Abstract
Few data from sub‐Saharan Africa exist on the effects of hypertension on the organs of the human body. We aimed to establish the prevalence of hypertensive end organ damage (EOD) in an elderly cohort of Tanzanians. The population aged 70 years and over of 2 villages in northern Tanzania (n = 246), had blood pressure (BP) data available from 2010 and 2013, and underwent in‐depth follow‐up for markers of hypertensive EOD in 2016. Assessment included ankle‐brachial pressure index, lying‐standing BP, electrocardiogram, and mid‐stream urine dip. Sustained hypertension (those with hypertension at all 3 assessments) was found in 129 (52.4% subjects). Of the entire cohort, 13.9% had left ventricular hypertrophy and 26.4% had peripheral arterial disease, both of which were associated with sustained hypertension, although orthostatic hypotension, stroke, proteinuria, and arterial stiffening were not. Further investigation, particularly in younger age groups, is merited if hypertension‐associated morbidity is to be controlled.
Keywords: end organ damage, hypertension, low‐ and middle‐income countries, sub‐Saharan Africa, Tanzania
1. INTRODUCTION
1.1. Hypertension in Africa
Globally, hypertension is associated with 9.4 million deaths each year.1 In 2010, hypertension was identified as the single greatest risk factor for death and disability.1 In 2000, 26.4% of the adult population globally had hypertension and this figure is estimated to increase to 29.2% by 2025.2 Until recently, it was considered that conditions such as hypertension and diabetes were diseases of the developed world, and the focus in developing countries remained on infectious diseases such as HIV and malaria. However, in a recent review of hypertension in Africa, Adeloye and Basquill3 reported that the pooled prevalence of hypertension in adults in Africa had risen from 19.7% in 1990 to 27.4% in 2000 and 30.8% in 2010. The number of cases in 2030 was projected to be 216.8 million. A growing and ageing population, urbanization, and increased uptake of a more western diet and lifestyle are all factors that are thought to have contributed to this rise.3
1.2. Hypertension and end organ damage
Over time, chronic, sustained hypertension can result in a process of vascular remodelling whereby hypertrophy and hyperplasia of vascular smooth muscle and fibrosis of vessel walls leads to reduced blood flow and under‐perfusion of organ tissue, resulting in organ damage. Commonly affected organs are the brain (increased stroke risk, cognitive decline), heart (left ventricular hypertrophy [LVH]), kidneys (renal impairment), eyes (hypertensive retinopathy), and extremities (peripheral arterial disease [PAD] and arterial stiffening).4 The combination of these effects is often referred to as end organ damage (EOD).
Given the increased potential for late diagnosis and delayed treatment and the influence of genetic and lifestyle factors, patterns of EOD are likely to vary substantially in sub‐Saharan Africa (SSA) compared to those seen in high‐income countries. However, very few studies have been published on the prevalence and patterns of hypertensive EOD in SSA. The most substantial evidence base comes from Nigeria, where LVH, renal impairment, and cerebrovascular disease are consistently reported as the most common forms of EOD. In a tertiary hospital in Nigeria in 2007, Ayodele et al5 reported high rates of EOD amongst 147 newly diagnosed hypertensives (mean age: 56 years). Most common were LVH (42.2%) and chronic kidney disease (23.8%). Cerebrovascular disease (10.9%), symptomatic heart failure (8.8%), advanced retinopathy (2.7%), and transient ischaemic attack (1.4%) was also reported. Busari et al reported similar results when he screened 96 hypertensives and age‐matched controls that were attending a clinic in Nigeria. Significantly higher rates of microalbuminuria (a known marker for vascular damage) were noted in hypertensives compared to controls (32.3% and 6.3%, respectively).6 In 2014, Nelissen et al7 published data from screening of 375 rural‐dwelling Nigerians, 52% of whom were hypertensive. The prevalence of some form of EOD was 32% amongst the hypertensives, with 15.9% having LVH, 10.8% having had myocardial infarction, and 6.6% having impaired glomerular filtration rate (GFR). This was in comparison to 15.4% of non‐hypertensives with EOD. Whether the individuals were treated for hypertension or not made no significant difference to rates of EOD. However, the age differences between hypertensives and non‐hypertensives were substantial, with the majority of hypertensives aged over 65 years and the majority of normotensives aged 18‐44 years. These results support those of Oladapo et al8 who investigated 415 hypertensives living in rural Nigeria (mean age: 46.9 years), of whom 27.9% had LVH, 15.2% had evidence of renal impairment (proteinuria), 6.3% had history of stroke, and 3.6% had a previous diagnosis of PAD. No comparison was made with normotensives. In the Democratic Republic of Congo, Kabedi et al9 found high rates of EOD amongst hypertensives (53.6% had LVH, 31.8% had chronic kidney disease and 17.6% had a previous stroke), supporting the findings from Nigeria. Studies in Ethiopia and Ghana have also been conducted, with renal impairment, stroke, and LVH common in hypertensives.10, 11, 12
However, the majority of studies are carried out in outpatient clinics and large proportions of the population of SSA do not have access to such facilities and so the cohorts are likely to be unrepresentative of the background population. As organizations such as the World Health Organization push for early intervention to prevent the growth of the global endemic of non‐communicable disease, it is important that we develop a thorough understanding of the impact of hypertension on populations in low‐income settings.
