Abstract
Data on arterial stiffness in older populations, according to blood pressure (BP) levels, are scarce in Brazil. The objective of this study was to establish reference values for core measures of arterial stiffness, including carotid‐femoral pulse wave velocity (cf‐PWV) and aortic augmentation index (AIx), in a cohort of older individuals with normotension (NT) and hypertension. Cross‐sectional analysis was performed with applanation tonometry data from 1192 patients aged 60 years or older. The authors classified patients according to their BP levels as having NT, controlled hypertension (CH), and uncontrolled hypertension (UH). The cf‐PWV values were 9.11 ± 0.16 m/s (NT), 9.12 ± 0.18 m/s (CH), and 9.42 ± 2.2 m/s (UH) (P < 0.005; UH vs NT and CH). The AIx was 33.3% for the entire cohort and similar across all groups. The cf‐PWV increased with age but reached a ceiling at 75 years. Compared with men, women had a higher AIx but similar cf‐PWV levels. In conclusion, the markers of arterial stiffness were similar among individuals with NT/CH and higher among individuals with UH.
Keywords: arterial stiffness, hypertension in the elderly, pulse wave velocity
1. INTRODUCTION
Evaluation of vascular function is a key element in mapping cardiovascular health. Arterial pulse wave velocity (PWV) is a well‐established indirect measure of arterial wall stiffness and an independent predictor of cardiovascular events.1, 2 Human aging is among the most important cardiovascular risk factors and is associated with changes in the function and structure of the heart and vascular network.3 Changes in the walls of large arteries include calcium deposition, progressive substitution of elastic fibers by collagen, and stiffening of the walls, with segmental dilatation and presence of atherosclerosis.4, 5 These changes lead to an increased pulse pressure, which, in turn, is associated with increased morbidity and mortality in an older population. Aortic wall stiffness changes the PWV and AIx of the central pulse wave generated by the ventricular systole.6, 7 These factors are considered independent predictors of cardiovascular and total mortality, as demonstrated in several studies from different regions of the world.8, 9, 10, 11
In the literature, reference values for PWV and AIx are mainly from Asia, the United States, Australia, and Europe.10, 12, 13 However, compared with European or North American populations, Latin American populations14, 15, 16 differ in the stature, as well as in the arterial stiffness. Latin American countries are traditionally characterized by a high degree of miscegenation between whites and blacks, resulting in a high percentage of “morenos” (brown), which makes the assessment of ethnic differences challenging.
Additionally, there are scarce data on PWV reference values, especially for the elderly population, according to hypertensive status and race. Measuring the carotid‐femoral PWV (cf‐PWV) is a simple, noninvasive, and reproducible method that is considered by various authors the gold standard for evaluating central artery stiffness.17, 18, 19 The objective of the present analysis was to establish the reference PWV values in a large cohort of elderly persons with NT and hypertension in both sexes and different races in an urban area of Brazil.
2. METHODS
The present study is a cross‐sectional analysis of the data obtained during the first medical visit of the Study of PWV in Elderly Individuals in Uberlandia, a large urban area of Brazil (EVOPIU [Estudo da Velocidade da Onda de Pulso em Idosos de Uberlandia], Uberlândia, MG; Brazil). EVOPIU is a longitudinal, prospective, observational, multiclinic study with a planned 4‐year follow‐up. Enrollment occurred from August 2014 to October 2015, and the end of the study is scheduled for 2018. Participants are followed biannually. During the follow‐up visits, clinical history, biochemical/hematological tests, electrocardiograms (ECGs), and applanation tonometry are assessed. All collected data are stored electronically and are the responsibility of the Federal University of Uberlândia, MG, Brazil. This study was approved by the research ethics committee under CAAE number 37440114.3.0000.5152 and was financed by the Minas Gerais State Agency for Research and Development (FAPEMIG).
