Abstract
The presence of abdominal obesity and lack of physical activity are both risk factors for the development of hypertension. The aim of this study was to analyze the risk of developing hypertension according to baseline waist circumference (WC). In total, 16 312 476 non‐hypertensive participants who were covered by the National Health Insurance Service (NHIS) from 2009 to 2012 in Korea were included in the study. The participants were divided into six groups according to the level of baseline WC with a 5‐cm interval starting from 80 cm in men and 75 cm in women. The risk for the future development of hypertension was assessed in 2015 using the claims data on the diagnosis of hypertension and prescription of anti‐hypertensive medications. Approximately 7.8% of the participants developed hypertension over a median follow‐up of 5.48 years. The proportion of participants who developed hypertension significantly increased from 4.2% in the WC level 1% to 17.5% in the WC level 6. After adjusting for confounding factors, level 6 of the baseline WC had a higher hazard ratio (HR) for the development of hypertension among the 6 levels of baseline with level 3 as the reference (1736; 95% confidence interval [95% CI]: 1.72‐1.753). The participants with abdominal obesity had a significantly higher HR than those without abdominal obesity regardless of whether they engage in high‐ or moderate‐intensity physical intensity (1.741; 95% CI: 1.718‐1.764). WC had a linear association with the development of hypertension based on this large nationwide population‐based cohort study, which was not influenced by physical activity.
Keywords: abdominal obesity, hypertension, nationwide population‐based study
1. INTRODUCTION
The prevalence of obesity is significantly increasing worldwide, and one in two adults and nearly one in six children are overweight or obese in countries in the Organization for Economic Co‐operation and Development area.1 Because of its impact on cardiovascular and metabolic diseases, such as diabetes, coronary heart diseases, ischemic stroke, hypertension, and sleep apnea, obesity is becoming one of the most serious health problems that are threatening global health.2 Moreover, it is considered the most threatening non‐communicable disease in Asia due to the susceptibility of Asians to metabolic diseases in the same amount of fat.3
Abdominal obesity is a well‐known risk factor for the development of cardiometabolic diseases because it reflects insulin resistance and ectopic fat accumulation with the subsequent development of metabolic syndrome and diabetes.4 Among the components of metabolic syndrome, hypertension has diverse pathogenic mechanisms other than insulin resistance.5 However, it is obvious that insulin resistance caused by abdominal obesity is a significant pathological mechanism that causes the development of hypertension.6
Previous studies have suggested the association between obesity and the development of hypertension in various populations.7, 8, 9, 10 A recent study showed that the co‐existence of abdominal obesity and hypertension significantly affected cardiovascular hemodynamics.11 In a recent meta‐analysis of more than 2.3 million pooled participants in 57 prospective cohort studies, there was a onefold to twofold increased risk of developing hypertension with the increment of various obesity indices, such as body mass index (BMI), waist circumference (WC), waist‐to‐hip ratio, and waist‐to‐height ratio.9 Another study involving 10 265 non‐hypertensive Chinese adults who were followed up for 6 years reported a 1.3‐fold increased risk of developing hypertension in those with 5% WC gain from baseline.8 However, it is still controversial whether overall fatness as reflected by a high BMI or abdominal obesity that is assessed using WC, waist‐to‐hip ratio, or waist‐to‐height ratio would predict the development of hypertension better.
The effects of exercise on the development or prevention of hypertension are already well known.12, 13 Increasing long‐term physical activity is known to reduce visceral fat, increase insulin sensitivity, and prevent weight gain, thus preventing metabolic syndrome and diabetes.14 However, whether abdominal obesity alone would have a major impact on the development of hypertension or engaging in a physical activity of certain intensity would attenuate the effect of abdominal obesity on the development of hypertension is not known.
Therefore, in this study, we first analyzed the risk for the development of hypertension according to the six levels of baseline WC with a median follow‐up of 5.5 years in a large nationwide population‐based cohort of 16 312 476 Korean adults. Furthermore, we analyzed the risk of developing hypertension considering the baseline physical activity in these adults.
2. METHODS
2.1. National Health Insurance Service Database
Nearly all Koreans (97.2% of the Korean population, approximately 50 million) are covered by the National Health Insurance Service (NHIS), which is a nonprofit, single‐payer organization that is run by the Korean government. The NHIS keeps patients’ information, such as demographics, medical examination results, claims for the disease diagnosis codes of the International Classification of Diseases (ICD‐10), and treatment that can be used to produce a population‐based cohort.15 Insured Korean adults older than 40 years and employees older than 20 years undergo regular health checkups provided by the NHIS every 1 or 2 years. Data that were obtained from the Korean National Health Screening databases through these checkups include anthropometric measurements, responses to health questionnaires, and laboratory findings. Moreover, data obtained from these databases and the nationwide medical records were combined and analyzed to establish a cohort for the investigation of health problems after the NHIS approved the use of its database for the research (research number: NHIS‐2017‐1‐201).
Our study protocol was approved by the official review committee and the institutional review board of the Korea National Institute for Bioethics Policy (P01‐201603‐21‐005).
2.2. Anthropometric measurements and baseline characteristics
Body weight (kg) and height (cm) were measured using an electronic scale, and WC (cm) was measured at the middle point between the rib cage and iliac crest by trained examiners. BMI was calculated with body weight divided by height in meters squared (m2). BP measurement was taken according to the Korean Society of Hypertension guideline.16 In brief, BP was measured twice or more using a mercury or automatic sphygmomanometer in a sitting position after a minimum 5‐minute rest, after the anthropometric measurement. Blood was sampled after a minimum 8‐hour overnight fasting.