1.3. Aims
We aimed to establish the effect of hypertension at the organ level by measuring the prevalence of EOD in a cohort of community‐dwelling older adults living in rural Tanzania.
2. METHODS
Ethical approval for baseline assessment and follow‐up was obtained from the National Institute of Medical Research (NIMR), Dar‐es‐Salaam, Tanzania. Informed written consent was obtained from all participants. Participants gave a signature, or a thumbprint if they could not read and write. For those who were not able to consent due to cognitive impairment, poor hearing, or poor vision, assent was gained from a close relative.
2.1. Timing, setting, and study population
This was a follow‐up study of a sub‐sample of a cohort of 2232 people aged 70 years and over who were seen at baseline in 2009/10, and also in 2012/13. Data from the baseline assessments and the 2013 follow‐up have been published.13, 14 The 6‐year follow‐up data were collected between 29th February and 13th May 2016. The baseline cohort was recruited from 12 randomly selected villages within a Demographic Surveillance Site (DSS) in the rural Hai District of Northern Tanzania. The economy of the area is largely based on agriculture and this is usually at a subsistence level. Some crops, such as coffee, tomatoes, and fruit are grown for sale in more fertile areas. Based on census data from June 2009, at baseline assessment, 8869 people aged ≥ 70 years old lived within the 52 villages in the DSS, accounting for 5.5% of the total population.13
For this 6‐year follow‐up, data were collected from 2 of the villages, randomly selected from the original 12. Due to resource limitations and time constraints, not all the participants in both villages could be followed up. All baseline participants were followed up from one of the villages and approximately 50% of baseline participants from the second village. In the second village, a statistician randomized participants into blocks of 30 (WKG). Half of the randomised blocks were followed up as a way of minimising selection bias.
2.2. Assessments
We used local village enumerators to identify the participants to be seen. Assessments were conducted either at the local health clinic, or at the participant's home depending on their health and ability to travel. Any participant who failed to attend clinic after 3 invitations, or who refused to be seen at home, was considered to have refused participation. For those participants who were found to have died since the previous follow‐up, mortality data were sought from the village enumerators.
Two UK medical students (HP and RJ), enumerators, and an experienced translator collected data. All interviews were conducted in Swahili or the local tribal language (Chagga) and translated into English by the translator. The same translator was used for every interview. Given the rural setting of this study, invasive procedures and procedures requiring non‐portable technical equipment were not possible.
2.2.1. Demographics, lifestyle, and self‐reported health status
Demographic data were collected. Assessment of alcoholic unit intake can be difficult in this region. The most common alcoholic drink is “mbege,” a local beer brewed from bananas using homemade equipment. As such, its strength varies enormously. Therefore drinking status was recorded simply as current (within the last 12 months), previous or never. Since the strength of cigarettes also varies depending on the source of tobacco, the same categorisation was used for tobacco smoking. The interview also involved a brief medical history, including history of medication use and current and past medical problems. Patients were asked directly if they had noticed any recent problems with their vision, breathing or any chest pain.
2.2.2. Seated blood pressure measurement
Blood pressure (BP) was measured in accordance with the WHO STEPwise Approach to Surveillance (STEPS) protocol.15 After 5 minutes of sitting quietly, BP was recorded from the supported right arm, in the seated position with an appropriately sized cuff. Three measurements were taken, with 1 minute between measurements. An average was taken of the second and third measurements and this was recorded as the patient's BP. In cases where there was a marked discrepancy between the second and third readings (> 20 mm Hg systolic or > 10 mm Hg diastolic) further measurements were taken until 2 consistent readings were available. All BP readings were taken using a calibrated A&D Medical UA‐1020 Digital BP Monitor. Hypertension was defined in alignment with current UK National Institute for Health and Care Excellence (NICE) guidelines as average systolic BP ≥ 140 mm Hg, average diastolic BP ≥ 90 mm Hg or currently taking antihypertensive medication.16 An additional cutoff for grade II hypertension or above (average systolic BP ≥ 160 mm Hg, average diastolic BP ≥ 100 mm Hg) was also used.
