. [DOI] [PubMed] [Google Scholar]
- 45. Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension. 2013;62:810‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stubendorff K, Aarsland D, Minthon L, Londos E. The impact of autonomic dysfunction on survival in patients with dementia with Lewy bodies and Parkinson's disease with dementia. PLoS ONE. 2012;7:e45451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hoshide S, Ishikawa J, Eguchi K, Oowada T, Shimada K, Kario K. Cognitive dysfunction and physical disability are associated wth mortality in extremely elderly patients. Hypertens Res. 2008;31:1331‐1338. [DOI] [PubMed] [Google Scholar]
- 48. Woodard JL, Goldstein FC, Robert VJ, McGuire C. Convergent and discriminant validity of the CVLT (dementia version). California Verbal Learning Test. J Clin Exp Neuropsychol. 1999;21:553‐558. [DOI] [PubMed] [Google Scholar]
- 49. Wechsler D. Wechsler memory scale‐IV. Pearson. 2009. http://images.pearsonclinical.com/images/Assets/WMS-IV/WMSIV_2_06_8.pdf. Accessed December 1, 2017.
- 50. Foreman N, Hemmings R. The Gollin incomplete figures test: a flexible, computerized version. Perception. 1987;16:543‐548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