Diabetes was defined as fasting blood glucose level ≥126 mg/dL (≥7 mmol/L) or the presence of one or more claims per year for anti‐hyperglycemic medications with ICD‐10 code E10‐14. Dyslipidemia was defined as total cholesterol level ≥240 mg/dL (≥6.21 mmol/L) or the presence of one or more claims per year for anti‐hyperlipidemic medications with ICD‐10 code E78. The diagnoses of hypertension, optimal, high‐normal, and pre‐hypertension, were made according to the 2013 Korean Society of Hypertension guidelines.16
Data on socioeconomic characteristics, such as income level, and lifestyle factors, including smoking, alcohol drinking, and exercise, were obtained using standardized questionnaires. Low income level was defined as being in the lower one‐fifth of the whole population, and current smoker was defined as someone who replied yes to the question “Are you currently smoking?” Alcohol drinking was defined as drinking more than 30 g/d, and this definition was based on the predefined questionnaire in the national health examination.
Physical activity was also defined as engaging in a regular exercise (either of the next intensity): physical activity with high intensity for more than 20 minutes per session ≥3 days a week and physical activity with moderate intensity for more than 30 minutes per session ≥5 days a week. The high‐intensity physical activity is defined as a physical activity that causes extreme shortness of breath (eg, running, aerobic dance, bicycling in high speed, working in construction sites, carrying boxes through stairs), and moderate‐intensity physical activity is defined as a physical activity that causes substantial shortness of breath (eg, brisk walking, tennis, bicycling, carrying light boxes, cleaning). Those who replied yes to do the physical activity in high intensity would not answer yes to the questions asking for moderate‐intensity physical activity.
2.3. Study design and outcomes
Baseline enrollment was conducted for participants who underwent at least one national health screening examination within the enrollment period from 2009 to 2012 (n = 23 503 802). After vigorous exclusions, a total of 16 312 476 participants were enrolled from 2009 to 2012. The development of hypertension was investigated in 2015 with the time point of diagnosis using the claims records of NHIS with ICD‐10 code I10‐I15 and claims for anti‐hypertensive medications during the study period. The median follow‐up of incident hypertension was 2.76 (1.43‐4.21) years.
The WC measurements of the participants were divided into six levels with 5‐cm intervals: level 1: men <80 cm, women <75 cm; level 2: 80 ≤ men <85 cm, 75 ≤ women <80 cm; level 3: 85 ≤ men <90 cm, 80 ≤ women <85 cm; level 4: 90 ≤ men <95 cm, 85 ≤ women <90 cm; level 5: 95 ≤ men <100 cm, 90 ≤ women <95 cm; and level 6: men ≥100 cm, women ≥95 cm. The subgroup analyses for hypertension development in the presence or absence of various risk factors were performed according to the presence or absence of abdominal obesity by the definitions of Korean Society for the Study of Obesity (≤90 cm in men, ≤85 cm in women).17
2.4. Statistical analysis
Using the Cox proportional hazards model with 95% confidence interval (CI), HRs were assessed by analyzing the risk of developing hypertension according to the six groups of baseline WC. A multivariable adjustment for age, sex, current smoking, alcohol drinking, exercise, income level, and history of diabetes and hyperlipidemia was made. Categorical variables were analyzed using the chi‐square test, and continuous variables were analyzed using the one‐way analysis of variance (ANOVA). SAS version 9.3 (SAS Institute Inc, Cary, NC, USA) was used for all statistical analyses and subgroup analysis.
3. RESULTS
The exclusion criteria and the number of participants excluded from the study are shown in Figure 1. The participants with missing data on baseline characteristics and covariates (n = 125 699) or those who were younger than 20 years (n = 50 430) were excluded from the study. Furthermore, those who had one or more claims per year for anti‐hypertensive medications with ICD‐10 code I10‐I15 before the enrollment period (n = 5 243 431), and those with baseline systolic blood pressure (SBP) ≥140 mm Hg or diastolic blood pressure (DBP) ≥90 mm Hg at entry (n = 1 771 766) were further excluded from the study. In total, 16 312 476 participants were finally enrolled (Figure 1).
Figure 1.
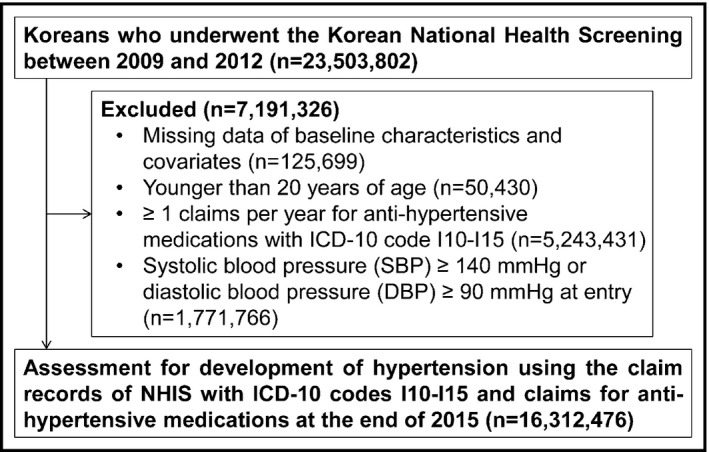
Selection of the study participants
The mean age of the participants was 43 years, and approximately 49.5% were men (Table 1). Median follow‐up time of the total study population was 5.48 years, and median follow‐up time of the participants who developed hypertension was 2.76 years. Over a median follow‐up of 5.48 years, approximately 7.8% of the participants developed hypertension, and the median time to the occurrence of the incident hypertension was 2.76 years. More than 93% of the study participants were younger than 65 years. The baseline SBP and DBP of the participants were 117 and 73 mm Hg, respectively, which are within normal range. The mean body mass index of the participants was 23.2 kg/m2, which shows that they are slightly overweight, and their mean WC was 78 cm. One‐fourth of the participants were in the obese range (Table 1).
Table 1.