2.2.3. Ankle‐brachial pressure index (ABPI)
ABPI measurement was carried out with the patient in a comfortable lying position, and having rested for 5 minutes. Brachial systolic pressure was measured using a NCD CE 0120 medical professional aneroid sphygmomanometer with an appropriately sized cuff and a Diaped Flux BT200 vascular Doppler machine. This was repeated on the opposite arm and on both ankles, taking Doppler readings from both the posterior tibialis and dorsalis pedis pulses. The ABPI was calculated for each ankle by dividing the highest brachial pressure by the highest of the 2 ankle pressures on that side. An ABPI of ≤ 0.9 in either limb was considered to be indicative of PAD, in alignment with NICE guidelines, and a score of ≥ 1.3 in either limb was considered suggestive of arterial stiffening. In a literature review it was concluded that, as a diagnostic measure of PAD, an ABPI of 0.90 or less was highly specific (83% to 99%), but sensitivity varied hugely (15% to 79%).17 Sensitivity was noted to be lower in older individuals. One proposed explanation for the reduced sensitivity in elderly people is that higher rates of arterial wall calcification can lead to an overestimation of arterial pressure.
2.2.4. Lying to standing blood pressure measurement
Whilst lying down, a BP reading was taken from the right arm using the same automated BP monitor. The participant was then asked to stand as quickly as they safely could, with support or assistance if needed, and further BP measurements were recorded at 30 seconds, 1, 2, and 3 minutes after standing. In some circumstances, due to the relative immobility of the participant, some readings were missed. In those unable to stand, a sitting BP was recorded in the same fashion where possible. Orthostatic hypotension was defined using lying‐standing BP as a drop in systolic BP of ≥ 20 mm Hg or in diastolic BP of ≥ 10 mm Hg.
2.2.5. Electrocardiogram (ECG)
Resting 12‐lead ECGs were recorded using a GE MAC 1200™ machine LVH was defined using the Sokolow‐Lyon criteria as the sum of the amplitude of the S wave in V1 and the larger of the R waves in either V5 or V6 being greater than 35 mV.18 The diagnosis of LVH on ECG using the Sokolow‐Lyon criteria has been shown to be highly specific (> 90%), although sensitivity is moderate to poor (range 20%‐60%).19
2.2.6. Urine sample analysis
All participants were asked to produce a midstream urine sample, which was analysed for protein using Siemens Multistix 10 SG urine dip strips as soon after the sample was taken as possible. Proteinuria was defined as a protein finding on urine dip of trace or higher in the absence of fever or other markers of urinary tract infection (ie, raised nitrites and leukocytes).
2.2.7. Cognition
Cognition was assessed using the IDEA cognitive screen. The screen has 6 items and takes 10‐15 minutes to administer. It has been validated for use in this setting.20, 21 A score of ≤ 7 is the recognised cutoff for probable dementia.
2.3. Statistical analysis
Data analysis was supported by SPSS for windows version 21. Data were summarised using standard descriptive statistics (eg, frequency, mean, median) as appropriate. When comparing 2 groups, statistical significance was assessed using Chi‐square tests for categorical variables and Mann‐Whitley U test for continuous variables, none of which were suitable for parametric analysis. For the purposes of this study, sustained hypertension was defined as having hypertension at grade I or above at all 3 assessments (2010, 2013, and 2016). An additional cutoff of hypertension at grade II or above at all 3 assessments was used in secondary analysis to investigate the role of moderate/severe hypertension compared to mild/no‐hypertension. Data were missing for 15 BP recordings in 2013. Only 1 person had missing BP data in 2016 and they also had missing data in 2013. In these cases data were imputed using the value from the previous recording. Using this technique, 8 of 15 subjects were identified as sustained hypertensives.
Binary logistic regression analysis was used to assess the influence of potentially confounding variables on the role of sustained hypertension in EOD. All markers for EOD that were associated with sustained hypertension at either of the 2 hypertension cutoffs at the 10% significance level were investigated. Five‐year age band, sex, educational level (any formal education, no formal education), smoking status (current, former, never), alcohol consumption (current, former, never), and weight were forced into the model as covariates. Statistical significance was set at 5%, unless stated otherwise, and two‐sided tests used throughout.
3. RESULTS
In the 2 villages, of the 663 people assessed at baseline in 2010, 82 were known to have died by March 2013 and so 581 were potentially available for follow‐up in 2016, of whom 412 were randomised for follow‐up and 246 were assessed. The study recruitment process is summarised in the Figure.
Figure 1.