2.1. Inclusion/Exclusion criteria
A total of 1204 elderly individuals were invited to enroll in the study and were required to meet the following inclusion criteria: age 60 years and older, ambulatory, able to perform activities of daily living without assistance, and not hospitalized. Exclusion criteria were chronic kidney failure (on dialysis), known malignant neoplasms expected to result in death during follow‐up, inability to remain in a supine position, and disagreement to participate in the study. The recruitment resulted in a final sample of 1192 patients. The patients came from nine different outpatient clinics (eight public and one private).
2.2. Anthropometric/biochemical/hematological data and ECG
General demographic and clinical data were collected for each patient. The color/race (white, black or nonblack) of each participant was determined based on skin color, as reported by the researcher. The ethnic classification was performed by interviewers based on skin color, hair pattern, and facial features. These criteria were arbitrarily used as follows: individuals with white skin and light eyes were labeled as white, those with dark skin and curly hair were labeled as black, and those who did not meet the two previous criteria were labeled as nonblack. In the present study, no participants were considered indigenous or Asian. Serum levels of uric acid, urea, and creatinine; blood glucose; and the lipid profile were assessed using colorimetric methods (Cobas® 6000; Roche Hitachi, Brazil), whereas hematological examination was performed with a Sysmex® XED‐2100, São Paulo, Brazil. The ECG was obtained with an Innomed Heart Screen device, model EKG HS 60G (Innomed®, São Paulo, Brazil). Glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.20 Patients were considered to have hypercholesterolemia when they had total fasting cholesterol >200 mg/dL, high‐density lipoprotein cholesterol <40 mg/dL, triglycerides >190 mg/dL, or used statins; diabetes mellitus was considered when fasting plasma glucose was ≥ 126 mg/dL or when patients were using insulin/oral hypoglycemic drugs. Smoking status was defined as never, prior, or current smoker.
2.3. BP measurements
2.3.1. Brachial BP
After 10 minutes of rest, brachial blood pressure (bBP) was assessed in a seated position by means of three consecutive measurements at 3‐minute intervals. For the first two measurements, an automatic digital oscillometric blood pressure (BP) device (HE 7200 Intelli Sense Omron Hem, Brazil) was used; the third measurement was performed with a SphygmoCor device. Individual values represented the arithmetic means of the three measurements in millimeters of mercury. The sizes of BP cuffs were adjusted to the arm circumference. We used the bBP (systolic/diastolic) levels for the classification of the hypertensive status of the patients. Patients with bBP <140/90 mm Hg were considered to have NT; those with bBP <140/90 mm Hg and using antihypertensive drugs were considered to have controlled hypertension (CH); and those with bBP ≥ 140/90 mm Hg, whether using antihypertensive drugs or not, were considered to have uncontrolled hypertension (UH).
2.3.2. Central BP, PWV, and AIx
Central BP values, cf‐PWV, and the aortic augmentation index (AIx) were obtained by applanation tonometry with a SphygmoCor XCEL device, model EM4C (AtCor Medical, Sydney, NSW, Australia); cf‐PWV was measured in meters per second, with the patient in a supine position. The carotid‐femoral distance (centimeters) was obtained and multiplied by 0.8 (direct method).21 The device automatically determines the best wave for the calculation and generates cf‐PWV values, central pulse pressure, central systolic BP (cSBP), central diastolic BP (cDBP), and AIx values. The AIx was automatically adjusted for a heart rate of 75 beats per minute since the heart rate is an important modifier of AIx. Applanation tonometry was performed in a single measurement, based on our own pilot study, which demonstrated high measurement reproducibility in this patient population.22
2.4. Sample Size
The sample size was calculated for the analysis of cardiovascular outcomes in this cohort and is therefore not applicable to the present analysis. The present analysis represents the evaluation of the entire cohort at baseline.
2.5. Statistical analysis
We assessed the normality of the data set using the Kolmogorov‐Smirnov test and found that all variables were normally distributed. Thus, the data are expressed as means and standard deviations. Two groups were compared with Student t test, whereas three or more groups were compared by analysis of variance and the Bonferroni posttest. The cf‐PWV, AIx, and augmentation pressure values were adjusted for sex, age, and mean arterial pressure (MAP). Univariate analyses were performed between cf‐PWV and age for the different groups studied (Figure 1). To estimate cf‐PWV values at predetermined ages (60, 70, and 80 years), the linear regression was performed, adjusted for sex and MAP, and their respective 95% confidence intervals were determined for the different ages (Figure 3). Significance was set at 0.05 in all analyses. STATA software version 14.0 (StataCorp, College Station, TX, USA) was used for statistical analyses.