Baseline characteristics of the participants according to the levels of waist circumference
| Total (N = 16 312 476) | Level 1 (N = 7 377 739) | Level 2 (N = 3 849 334) | Level 3 (N = 2 809 184) | Level 4 (N = 1 436 726) | Level 5 (N = 566 713) | Level 6 (N = 272 780) | P valuea | |
|---|---|---|---|---|---|---|---|---|
| Age (y) | 43.4 ± 12.8 | 40.4 ± 12.5 | 44.8 ± 12.3 | 46.6 ± 12.4 | 47.1 ± 12.6 | 46.9 ± 13.2 | 44.5 ± 13.6 | <0.0001 |
| <40 | 6 200 717 (38.0) | 3 460 565 (46.9) | 1 263 597 (32.8) | 797 780 (28.4) | 401 977 (28.0) | 171 270 (30.2) | 105 528 (38.7) | |
| 40‐64 | 9 095 567 (55.7) | 3 603 170 (48.8) | 2 338 464 (60.8) | 1 778 942 (63.3) | 897 298 (62.5) | 335 946 (59.3) | 141 747 (52.0) | |
| ≥65 | 1 016 192 (6.2) | 314 004 (4.3) | 247 273 (6.4) | 232 462 (8.3) | 137 451 (9.6) | 59 497 (10.5) | 25 505 (9.4) | |
| Sex: male (%) | 8 079 389 (49.5) | 2 819 090 (38.2) | 2 215 133 (57.6) | 1 684 071 (60.0) | 886 603 (61.7) | 329 560 (58.2) | 144 932 (53.1) | <0.0001 |
| Body weight (kg) | 62.7 ± 11.5 | 55.7 ± 7.7 | 64.1 ± 8.4 | 68.7 ± 9.3 | 73.5 ± 10.3 | 78.1 ± 11.4 | 85.7 ± 13.8 | <0.0001 |
| BMI (kg/m2) | 23.2 ± 3.1 | 21.0 ± 2.0 | 23.5 ± 1.9 | 25.0 ± 2.0 | 26.5 ± 2.2 | 28.1 ± 2.4 | 30.7 ± 3.2 | <0.0001 |
| Proportion of participants with body mass index ≥25 kg/m2 (%) | 4 202 597 (25.8) | 177 764 (2.4) | 766 634 (19.9) | 1 370 898 (48.8) | 1 103 611 (76.8) | 519 456 (91.7) | 264 234 (96.9) | <0.0001 |
| Waist circumference (cm) | 78.2 ± 8.9 | 70.6 ± 5.0 | 79.8 ± 2.9 | 84.8 ± 2.9 | 89.7 ± 2.8 | 94.5 ± 2.8 | 101.3 ± 4.6 | <0.0001 |
| Fasting blood glucose level (mg/dL) | 94.2 ± 19.5 | 91.0 ± 15.9 | 95.0 ± 19.7 | 97.2 ± 21.6 | 99.1 ± 23.3 | 100.5 ± 24.9 | 102.1 ± 27.6 | <0.0001 |
| Systolic blood pressure (mm Hg) | 117.0 ± 11.4 | 114.0 ± 11.4 | 118.0 ± 11.0 | 119.7 ± 10.7 | 121.0 ± 10.4 | 122.0 ± 10.2 | 123.1 ± 10.1 | <0.0001 |
| Diastolic blood pressure (mm Hg) | 73.0 ± 8.0 | 71.3 ± 8.1 | 73.6 ± 7.8 | 74.6 ± 7.6 | 75.5 ± 7.4 | 76.0 ± 7.3 | 76.8 ± 7.1 | <0.0001 |
| Total cholesterol level (mg/dL) | 193.0 ± 35.9 | 185.2 ± 33.5 | 195.6 ± 35.6 | 200.7 ± 36.4 | 203.8 ± 37.0 | 206.0 ± 37.7 | 207.4 ± 38.2 | <0.0001 |
| Current smoker (%) | 4 257 145 (26.1) | 1 638 000 (22.2) | 1 105 414 (28.7) | 821 275 (29.2) | 436 793 (30.4) | 171 181 (30.2) | 84 482 (31.0) | <0.0001 |
| Alcohol drinker (%) | 1 158 030 (7.1) | 368 530 (5.0) | 304 923 (7.9) | 253 207 (9.0) | 145 820 (10.2) | 58 190 (10.3) | 27 360 (10.0) | <0.0001 |
| Regular exercise (%) | 2 728 949 (16.7) | 1 145 302 (15.5) | 700 067 (18.2) | 503 887 (17.9) | 246 917 (17.2) | 92 087 (16.3) | 40 689 (14.9) | <0.0001 |
| Number of high‐intensity exercise/week | 0.91 ± 1.6 | 0.84 ± 1.5 | 0.99 ± 1.6 | 0.98 ± 1.6 | 0.95 ± 1.6 | 0.9 ± 1.6 | 0.83 ± 1.5 | <0.0001 |
| Proportion of participants who do high‐intensity exercise ≥3 times a week | 2 254 827 (13.8) | 934 327 (12.7) | 585 746 (15.2) | 420 539 (15.0) | 205 324 (14.3) | 75 785 (13.4) | 33 106 (12.1) | <0.0001 |
| Number of moderate‐intensity exercise/week | 1.12 ± 1.7 | 1.08 ± 1.7 | 1.18 ± 1.7 | 1.17 ± 1.7 | 1.13 ± 1.7 | 1.1 ± 1.7 | 1.05 ± 1.7 | <0.0001 |
| Proportion of participants who do moderate‐intensity exercise ≥5 times a week | 1 087 093 (6.7) | 464 770 (6.3) | 273 826 (7.1) | 197 103 (7.0) | 97 463 (6.8) | 37 115 (6.6) | 16 816 (6.2) | <0.0001 |
| Low income (%) | 3 406 679 (20.9) | 1 631 664 (22.1) | 766 133 (19.9) | 547 337 (19.5) | 282 746 (19.7) | 116 872 (20.6) | 61 927 (22.7) | <0.0001 |
| Proportion of blood pressure status at baseline (%)b | ||||||||
| Optimal | 11 591 229 (71.1) | 5 804 037 (78.7) | 2 654 366 (69.0) | 1 807 135 (64.3) | 862 252 (60.0) | 321 441 (56.7) | 141 998 (52.1) | <0.0001 |
| High‐normal | 13 348 012 (81.8) | 6 428 534 (87.1) | 3 104 104 (80.6) | 2 167 574 (77.2) | 1 061 701 (73.9) | 403 323 (71.2) | 182 776 (67.0) | <0.0001 |
| Pre‐hypertension | 6 160 496 (37.8) | 2 098 812 (28.5) | 1 554 716 (40.4) | 1 293 604 (46.1) | 736 021 (51.2) | 312 782 (55.2) | 164 561 (60.3) | <0.0001 |
| Proportion of participants who developed hypertension (%) | 1 265 644 (7.8) | 308 199 (4.2) | 313 296 (8.1) | 309 886 (11.0) | 195 996 (13.6) | 90 417 (16.0) | 47 850 (17.5) | <0.0001 |
| Proportion of participants with diabetes (%) | 757 278 (4.6) | 171 885 (2.3) | 186 016 (4.8) | 187 736 (6.7) | 121 898 (8.5) | 57 094 (10.1) | 32 649 (12.0) | <0.0001 |
| Proportion of participants with hyperlipidemia (%) | 2 109 335 (12.9) | 566 967 (7.7) | 538 886 (14.0) | 505 354 (18.0) | 300 539 (20.9) | 131 048 (23.1) | 66 541 (24.4) | <0.0001 |
Level 1: men <80 cm, women <75 cm; level 2: 80 ≤ men <85 cm, 75 ≤ women <80 cm; level 3: 85 ≤ men <90 cm, 80 ≤ women <85 cm; level 4: 90 ≤ men <95 cm, 85 ≤ women <90 cm; level 5: 95 ≤ men <100 cm, 90 ≤ women <95 cm; and level 6: men ≥100 cm, women ≥95 cm.