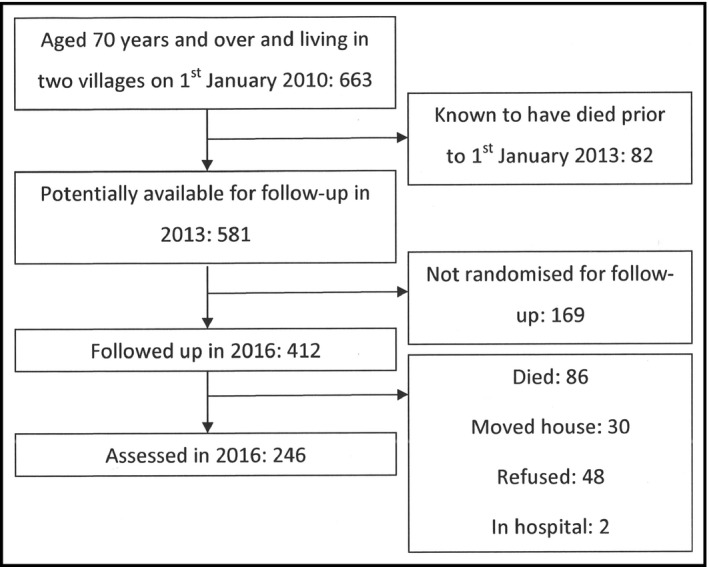
Recruitment process
3.1. Demographics and lifestyle characteristics of the group
Demographics in terms of age and sex were similar between the 246 people assessed at follow‐up and the 417 people not followed up from the baseline cohort. The median age at baseline of those followed up and assessed was 76 years (Inter‐quartile range (IQR) 72 to 80) and of those not assessed was also 76 years (IQR 73 to 82). However, this difference was significant, with those not followed up being significantly older (U = 43984.5, z = 3.073, P = .002). The number of females in those assessed was 130 (52.8%) and in those not assessed was 233 (55.9%); not significant (X 2 (1) = 0.573, P = .449).
Of those seen, 108 (43.9%) reported that they could not read or write and 103 (41.9%) had never attended school, of which 78 (75.7%) were female. Only 29 (11.8%) people had stayed at school for more than 4 years. The vast majority of participants worked in farming in some capacity (n = 227 (92.3%)), although many had other paid work. Only 7 (2.8%) people described themselves as retired (in all cases from paid work), although 6 of these said they still worked on the family farm. Although only 7 (2.8%) people described themselves as current smokers, 70 (28.5%) were ex‐smokers. Ninety‐three (37.8%) people were current drinkers of alcohol, 111 (45.1%) were former drinkers and only 42 (17.1%) said they had never drank alcohol.
3.2. Hypertension
Hypertension at grade I or above was highly prevalent, and affected a consistent proportion of the population at all 3 time points; 182 (74.0%) participants were hypertensive at baseline and the same number at follow‐up in 2013 and 166 (67.5%) were hypertensive in 2016. One‐hundred and twenty‐nine (52.4%) people were identified as sustained hypertensives. Fifty‐six (22.8%) people had hypertension on 2 of the assessments, 31 (12.6%) had hypertension on 1 of the assessments, and only 30 (12.2%) were not hypertensive at any of the assessments. Demographic and life style data are presented in Table 1 for sustained hypertensives and non‐sustained hypertensives. Sustained hypertensives were significantly more likely to be female and were significantly heavier than non‐hypertensives.
Table 1.
Characteristics of sustained and non‐sustained hypertensives at grade I or above
| Sustained hypertensives (n = 129) | Non‐sustained hypertensives (n = 117) | Significance | |
|---|---|---|---|
| Median age at baseline (y) | 75 (72 to 80) | 76 (73 to 80) | U = 7124.0, z = 0.760, P = .447 |
| Number of females | 77 (59.7%) | 53 (45.3%) | X 2 (1) = 5.099, P = .024 |
| No formal education | 56 (43.4%) | 47 (40.2%) | X 2 (1) = 0.405, P = .524 |
| Occupation as a farmer | 122 (94.6%) | 105 (89.7%) | X 2 (2) = 2.008, P = .156 |
| Smoking status |
Former: 36 (27.9%) Current: 6 (4.7%) |
Former: 34 (29.1%) Current: 1 (0.9%) |
X 2 (2) = 3.199, P = .202 |
| Alcohol consumption |
Former: 55 (42.6%) Current: 51 (39.5%) |
Former: 56 (47.9%) Current: 42 (35.9%) |
X 2 (2) = 0.677, P = .713 |
| Median weight (kg) | 55 (47 to 63) | 50 (45 to 60) | U = 6.032.0, z = 2.720, P = .007 |
| Currently taking anti‐hypertensive medication | 48 (37.2%) | 4 (3.4%) | X 2 (1) = 42.024, P < .001 |
Although 52 (21.1%) participants claimed to be currently taking anti‐hypertensive medication, 48 (92.3%) of these were identified as sustained hypertensives and all were hypertensive at 2016 follow‐up, suggesting medication, if taken, was not controlling their BP. Rates of hypertension control in this population are known to be very low.13
3.3. End organ damage
Data for the prevalence of markers for EOD in sustained hypertensives and non‐sustained hypertensives are presented in Table 2 using the cutoff of grade I or above and in Table 3 for a cutoff of grade II or above. Using the lower cutoff, PAD, and LVH were associated with sustained hypertension and using the higher cutoff only LVH was associated with sustained hypertension. These associations remained after adjustment for potential demographic and lifestyle variables that could be confounding (Table 4). Current smoking and greater age were independently associated with PAD; greater age was independently associated with proteinuria and alcohol consumption with arterial stiffness.