Figure 1.
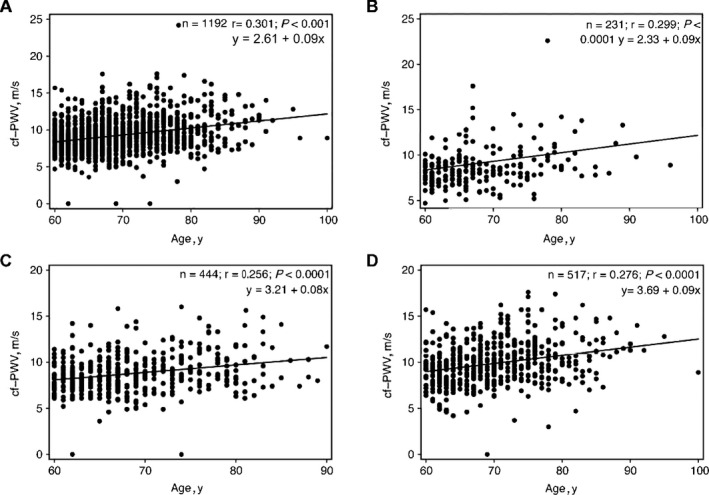
Linear regression between age and unadjusted carotid‐femoral pulse wave velocity values in all patients (A) and in the normotension (B), controlled hypertension (C), and uncontrolled hypertension D) groups
Figure 3.
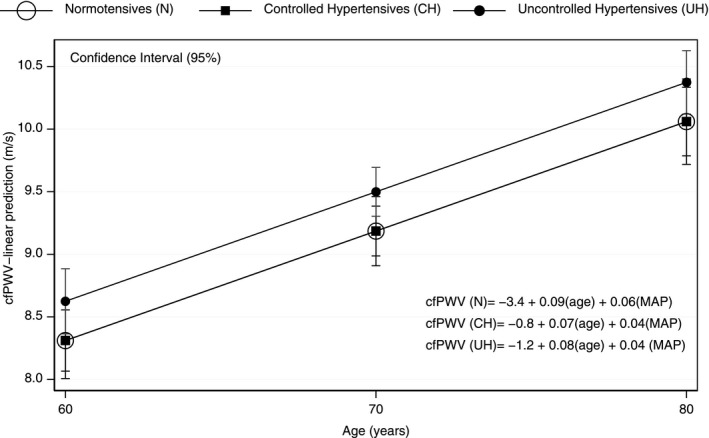
Predictive values of adjusted carotid‐femoral pulse wave velocity, stratified by group (normotension, controlled hypertension, and uncontrolled hypertension) at 60, 70, and 80 y of age
3. RESULTS
Of all participants, 81% were diagnosed with systemic arterial hypertension and 60% were women. Table 1 lists the clinical and laboratory characteristics of the participants according to their BP status. The population was generally overweight and of relatively short stature. In general, both peripheral and central BP values and the measures of arterial stiffness were similar between the patients with NT and those with CH. By contrast, patients with UH were older than both patients with NT and those with CH and had higher values for all BP parameters, as well as for cf‐PWV. The distending pressure has a direct effect on cf‐PWV (higher pressure, higher PWV); therefore, all analyses of cf‐PWV were adjusted for central MAP (cMAP).
Table 1.