The significant differences in continuous variables among the six groups were analyzed with one‐way ANOVA test and those in categorical variables were analyzed by chi‐square test.
According to the 2013 Korean Society of Hypertension guidelines,16 optimal: <120/80 mm Hg, high‐normal: 120‐129/<80 mm Hg, and pre‐hypertension: 130‐139/80‐90 mm Hg.
When the baseline WC values of the participants were divided into six levels, approximately 14.0% of the total study population was classified within the range of abdominal obesity (men ≥90 cm, women ≥85 cm, and higher than level 4) according to the cutoff values for abdominal obesity in Koreans (Table 1).17 The proportion of participants who are older than 65 years significantly increased as the WC levels increased from 1 to 6. On the other hand, when the distribution of WC levels was analyzed according to age‐groups, those with a high level of WC increased, and those with a low level of WC decreased as the participants get older (Figure S1).
The mean BMI and proportion of participants within the obese range (BMI ≥25 kg/m2) linearly increased as the WC level increased from 1 to 6. Mean fasting blood glucose, SBP, and DBP linearly increased as the level of WC level increased from 1 to 6.
The proportions of participants who replied to perform regular exercise, high‐intensity exercise ≥3 times a week, and moderate‐intensity exercise ≥5 times a week were 16.7%, 13.8%, and 6.7% of the total study population (Table 1). Although there were statistically significant differences, there were no trends for any increase or decrease in the proportion or the mean exercise frequency among the six levels of WC (Table 1).
The proportion of participants who developed hypertension significantly increased from 4.2% in WC level 1% to 17.5% in WC level 6 (Table 1). The proportion of participants with optimal BP significantly decreased, and the proportion of participants significantly increased as the WC levels increased from 1 to 6 (Table 1).
The incidence rate of hypertension linearly increased as the WC level increased from 4 to 6, with level 1 participants having the lowest and level 6 participants having the highest incidence rate of hypertension among the six levels (Figure 2). The HRs for the development of hypertension were assessed according to the 6 levels of WC, and the WC measurements in level three participants (85 ≤ men <90 cm, 80 ≤ women <85 cm) were used as the reference range because WC ≥90 cm in men and ≥85 cm in women are the cutoff values of abdominal obesity in Koreans.17 The HRs gradually increased from level 1 to 6, with level 6 participants having the highest HR among the six groups (1736; 95% CI: 1.72‐1.753) (Table 2). The HRs in levels 1 and 2 were lower than 1.00, suggesting a reduced risk of developing hypertension in those with smaller WC measurements than level 3 (Table 2).
Figure 2.
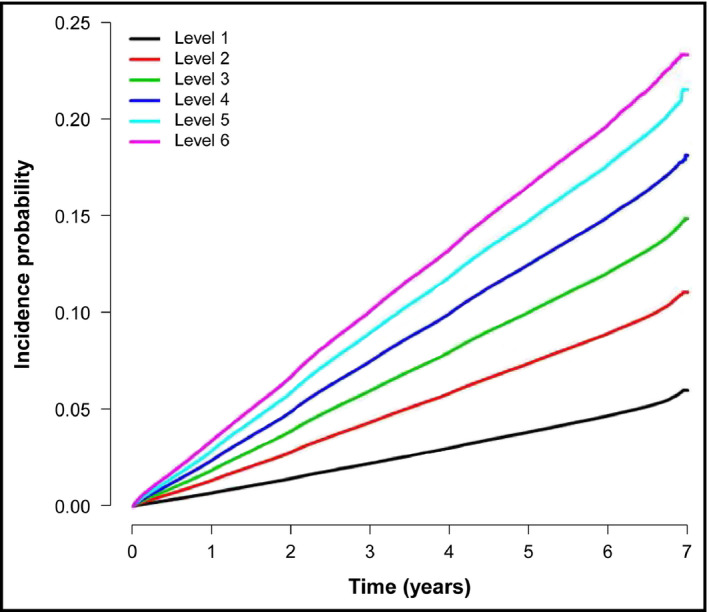
Incidence of hypertension according to the baseline levels of waist circumference
Table 2.