Table 2.
Prevalence of end organ damage in sustained and non‐sustained hypertensives
| Sustained hypertensives (n = 129) | Non‐sustained hypertensives (n = 117) | Significance | |
|---|---|---|---|
| Self reported visual loss | 77 (59.7%) | 68 (58.1%) | X 2 (1) = 0.063, P = .803 |
| LVH | 24 of 128 (18.8%) | 10 of 116 (8.6%) | X 2 (1) = 5.206, P = .023 |
| PAD | 45 (34.9%) | 20 (17.1%) | X 2 (1) = 9.987, P = .002 |
| Proteinuria | 24 of 120 (20.0%) | 13 of 110 (11.8%) | X 2 (1) = 2.846, P = .092 |
| Orthostatic hypotension | 34 of 127 (26.8%) | 26 (22.2%) | X 2 (1) = 0.680, P = .410 |
| Cognitive impairment | 28 of 127 (22.0%) | 30 of 115 (26.1%) | X 2 (1) = 0.540, P = .462 |
| Arterial stiffening | 13 (10.1%) | 19 (16.2%) | X 2 (1) = 2.059, P = .151 |
| Previous stroke | 8 (6.2%) | 5 (4.3%) | X 2 (1) = 0.456, P = .500 |
Table 3.
Prevalence of end organ damage in sustained and non‐sustained hypertensives at grade II or above
| Sustained hypertensives (n = 61) | Non‐sustained hypertensives (n = 185) | Significance | |
|---|---|---|---|
| Self reported visual loss | 36 (59.0%) | 109 (58.9%) | X 2 (1) = 0.000, P = .989 |
| LVH | 17 of 60 (28.3%) | 17 of 184 (9.2%) | X 2 (1) = 13.755, P < .001 |
| PAD | 21 (34.4%) | 44 (23.8%) | X 2 (1) = 2.673, P = .102 |
| Proteinuria | 14 of 58 (24.1%) | 23 of 172 (13.4%) | X 2 (1) = 3.724, P = .054 |
| Orthostatic hypotension | 14 of 60 (23.3%) | 46 of 184 (25.0%) | X 2 (1) = 0.068, P = .795 |
| Cognitive impairment | 15 (24.6%) | 43 of 181 (26.1%) | X 2 (1) = 0.017, P = .895 |
| Arterial stiffening | 4 (6.6%) | 28 (15.1%) | X 2 (1) = 2.983, P = .084 |
| Previous stroke | 2 (3.3%) | 11 (5.9%) | X 2 (1) = 0.652, P = .419 |
Table 4.
Multivariable analysis of independent factors associated with markers for end organ damage
| Odds ratio for sustained hypertension (95% CI, P value) | Odds ratio for other significant variables (95% CI, P value) | |
|---|---|---|
| Sustained hypertension at ≥140/90 mm Hg | ||
| LVH | 2.36 (1.02 to 5.36, P = .045) | — |
| PAD | 2.55 (1.30 to 5.01, P = .006) |
Smoking (never as reference) Former smoker: 1.58 (0.64 to 3.93, P = .323) Current smoker: 6.86 (1.15 to 41.07, P = .035) Age band (70‐74 y as reference) 75‐79 y: 2.06 (0.96 to 4.44, P = .065) 80‐84 y: 3.54 (1.34 to 9.33, P = .011) 85 y and over: 5.12 (1.90 to 13.79, P = .001) |
| Proteinuria | 1.93 (0.86 to 4.32, P = .111) |
Age band (70‐74 y as reference) 75‐79 y: 1.41 (0.57 to 3.46, P = .461) 80‐84 y: 1.84 (0.54 to 6.33, P = .332) 85 y and over: 4.20 (1.35 to 13.02, P = .013) |
| Arterial stiffness | 0.81 (0.36 to 1.84, P = .610) |
Alcohol (never as reference) Former drinker: 3.47 (0.66 to 18.26, P = .142) Current drinker: 6.42 (1.29 to 31.90, P = .023) |
| Sustained hypertension at ≥160/100 mm Hg | ||
| LVH | 4.26 (1.93 to 9.42, P < .001) | — |
| PAD | 1.46 (0.75 to 2.87, P = .269) |
Smoking (never as reference) Former smoker: 1.80 (0.73 to 4.44, P = .200) Current smoker: 9.71 (1.66 to 57.01, P = .012) Age band (70‐74 y as reference) 75‐79 y: 1.96 (0.92 to 4.17, P = .082) 80‐84 y: 3.68 (1.41 to 9.62, P = .008) 85 y and over: 4.93 (1.85 to 13.12, P = .001) |
| Proteinuria | 2.18 (0.99 to 4.78, P = .053) |
Age band (70‐74 y as reference) 75‐79 y: 1.37 (0.55 to 3.38, P = .499) 80‐84 y: 1.97 (0.57 to 6.79, P = .285) 85 y and over: 4.15 (1.34 to 12.84, P = .014) |
| Arterial stiffness | 0.48 (0.15 to 1.50, P = .205) |
Alcohol (never as reference) Former drinker: 3.64 (0.68 to 19.48, P = .131) Current drinker: 6.58 (1.31 to 33.11, P = .022) |
4. DISCUSSION
This is the first published longitudinal study to investigate the prevalence and nature of EOD in a community‐dwelling cohort living in SSA. Our data adds significantly to the current understanding of the impact of hypertension on the organs of the body in SSA populations. We have previously reported on the prevalence of hypertension in this cohort.13 The prevalence reported at both follow‐up points is similar to that reported at baseline and towards the upper end of global estimates in this age group.2 One explanation for the lack of variation in the prevalence of hypertension over time is that having hypertension is not associated with an increased mortality risk, and this is supported by previous work by our team.14 One possible explanation for this apparently contradictory finding is that, in this resource‐poor setting, many deaths due to hypertension will already have occurred to individuals prior reaching 70 years and that our cohort represent “survivors.” If this is the case, then one might expect the prevalence of hypertensive EOD to be low and similar in both sustained hypertensives and non‐sustained hypertensives.