Peripheral and central blood pressure, pulse wave velocity, and AIx in patients with NT, CH, and UH
| All patients (N = 1192) | NT (n = 231) | CH (n = 444) | UH (n = 517) | P valuea | P valueb | |
|---|---|---|---|---|---|---|
| Age, years | 69.2 ± 7.0 | 68.1 ± 7.0 | 68.7 ± 6.6 | 70.2 ± 7.2 | 0.636 | 0.002 |
| Male/female sex, % | 39.5/60.5 | 43/57 | 32/68 | 39/61 | 0.0001 | 0.0001 |
| Race, No./% | ||||||
| White | 99/8.3 | 22/9.5 | 38/8.6 | 39/7.5 | 0.0001 | 0.0001 |
| Black | 237/20 | 36/15 | 95/21.4 | 106/20.5 | 0.0001 | 0.0001 |
| Nonblack | 834/70 | 171/74 | 301/67.8 | 362/70 | 0.0001 | 0.0001 |
| Height, m | 1.57 ± 0.09 | 1.58 ± .09 | 1.58 ± .08 | 1.56 ± .0.9 | 1 | 0.015 |
| Weight, kg | 70.4 ± 15.2 | 66.3 ± 13.3 | 71.8 ± 15.2 | 70.9 ± 15.8 | 1 | 0.258 |
| Abdominal circumference, cm | 98.2 ± 13.0 | 93.1 ± 12.1 | 99.4 ± 12.3 | 99.6 ± 13.5 | 0.347 | 0.463 |
| BMI, kg/m2 | 28.4 ± 6.0 | 26.6 ± 4.9 | 28.8 ± 6.2 | 28.9 ± 6.0 | <0.001 | 1 |
| Heart rate, beats per min | 69.2 ± 11.6 | 68.3 ± 10.1 | 69.2 ± 11.9 | 69.6 ± 11.9 | 0.696 | 1 |
| Blood pressure, mm Hg | ||||||
| bSBP | 138.7 ± 20.0 | 124.8 ± 10.1 | 125.1 ± 10.1a | 156.6 ± 15.1 | 1 | <0.0001 |
| bDBP | 78.1 ± 11.1 | 78.0 ± 9.9 | 78.7 ± 10.9 | 88.2 ± 12.5 | 1 | <0.0001 |
| bPP | 55.3 ± 19.3 | 47.3 ± 13.7 | 49.4 ± 13.3 | 64.3 ± 21.8 | 0.422 | <0.0001 |
| bMAP | 101.3 ± 12.9 | 93.6 ± 8.5 | 94.2 ± 8.8 | 110.9 ± 11.1 | 1 | <0.0001 |
| cSBP | 132.1 ± 19.2 | 119.2 ± 13.1 | 123.5 ± 14.3 | 144.4 ± 17.6 | 0.004 | <0.0001 |
| cDBP | 84.0 ± 12.6 | 78.9 ± 9.9 | 79.6 ± 10.8 | 98.8 ± 12.6 | 0.742 | <0.0001 |
| cPP | 48.1 ± 14.4 | 41.0 ± 10.7 | 43.9 ± 11.6 | 54.4 ± 15.2 | 0.018 | <0.0001 |
| cMAP | 100.1 ± 13.5 | 92.5 ± 9.9 | 94.6 ± 10.8 | 108.1 ± 12.7 | 0.076 | <0.0001 |
| Arterial stiffnessc | ||||||
| AP, mm Hg | 16.7 ± 0.59 | 14.8 ± 0.73 | 16.0 ± 0.53 | 19.4 ± 0.52 | 0.165 | <0.0001 |
| AIx, % | 33.3 ± 0.77 | 33.0 ± 0.96 | 33.3 ± 0.69 | 33.7 ± 0.68 | 0.803 | 0.76 |
| cf‐PWV, m/s | 9.21 ± 0.84 | 9.11 ± 0.16 | 9.12 ± 0.18 | 9.42 ± 2.2 | 0.924 | 0.043 |
| Medications in use, No./% | ||||||
| Diuretic | 502/42 | 0 | 260/58.5 | 242/46.8 | 0.0002 | |
| ACEI | 347/29 | 0 | 194/43.6 | 153/29.6 | 0.0001 | |
| ARB | 321/27 | 0 | 163/36.7 | 161/31.1 | 0.0649 | |
| β‐Blocker | 297/25 | 0 | 147/33.1 | 149/28.8 | 0.1502 | |
| CCB | 179/15 | 0 | 78/17.5 | 101/19.5 | 0.4446 | |
| Direct vasodilators | 37/3 | 0 | 16/3.1 | 21/4.0 | 0.7174 | |
| Statins | 348/29.1 | 36/15.5 | 165/37.1 | 147/28.4 | 0.0001 | 0.0037 |
| Comorbidities, No./% | ||||||
| Diabetes mellitus | 524/44 | 75/32 | 213/48 | 236/46 | 0.0001 | 0.4717 |
| Obesity (BMI ≥ 30 kg/m2) | 382/32 | 20/8.6 | 161/36.2 | 201/38.9 | 0.0001 | 0.0001 |
| Dyslipidemia | 365/31 | 39/18 | 173/39 | 153/30 | 0.0001 | 0.4717 |
| Current and ex‐smokers | 645/54 | 136/58.8 | 233/52.4 | 276/53.3 | 1 | 1 |
| Previous CVE | 184/15.4 | 38/16.4 | 45/10.1 | 101/19.5 | 0.0179 | 0.0001 |
Values are expressed as mean ± standard error.