Hazard ratio (95% CI) for the development of hypertension according to the level of waist circumference
| Levels of WC | Number of participants | Number of participants who developed hypertension | IR (per 1000 person‐years) | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|---|---|---|
| Total | 1 | 7 377 739 | 308 199 | 7.9 | 0.519 (0.516, 0.521) | 0.52 (0.517, 0.522) | 0.546 (0.544, 0.549) |
| 2 | 3 849 334 | 313 296 | 15.6 | 0.798 (0.794, 0.802) | 0.799 (0.795, 0.803) | 0.815 (0.811, 0.819) | |
| 3 | 2 809 184 | 309 886 | 21.4 | 1 (ref.) | 1 (ref.) | 1 (ref.) | |
| 4 | 1 436 726 | 195 996 | 27.0 | 1.212 (1.206, 1.219) | 1.209 (1.203, 1.216) | 1.187 (1.18, 1.194) | |
| 5 | 566 713 | 90 417 | 32.3 | 1.46 (1.449, 1.471) | 1.453 (1.442, 1.463) | 1.401 (1.391, 1.411) | |
| 6 | 272 780 | 47 850 | 36.6 | 1.856 (1.838, 1.874) | 1.839 (1.821, 1.857) | 1.736 (1.72, 1.753) | |
| Men | 1 | 2 819 090 | 141 345 | 9.4 | 0.525 (0.521, 0.529) | 0.525 (0.521, 0.529) | 0.559 (0.555, 0.563) |
| 2 | 2 215 133 | 178 466 | 15.2 | 0.789 (0.784, 0.794) | 0.79 (0.785, 0.795) | 0.809 (0.804, 0.815) | |
| 3 | 1 684 071 | 178 347 | 20.4 | 1 (ref.) | 1 (ref.) | 1 (ref.) | |
| 4 | 886 603 | 115 494 | 25.5 | 1.231 (1.222, 1.24) | 1.226 (1.217, 1.236) | 1.2 (1.191, 1.209) | |
| 5 | 329 560 | 49 154 | 29.8 | 1.498 (1.483, 1.513) | 1.488 (1.473, 1.503) | 1.428 (1.414, 1.442) | |
| 6 | 144 932 | 23 990 | 34.0 | 1.97 (1.944, 1.997) | 1.947 (1.92, 1.973) | 1.825 (1.8, 1.85) | |
| Women | 1 | 4 558 649 | 166 854 | 7.0 | 0.531 (0.528, 0.535) | 0.532 (0.528, 0.536) | 0.55 (0.546, 0.554) |
| 2 | 1 634 201 | 134 830 | 16.0 | 0.818 (0.811, 0.824) | 0.818 (0.812, 0.824) | 0.83 (0.824, 0.836) | |
| 3 | 1 125 113 | 131 539 | 23.1 | 1 (ref.) | 1 (ref.) | 1 (ref.) | |
| 4 | 550 123 | 80 502 | 29.5 | 1.182 (1.172, 1.192) | 1.181 (1.171, 1.192) | 1.165 (1.155, 1.175) | |
| 5 | 237 153 | 41 263 | 35.9 | 1.4 (1.384, 1.415) | 1.397 (1.382, 1.412) | 1.359 (1.344, 1.374) | |
| 6 | 127 848 | 23 860 | 39.6 | 1.728 (1.704, 1.752) | 1.718 (1.695, 1.742) | 1.644 (1.621, 1.667) | |
Model 1: adjusted for age and sex; model 2: adjusted for age, sex, current smoking, alcohol drinking, exercise, and income level; and model 3: adjusted for age, sex, current smoking, alcohol drinking, exercise, income level, and history of diabetes and hyperlipidemia.
Level 1: men <80 cm, women <75 cm; level 2: 80 ≤ men <85 cm, 75 ≤ women <80 cm; level 3: 85 ≤ men <90 cm, 80 ≤ women <85 cm; level 4: 90 ≤ men <95 cm, 85 ≤ women <90 cm; level 5: 95 ≤ men <100 cm, 90 ≤ women <95 cm; and level 6: men ≥100 cm, women ≥95 cm.
CI, confidence interval; IR, incidence rate; WC, waist circumference.
When the analyses were performed separately based on sex, men had a numerically higher HR than women, (HR: 1.825; 95% CI: 1.8‐1.85 vs HR: 1.644; 95% CI: 1.621‐1.667) (Table 2). In similar analyses that involved various subgroups with a risk of incident hypertension according to the presence of abdominal obesity, results showed an increased risk of incident hypertension regardless of various risk factors (Figure S2). Similar results were seen after further adjustment for additional risk factors, such as baseline blood pressure as a continuous variable or glomerular filtration rate (Table S1).
When the HRs of the subgroups were obtained based on BMI, the same obesity group had a significantly increased risk of developing hypertension with increasing WC levels (Figure 3). However, those with a normal BMI (<23 kg/m2) were not at an increased risk of incident hypertension. In contrast, the participants with BMI in overweight status had a significantly increased risk of incident hypertension with increasing WC levels. Interestingly, those in the obese group with BMI ≥25 kg/m2, had a significantly increased risk of incident hypertension according to the increment of WC, particularly those with level 6 WC measurements (≥100 cm in men and ≥95 cm in women), showing 2.349‐fold increased risk of incident hypertension with level 3 WC (85 ≤ men <90 cm, 80 ≤ women <85 cm) as the reference group (Figure 3).
Figure 3.
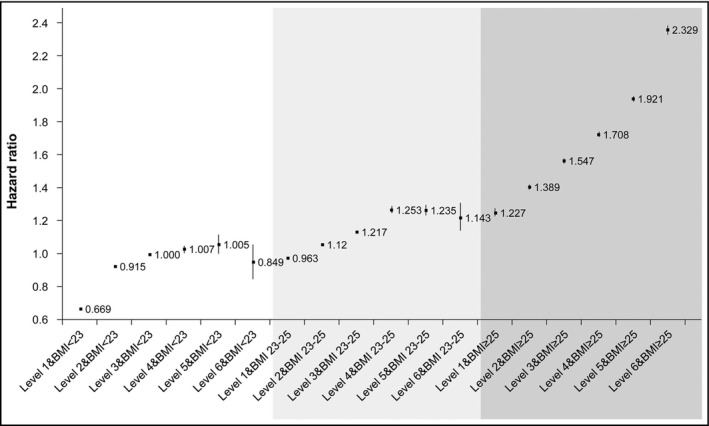
Hazard ratios for the development of hypertension according to obesity status and levels of waist circumference
When the HRs for the development of hypertension were analyzed with BMI included in the model, similar results were seen as other models (Table S1, model 4). However, when the analyses were performed in separate groups divided by BMI of 25 kg/m2, only those who were obese (BMI ≥25 kg/m2) showed significantly increased HRs for the development of hypertension as WC levels increased compared to the reference group (Table S2).