4.1. Prevalence of hypertensive EOD
LVH and PAD were the EOD markers most strongly associated with hypertension status across the 6 years of follow‐up. We observed a prevalence of PAD of 34.9% in sustained hypertensives. In a cross‐sectional study of rural community‐dwelling hypertensive Nigerians, a PAD prevalence of only 3.6% was reported.8 However, this was in a much younger population (18 to 64 years) and based on self‐report of previous diagnosis, rather than formal assessment and so is likely to represent an under‐estimate. In contrast, a study in the Netherlands found PAD to be present in 19.1% of people aged over 55 years. Prevalence increased with age with 52.0% of men and 59.6% of women having PAD in the 85 years and over group.22 The authors also found PAD to be more common in hypertensives, supporting our findings. In our study, arterial stiffness was more common in those without sustained hypertension, although the difference was not significant. Although the reasons for this are not clear, the findings merit further study.
Our observation that 18.8% of sustained hypertensives had LVH is in line with reports of LVH prevalence in Nigeria (15.9%).7 For our entire sample, rates of LVH were 13.9%, much lower than reported in people aged 70 years and over in the US Framingham Heart Study, in which LVH prevalence was 33% in men and 49% in women.23 Whether the much lower prevalence seen in Tanzania is due to poor survival rates in those with LVH, or due to demographic, lifestyle or genetic factors merits further investigation. Although self‐reported previous stroke was not associated with sustained hypertension in our cohort, historically low rates of diagnosis in this population are likely to mean that the reported prevalence is an underestimate.24 Hypertension has been previously identified as the single strongest predictor of stroke in Hai district.25 We found proteinuria to be present in a fifth of hypertensives, similar to rates of proteinuria reported in younger rural dwelling Nigerians and the prevalence of other markers for renal impairment reported in hypertensives from Nigeria and Congo.6, 8, 9, 26 Coresh et al27 reported the prevalence of chronic kidney disease, defined by reduced GFR and albuminuria, in the US population of people aged over 70 years as 13.1%, similar to that found in our complete cohort of a similar age.
LVH and PAD were more common in sustained hypertensives, even after adjusting for lifestyle and demographic factors. Age was an important predictor of hypertension, even in this “survivor” cohort. The link between hypertension and EOD may be complex in older people living in resource‐limited settings. Given the lack of association between hypertension and mortality in our cohort, it may be that the increased mortality risk associated with LVH and PAD in hypertensives is counteracted by the potential benefits of increased blood pressure, such as lower rates of cognitive impairment, arterial stiffness and orthostatic hypotension, due to increased cerebral perfusion.28 Alternatively, it may be argued that our cohort are survivors whose bodies have been able to adapt to chronic, uncontrolled hypertension and associated EOD, and that many people with EOD will already have died prior to recruitment. Although our study provides little direct support for either of these hypotheses, further investigation of the potentially beneficial effects of hypertension in this setting should be considered. The possibility that late life hypertension may be beneficial for many people is supported by data from high‐income settings. Findings from the Leiden 85‐plus study suggested that at 85 years and older, higher systolic BP was associated with resilience to physical and cognitive decline.28 This supports findings by Diao et al,29 who in 2012 reviewed 4 randomised clinical trials to conclude that there is no significant benefit to treating grade I hypertensives with no previous cardiovascular disease on the outcomes of mortality or cardiovascular events. However, recent evidence published by Williamson et al30 reports that lowering the systolic BP from < 140 to < 120 mm Hg in people aged over 75 years results in significantly lower rates of cardiovascular events and death. Despite the conflicting evidence for the benefits of treating hypertension in the very old, there can be little doubt of the need to consider hypertension status within the framework of overall cardiovascular risk, particularly within the resource limited setting of much of SSA.31
4.2. Limitations
Although the 2 villages were selected for follow‐up at random, and we have no reason to suspect that this has significantly biased our findings, it is possible that they were not fully representative of the whole DSS. The community‐based nature of this study required the use of non‐invasive procedures to assess the prevalence of EOD. In the absence of technical resources, relatively crude techniques were utilized. This makes comparison of prevalence of some markers for EOD reported here with previous work in other world regions difficult. In particular, using self‐reported visual loss as an indicator of retinal damage is likely to be too crude a measure to accurately assess the impact of hypertension on visual loss. It is not surprising that over half the cohort reported some level of visual impairment given the age structure. The validity of each of the chosen methods of identifying other markers of EOD is reviewed briefly in the methods section. We also acknowledge that, since a substantial “white coat effect” has been noted in this population with regard to BP measurement, recorded BPs may not reflect true values.32 To minimize the influence of the white coat effect, we have also looked at the association of EOD with hypertension at grade II and above. However, results were similar whichever cutoff was used. The use of a urine dipstick has been reported as a highly specific method for detecting proteinuria, but relatively non‐sensitive (32%‐46%).33 Ideally, positive proteinuria findings should be repeated to exclude transient proteinuria. Due to time constraints and logistical reasons, repeat sampling was not possible in this study.