ACEI, angiotensin‐converting enzyme inhibitor; AIx, augmentation index; AP, augmentation pressure; ARB, angiotensin receptor blocker; bDBP, brachial diastolic blood pressure; bMAP, brachial mean arterial pressure; BMI, body mass index; bPP, brachial pulse pressure; bSBP, brachial systolic blood pressure; CCB, calcium channel blocker; cDBP, central diastolic blood pressure; cf‐PWV, carotid‐femoral pulse wave velocity; cPP, central pulse pressure; cSBP, central systolic blood pressure; CVE, cardiovascular event.
P: normotension (NT) vs controlled hypertension (CH).
P: CH vs uncontrolled hypertension (UH).
Adjusted by central mean arterial pressure (cMAP), sex, and age.
The CH group had a higher number of patients using antihypertensive medications than the UH group. The NT group had a lower number of comorbidities than the other groups.
Table 2 lists the BP values in the NT, CH, and UH groups, stratified by sex. In general, women were younger (except in the UH group) and had a higher central BP and higher AIx but not a higher cf‐PWV. Table 3 shows the hemodynamic data stratified by color/race. Although nonblack patients were the majority, no significant differences were observed between the listed categories. Table 4 shows the BP values, cf‐PWV, AIx, and augmentation pressure according to antihypertensive drug classes. Compared with that of other antihypertensive medications, the use of β‐blockers was associated with lower cf‐PWV values. The most frequent β‐blocker used by the patients was atenolol. Figure 1 shows the overall correlation between age and unadjusted cf‐PWV in all patients (A) and in the NT (B), CH (C), and UH (D) groups. Despite the wide variability in the distribution, there was a positive linear relationship between age and cf‐PWV (r = 0.301; P < 0.001). Figure 2 shows the cf‐PWV values adjusted for sex and cMAP and separated by age (in 5‐year strata). The values in the highest age groups (75–80 and >80 years) were similar (P = 0.99), thus indicating a “ceiling” for the cf‐PWV. Figure 3 shows the cf‐PWV slope of predictive margins of cf‐PWV, stratified by group (NT, CH, and UH) at 60, 70, and 80 years of age, and the respective confidence intervals, adjusted by MAP and age.
Table 2.