The HRs for the development of hypertension were analyzed in terms of the presence of abdominal obesity and lack of physical activity (Table 3). The participants with abdominal obesity (≥90 cm in men and ≥85 cm in women) and not engaged in any physical activity of high intensity had an HR of 1.74 (95% CI: 1.732 and 1.747), suggesting that these participants have a higher risk of developing hypertension than those without abdominal obesity and not engaged in any physical activity (Table 3). The participants without abdominal obesity who were performing high‐intensity physical activity showed a slightly increased risk of developing hypertension, with an HR of 1.043 (95% CI: 1.032 and 1.055). However, if these participants have abdominal obesity, the HR for incident hypertension will increase to 1.741 (95% CI: 1.718 and 1.764), suggesting a more significant correlation between the presence of abdominal obesity and the development of hypertension than that between engaging in a physical activity and the development of hypertension. Similar results were obtained in the analysis for participants performing moderate‐intensity physical activity (Table 3).
Table 3.
Hazard ratio (95% CI) for the development of hypertension according to abdominal obesity and physical activity
| Level of physical activitya | Abdominal obesityb | Number of participants who developed hypertension | Incidence rate (per 1000 person‐years) | Model 1c |
|---|---|---|---|---|
| High intensity | ||||
| No | No | 782 194 | 12.4 | 1 (Ref.) |
| Yes | 285 087 | 29.2 | 1.74 (1.732, 1.747) | |
| Yes | No | 148 426 | 14.5 | 1.043 (1.032, 1.055) |
| Yes | 48 917 | 30.9 | 1.741 (1.718, 1.764) | |
| Moderate intensity | ||||
| No | No | 852 032 | 12.5 | 1 (Ref.) |
| Yes | 308 371 | 29.1 | 1.738 (1.73, 1.745) | |
| Yes | No | 78 065 | 16.0 | 0.995 (0.986, 1.004) |
| Yes | 25 482 | 33.9 | 1.621 (1.599, 1.643) | |
CI, confidence interval.
High‐intensity physical activity was defined as vigorous exercise for more than 3 times/week; moderate‐intensity physical activity was defined as exercise with moderate intensity for more than 5 d/wk.
Abdominal obesity was defined as waist circumference ≥90 cm in men and ≥85 cm in women.
Adjusted for age, sex, current smoking, alcohol drinking, exercise, income level, and history of diabetes and hyperlipidemia.
4. DISCUSSION
The present study included a large nationwide population‐based cohort of 16 312 476 Korean adults, and their baseline WC showed a linear association with the development of hypertension in a median follow‐up period of 5.48 years. When analyzed separately based on sex, the association between WC and the risk for hypertension was more significant in men than in women. In addition, an increased risk of incident hypertension according to increasing WC was more evident in those who are overweight and obese than those with normal BMI, suggesting an association between an increased risk of incident hypertension and higher BMI. Furthermore, the association between an increased risk of incident hypertension and increased WC was consistently significant even when physical activity was used in the analyses. Therefore, we could assume that the presence of abdominal obesity was stronger than the protective effect of physical activity on the development of hypertension.
Although numerous studies and even meta‐analysis have already been published, there are still controversies regarding which obesity index can be used as a predictor for the development of hypertension superior to one another. In a recent systemic review and dose‐response meta‐analysis of more than 2.3 million participants in 57 prospective cohort study, 1.49‐fold and 1.27‐fold increased risks for the development of hypertension were noted for every 5‐unit increment in BMI and 10‐cm increment in WC, respectively.9 In this study, the risk for hypertension increased continuously with increasing anthropometric measurements, along with weight gain, suggesting that maintaining a normal BMI prevents hypertension. In another study by Zhao et al, which involved 10 265 non‐hypertensive Chinese individuals, those who gained >5% WC had 1.34‐fold increased risk of incident hypertension than those who only gained −2.5%‐2.5% WC over 6 years.8 In our study, increasing WC with a 5‐cm interval significantly increased the risk of incident hypertension in a median follow‐up of 5 years. In addition, the risk of incident hypertension was more evident in those with a higher BMI (overweight and obese), which is consistent with a previous study by Jayedi et al.9 Therefore, staying lean and controlling abdominal obesity might be the best strategies for preventing the development of hypertension.
The association between abdominal obesity and the development of hypertension is not attributed to one single mechanism. Adiposity is considered as the main cause of several cardiometabolic conditions, particularly diseases related to insulin resistance, such as diabetes and metabolic syndrome.18 Recent studies have focused on the depot‐specific role of adipose tissues in the development of metabolic diseases. That is, visceral fats play a deleterious role, whereas subcutaneous fats play a protective role in the development of metabolic diseases.19, 20 Since WC reflects the accumulation of visceral fats, which is the representative depot for ectopic fat accumulation, increased WC indicates ectopic accumulation caused by insulin resistance and excessive lipolysis.21 Insulin resistance is one of the mechanisms that cause hypertension in metabolic syndrome.22 Other studies have reported an increased systemic inflammation and the activation of the sympathetic nervous system caused by inflamed adipose tissues, which could be important factors for the development of hypertension.5 Furthermore, hyperactivity of the renin‐angiotensin‐aldosterone system in obese individuals can cause hypertension.23 The linear association between increased WC and the risk of incident hypertension is attributed to insulin resistance and subsequent increases in systemic inflammation and the activation of the sympathetic nervous system.