5. CONCLUSIONS
The prevalence of markers for EOD was similar to those previously reported in other areas of SSA. The prevalence of LVH and PAD was significantly higher in sustained hypertensives. We plan to follow‐up this cohort to investigate the link between EOD and morbidity and mortality. Further work on younger cohorts is needed in order to investigate if our findings are unique to this “survivor” cohort or whether similar patterns of EOD are present in middle age.
CONFLICTS OF INTEREST
There were no conflicts of interest.
AUTHOR CONTRIBUTION
Study concept and design: RW, WG, HP, RJ. Literature search: HP, RJ. Data collection: HP, RJ, JR, MD, FD. Data analysis: HP, WG. Interpretation of results: RW, WG, HP, RJ. Writing of paper and review: RW, WG, HP, RJ, BS.
ACKNOWLEDGMENTS
We wish to acknowledge all the help we have received from the health care workers, translators, village enumerators, officials, carers, family members, and participants in Hai district who helped with assessments, examinations, data collection, and input. We would like to thank Norma Cardill and Victoria Ferguson for assisting in data entry for the 2013 follow‐up and Gillian Tough for administrative support (all Northumbria Healthcare NHS Foundation Trust).
Putnam HWI, Jones R, Rogathi J, et al. Hypertension in a resource‐limited setting: Is it associated with end organ damage in older adults in rural Tanzania?. J Clin Hypertens. 2018;20:217–224. 10.1111/jch.13187
REFERENCES
- 1. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224‐2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217‐223. [DOI] [PubMed] [Google Scholar]
- 3. Adeloye D, Basquill C. Estimating the prevalence and awareness rates of hypertension in Africa: a systematic analysis. PLoS ONE. 2014;9:e104300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898‐902. [DOI] [PubMed] [Google Scholar]
- 5. Ayodele OE, Alebiosu CO, Akinwusi PO, Akinsola A, Mejiuni A. Target organ damage and associated clinical conditions in newly diagnosed hypertensives attending a tertiary health facility. Niger J Clin Pract. 2007;10:319‐325. [PubMed] [Google Scholar]
- 6. Busari OA, Opadijo OG, Omotoso AB. Microalbuminuria and hypertensive retinopathy among newly diagnosed nondiabetic hypertensive adult Nigerians. Niger J Clin Pract. 2011;14:436‐439. [DOI] [PubMed] [Google Scholar]
- 7. Nelissen HE, Hendriks ME, Wit FW, et al. Target organ damage among hypertensive adults in rural Nigeria: a cross‐sectional study. J Hypertens. 2014;32:487‐494. [DOI] [PubMed] [Google Scholar]
- 8. Oladapo OO, Salako L, Sadiq L, Shoyinka K, Adedapo K, Falase AO. Target‐organ damage and cardiovascular complications in hypertensive Nigerian Yoruba adults: a cross‐sectional study. Cardiovasc J Afr. 2012;23:379‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kabedi NN, Mwanza JC, Lepira FB, Kayembe TK, Kayembe DL. Hypertensive retinopathy and its association with cardiovascular, renal and cerebrovascular morbidity in Congolese patients. Cardiovasc J Afr. 2014;25:228‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Worku T, Tadesse Y, Hughes P, Lemessa T. Patterns of complications seen in patients with hypertension admitted to Tikur Anbessa Hospital: a retrospective analysis. Ethiop Med J. 2015;53(Suppl 2):51‐56. [PubMed] [Google Scholar]
- 11. Osafo C, Mate‐Kole M, Affram K, Adu D. Prevalence of chronic kidney disease in hypertensive patients in Ghana. Ren Fail. 2011;33:388‐392. [DOI] [PubMed] [Google Scholar]
- 12. Halle MP, Takongue C, Kengne AP, Kaze FF, Ngu KB. Epidemiological profile of patients with end stage renal disease in a referral hospital in Cameroon. BMC Nephrol. 2015;16:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dewhurst MJ, Dewhurst F, Gray WK, Chaote P, Orega GP, Walker RW. The high prevalence of hypertension in rural‐dwelling Tanzanian older adults and the disparity between detection, treatment and control: a rule of sixths? J Hum Hypertens. 2013;27:374‐380. [DOI] [PubMed] [Google Scholar]