In our study, we aimed at analyzing whether physical activity could attenuate the association between abdominal obesity and hypertension. Studies on this triangular relationship are limited. In a systemic review on the association between exercise training and blood pressure, which involved 5223 participants, endurance, dynamic resistance, and isometric resistance trainings lowered both SBP and DBP, whereas combined training only lowered DBP.12 However, the association between exercise training and the development of hypertension as well as that between abdominal obesity and the development of hypertension was not analyzed. In a study by Sosner et al, engaging in lifestyle programs for 9 months, which include high‐intensity interval trainings, improved the BP of 115 individuals with abdominal obesity.24 However, whether exercise alone could affect abdominal obesity or blood pressure was not validated because lifestyle factors were not adjusted in the program. Similarly, in the previous studies that analyzed the association between baseline waist circumference and hypertension risk, adjustment for physical activity did not affect the association between abdominal obesity and hypertension.8, 25, 26 In our study, we observed that, even though this was not an intervention study, baseline physical activity did not affect the deleterious effects of abdominal obesity on incident hypertension. Thus, in preventing hypertension, prompt treatment of abdominal obesity is more important than solely being physically active. However, being physically active could help getting rid of abdominal obesity in the long term.
In our study, although the statistical comparison of HRs in different sex was not possible, men had numerically higher HRs for incident hypertension than women. In a study by Luz et al, which involved elderly populations in Brazil, all obesity indicators were associated with hypertension in women, with WC having the strongest association among all the parameters, including BMI.7 In contrast, WC was not associated with hypertension in men. The authors attributed this difference to the amount of body fat and hormonal changes because women go through menopause. However, the participants in the previous study were in their 70s, which is past the menopausal age. Therefore, the explanation cannot be applied in our study. Women lose more muscle than men as they get older, and they also easily gain more body fat than men.27 However, before menopause, estrogen can protect women from metabolic diseases caused by abdominal obesity.28 The mean age of the participants in our study is 43 years, which is relatively younger than that of a previous study. Therefore, in this age population, abdominal obesity would affect the development of metabolic diseases more in men than in premenopausal women.
Our study has limitations. First, only participants from a single ethnicity were included. Therefore, the results are only applicable to the population in our study. Second, only abdominal obesity measured using WC was examined, which did not include the use of tools, such as DEXA or fat computed tomography, for accurately measuring body fat. However, WC is an extremely simple and affordable method for the measurement of abdominal obesity, and it could be easily used in clinical practice if there is a small interobserver variation. Third, the amount and quality of physical activity were only assessed using self‐administered questionnaires, not with more specific tools, because this study was analyzed based on a health screening examination. However, numerous studies on exercise or physical activity were conducted, which used self‐administered questionnaires to assess the amount and quality of exercise.29, 30 Therefore, this might not be the limitations. However, important limitation is that the information on physical activity was obtained only at entry of the study, and no information is available during the follow‐up period. Therefore, the possibility for influence of any change in the amount of physical activity during the follow‐up period on the development of hypertension cannot be assessed. Fourth, much information on diet, such as sodium or calorie intake, and family history of hypertension could not be included in the analyses. Sodium intake and calorie intake are well‐known factors to affect the development of hypertension in various ethnic groups.31 Fifth, our study used only BP values at entry of the study for exclusion of participants with hypertension at baseline; no follow‐up BP values were available. Therefore, those who have high BP but not taking anti‐hypertensive medication could not be included in the study. There are possibilities that any changes in other parameters, such as WC or BMI, during the observational period could have affected the results. Sixth, as this is an observational cohort study, our study results cannot provide definitive cause‐and‐effect associations. Lastly, those with secondary hypertension could not be excluded from the analyses, as the database does not include more specific laboratory data on rare diseases. For all these limitations, our study has strength in that this is the largest study in the literature that analyzed the association between abdominal obesity and incident hypertension in relation to physical activity.
5. CONCLUSIONS
In conclusion, WC had a linear association with the risk of incident hypertension in adult participants with abdominal obesity, and a significant association was obtained after adjusting for confounding factors in 16 312 476 Koreans during a median follow‐up of 5.48 years. The linear association between WC and the risk of incident hypertension was more evident in participants who were overweight and obese than those with normal weight, as assessed using their BMI. Interestingly, the increased risk of incident hypertension in participants with abdominal obesity was not affected by physical activity. Long‐term physical activity and subsequent reduction in WC might prevent and treat the deleterious components of metabolic syndrome, such as hypertension.
CONFLICT OF INTEREST
The authors report no conflict of interests to disclose.
AUTHOR CONTRIBUTIONS
ER wrote the manuscript and researched data. WL and SY designed the study and commented on the manuscript. JJ, KH, and YP performed the statistical analyses. JC, HK, SP, HP, and YK researched data and commented on the manuscript.
Supporting information
Rhee E‐J, Cho J‐H, Kwon H, et al. Association between abdominal obesity and increased risk for the development of hypertension regardless of physical activity: A nationwide population‐based study. J Clin Hypertens. 2018;20:1417–1426. 10.1111/jch.13389
Yoo and Lee contributed equally to the work reported; therefore, they should be considered as the co‐corresponding authors.
Funding information
This study was supported by a grant of the Korean Health Technology R&D project, Ministry of Health and Welfare, Republic of Korea (HC16C2285).
Contributor Information
Soon‐Jib Yoo, Email: sjyoo@catholic.ac.kr.
Won‐Young Lee, Email: drlwy@hanmail.net.
REFERENCES
- 1.Available at https://www.oecd.org/health/health-systems/Obesity-Update-2017.pdf. Accessed February 17, 2018.
- 2. An R, Ji M, Zhang S. Global warming and obesity: a systematic review. Obes Rev. 2018;19:150‐163. [DOI] [PubMed] [Google Scholar]
- 3. Ramachandran A, Chamukuttan S, Shetty SA, Arun N, Susairaj P. Obesity in Asia–is it different from rest of the world. Diabetes Metab Res Rev. 2012;28(suppl 2):47‐51. [DOI] [PubMed] [Google Scholar]
- 4. Lim S. Ectopic fat assessment focusing on cardiometabolic and renal risk. Endocrinol Metab (Seoul). 2014;29:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Landsberg L, Aronne LJ, Beilin LJ, et al. Obesity‐related hypertension: pathogenesis, cardiovascular risk, and treatment – a position paper of the Obesity Society and The American Society of Hypertension. Obesity (Silver Spring). 2013;21:8‐24. [DOI] [PubMed] [Google Scholar]
- 6. Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clin North Am. 2014;43:1‐23. [DOI] [PubMed] [Google Scholar]
- 7. Luz RH, Barbosa AR, d'Orsi E. Waist circumference, body mass index and waist‐height ratio: are two indices better than one for identifying hypertension risk in older adults? Prev Med. 2016;93:76‐81. [DOI] [PubMed] [Google Scholar]
- 8. Zhao Y, Zhang M, Luo X, et al. Association of 6‐year waist circumference gain and incident hypertension. Heart. 2017;103:1347‐1352. [DOI] [PubMed] [Google Scholar]
- 9. Jayedi A, Rashidy‐Pour A, Khorshidi M, Shab‐Bidar S. Body mass index, abdominal adiposity, weight gain and risk of developing hypertension: a systematic review and dose‐response meta‐analysis of more than 2.3 million participants. Obes Rev. 2018; 19(5):654‐667. [DOI] [PubMed] [Google Scholar]
- 10. Seo DC, Choe S, Torabi MR. Is waist circumference ≥102/88 cm better than body mass index ≥30 to predict hypertension and diabetes development regardless of gender, age group, and race/ethnicity? Meta‐analysis. Prev Med. 2017;97:100‐108. [DOI] [PubMed] [Google Scholar]
- 11. Krzesiński P, Stańczyk A, Piotrowicz K, Gielerak G, Uziębło‐Zyczkowska B, Skrobowski A. Abdominal obesity and hypertension: a double burden to the heart. Hypertens Res. 2016;39:349‐355. [DOI] [PubMed] [Google Scholar]
- 12. Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta‐analysis. J Am Heart Assoc. 2013;2:e004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lund Rasmussen C, Nielsen L, Linander Henriksen M, et al. Acute effect on ambulatory blood pressure from aerobic exercise: a randomised cross‐over study among female cleaners. Eur J Appl Physiol. 2018;118:331‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Church T. Exercise in obesity, metabolic syndrome, and diabetes. Prog Cardiovasc Dis. 2011;53:412‐418. [DOI] [PubMed] [Google Scholar]
- 15. Song SO, Jung CH, Song YD, et al. Background and data configuration process of a nationwide population‐based study using the Korean National Health Insurance System. Diabetes Metab J. 2014;38:395‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shin J, Park JB, Kim KI et al. Guideline Committee of the Korean Society of Hypertension. 2013 Korean Society of Hypertension guidelines for the management of hypertension: part I‐epidemiology and diagnosis of hypertension. Clin Hypertens. 2015;21:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim MK, Lee WY, Kang JH et al. Committee of Clinical Practice Guidelines; Korean Society for the Study of Obesity. 2014 clinical practice guidelines for overweight and obesity in Korea. Endocrinol Metab (Seoul). 2014;29:405–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2456. [DOI] [PubMed] [Google Scholar]
- 19. Wajchenberg BL, Giannella‐Neto D, da Silva ME, Santos RF. Depot‐specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res. 2002;34:616–621. [DOI] [PubMed] [Google Scholar]
- 20. Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith U. Abdominal obesity: a marker of ectopic fat accumulation. J Clin Invest. 2015;125:1790–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park SE, Rhee EJ, Park CY, et al. Impact of hyperinsulinemia on the development of hypertension in normotensive, nondiabetic adults: a 4‐year follow‐up study. Metabolism. 2013;62:532–538. [DOI] [PubMed] [Google Scholar]
- 23. Kamide K. Role of renin‐angiotensin‐aldosterone system in metabolic syndrome and obesity‐related hypertension. Curr Hypertens Rev. 2014;9:238‐245. [PubMed] [Google Scholar]
- 24. Sosner P, Bosquet L, Herpin D, et al. Net blood pressure reduction following 9 months of lifestyle and high‐intensity interval training intervention in individuals with abdominal obesity. J Clin Hypertens (Greenwich). 2016;18:1128–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen X, Liu Y, Sun X, et al. Comparison of body mass index, waist circumference, conicity index, and waist‐to‐height ratio for predicting incidence of hypertension: the rural Chinese cohort study. J Hum Hypertens. 2018;32:228–235. [DOI] [PubMed] [Google Scholar]
- 26. Roka R, Michimi A, Macy G. Associations between hypertension and body mass index and waist circumference in U.S. adults: a comparative analysis by gender. High Blood Press Cardiovasc Prev. 2015;22:265–273. [DOI] [PubMed] [Google Scholar]
- 27. Kim SK, Kwon YH, Cho JH, et al. Changes in body composition according to age and sex among young non‐diabetic Korean adults: the Kangbuk Samsung Health Study. Endocrinol Metab (Seoul). 2017;32:442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond). 2008;32:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu X, Bastian K, Ohinmaa A, Veugelers P. Influence of physical activity, sedentary behavior, and diet quality in childhood on the incidence of internalizing and externalizing disorders during adolescence: a population‐based cohort study. Ann Epidemiol. 2018;28:86–94. [DOI] [PubMed] [Google Scholar]
- 30. Calvert M, Kyte D, Mercieca‐Bebber R, et al. Guidelines for Inclusion of Patient‐Reported Outcomes in Clinical Trial Protocols: The SPIRIT‐PRO Extension. JAMA. 2018;319:483–494. [DOI] [PubMed] [Google Scholar]
- 31. Murtaugh MA, Beasley JM, Appel LJ, et al. Relationship of sodium intake and blood pressure varies with energy intake: secondary analysis of the DASH (Dietary Approaches to Stop Hypertension)‐sodium trial. Hypertension. 2018;71:858–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