- 14. Gray WK, Dewhurst F, Dewhurst MJ, et al. Rates and predictors of three‐year mortality in older people in rural Tanzania. Arch Gerontol Geriatr. 2016;62:36‐42. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization . The WHO STEPS Instrument: The WHO STEPwise Approach to Chronic Disease Risk Factor Surveillance (STEPS). Geneva: World Health Organization; 2008. [Google Scholar]
- 16. National Institute for Health and Clinical Excellence (NICE) . Hypertension: Clinical Management of Primary Hypertension in Adults (CG127). London, UK: NICE; 2011. [Google Scholar]
- 17. Dachun X, Jue L, Liling Z, et al. Sensitivity and specificity of the ankle‐brachial index to diagnose peripheral artery disease: a structured review. Vasc Med. 2010;15:361‐369. [DOI] [PubMed] [Google Scholar]
- 18. Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161‐186. [DOI] [PubMed] [Google Scholar]
- 19. Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. 1990;81:815‐820. [DOI] [PubMed] [Google Scholar]
- 20. Gray WK, Paddick SM, Collingwood C, et al. Community validation of the IDEA study cognitive screen in rural Tanzania. Int J Geriatr Psychiatry. 2016;31:1199‐1207. [DOI] [PubMed] [Google Scholar]
- 21. Paddick SM, Gray WK, Ogunjimi L, et al. Validation of the Identification and Intervention for Dementia in Elderly Africans (IDEA) cognitive screen in Nigeria and Tanzania. BMC Geriatr. 2015;15:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meijer WT, Hoes AW, Rutgers D, Bots ML, Hofman A, Grobbee DE. Peripheral arterial disease in the elderly: The Rotterdam Study. Arterioscler Thromb Vasc Biol. 1998;18:185‐192. [DOI] [PubMed] [Google Scholar]
- 23. Levy D, Anderson KM, Savage DD, Kannel WB, Christiansen JC, Castelli WP. Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. The Framingham Heart Study. Ann Intern Med. 1988;108:7‐13. [DOI] [PubMed] [Google Scholar]
- 24. Walker R, Whiting D, Unwin N, et al. Stroke incidence in rural and urban Tanzania: a prospective, community‐based study. Lancet Neurol. 2010;9:786‐792. [DOI] [PubMed] [Google Scholar]
- 25. Walker RW, Jusabani A, Aris E, et al. Stroke risk factors within an incident population in urban and rural Tanzania: A prospective, community‐based case‐control study. Lancet Global Health. 2013;1:e282‐e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ayodele OE, Egbewale BE, Alebiosu CO. Kidney function and clinical correlates in newly diagnosed hypertensives attending a university teaching hospital in southwest Nigeria. Afr J Med Med Sci. 2007;36:95‐101. [PubMed] [Google Scholar]
- 27. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038‐2047. [DOI] [PubMed] [Google Scholar]
- 28. Sabayan B, Oleksik AM, Maier AB, et al. High blood pressure and resilience to physical and cognitive decline in the oldest old: the Leiden 85‐plus Study. J Am Geriatr Soc. 2012;60:2014‐2019. [DOI] [PubMed] [Google Scholar]
- 29. Diao D, Wright JM, Cundiff DK, Gueyffier F. Pharmacotherapy for mild hypertension. Cochrane Database Syst Rev. 2012;8:CD006742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged >/=75 years: a randomized clinical trial. JAMA. 2016;315:2673‐2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Damasceno A, Padrao P, Silva‐Matos C, Prista A, Azevedo A, Lunet N. Cardiovascular risk in Mozambique: who should be treated for hypertension? J Hypertens. 2013;31:2348‐2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ivy A, Tam J, Dewhurst MJ, et al. Ambulatory blood pressure monitoring to assess the white‐coat effect in an elderly East African population. J Clin Hypertens (Greenwich). 2015;17:389‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kashif W, Siddiqi N, Dincer AP, Dincer HE, Hirsch S. Proteinuria: how to evaluate an important finding. Cleve Clin J Med. 2003;70:535‐537 , 41‐4, 46‐7. [DOI] [PubMed] [Google Scholar]