Peripheral and central blood pressure, pulse wave velocity, and AIx in patients with NT, CH, and UH by sex
| NT (n = 231) | CH (n = 444) | UH (n = 517) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | P value | Male | Female | P value | Male | Female | P value | |
| No. | 100 | 131 | 170 | 274 | 201 | 316 | |||
| Age, y | 70.2 ± 7.6 | 66.3 ± 5.9 | <0.0001 | 70.5 ± 6.4 | 67.6 ± 6.4 | <0.0001 | 70.3 ± 7.6 | 70.1 ± 7.0 | 0.3861 |
| Heart rate, beats per min | 67.0 ± 9.7 | 69.2 ± 10.6 | 0.9478 | 65.9 ± 12.0 | 71.6 ± 11.7 | <0.0001 | 67.4 ± 11.9 | 70.5 ± 11.8 | <0.0001 |
| Race, No./% | |||||||||
| White | 10/10 | 12/9 | 1 | 19/11 | 19/7 | 1 | 21/10 | 18/6 | 1 |
| Black | 20/20 | 18/14 | 1 | 40/24 | 55/20 | 1 | 37/18 | 69/22 | 1 |
| Nonblack | 70/70 | 101/77 | 1 | 111/65 | 200/63 | 1 | 143/72 | 229/72 | 1 |
| Height, m | 1.6 ± 0.1 | 1.5 ± 0.1 | <0.0001 | 1.6 ± 0.1 | 1.5 ± 0.1 | <0.0001 | 1.6 ± 0 | 1.5 ± 0 | <0.0001 |
| Weight, kg | 70.5 ± 1.3 | 63 ± 0.1 | <0.0001 | 75.1 ± 1.1 | 69.7 ± 0.9 | 0.0002 | 75.2 ± 1.1 | 68.2 ± 0.8 | <0.0001 |
| Abdominal circumference, cm | 94.3 ± 1.2 | 92.4 ± 1.0 | 0.1043 | 99.9 ± 0.9 | 99.1 ± 0.7 | 0.2603 | 100.2 ± 1.0 | 99.2 ± 0.7 | 0.2115 |
| Body mass index, kg/m2 | 26 ± 0.4 | 27.1 ± 0.4 | 0.9335 | 27.3 ± 0.3 | 29.8 ± 0.4 | 1 | 27.9 ± 0.3 | 29.6 ± 0.3 | 0.9977 |
| Blood pressure, mm Hg | |||||||||
| bSBP | 126 ± 9.0 | 123 ± 9.7 | 0.0081 | 124 ± 11.8 | 125 ± 8.9 | 0.2706 | 154 ± 13.7 | 156 ± 16.5 | 0.0216 |
| bDBP | 74 ± 7.5 | 72 ± 7.7 | 0.0045 | 72 ± 8.1 | 72 ± 7.8 | 0.5877 | 86 ± 11.6 | 83 ± 10.3 | 0.0002 |
| bPP | 47 ± 14.4 | 48 ± 12.8 | 0.5096 | 49 ± 13.1 | 49 ± 12.6 | 0.4904 | 60 ± 21.2 | 64 ± 23.0 | <0.0001 |
| bMAP | 94 ± 8.1 | 92 ± 8.9 | 0.0865 | 94 ± 8.6 | 94 ± 8.9 | 0.7135 | 111 ± 11.0 | 110 ± 11.2 | 0.4331 |
| cSBP | 117 ± 12.0 | 120 ± 13.7 | 0.0121 | 121 ± 13.5 | 125 ± 14.6 | 0.0049 | 139 ± 16.2 | 147 ± 17.7 | <0.0001 |
| cDBP | 79 ± 9.1 | 77 ± 10.0 | 0.6897 | 79 ± 10.1 | 80 ± 11.1 | 0.1867 | 90 ± 12.6 | 89 ± 12.6 | 0.8598 |
| cPP | 38 ± 9.0 | 43 ± 11.4 | 0.0002 | 42 ± 11.7 | 45 ± 11.4 | 0.0064 | 48 ± 12.8 | 58 ± 15.5 | <0.0001 |
| cMAP | 92 ± 9.4 | 92 ± 10.3 | 0.6783 | 93 ± 10.2 | 95 ± 11.1 | 0.0269 | 107 ± 12.6 | 108 ± 12.7 | 0.0779 |
| Arterial stiffnessa | |||||||||
| AP, mm Hg | 11.6 ± 1.0 | 16.8 ± 0.96 | 0.0003 | 13.4 ± 0.84 | 17.7 ± 0.66 | <0.0001 | 15.4 ± 0.78 | 22.0 ± 0.64 | <0.0001 |
| AIx, % | 27.8 ± 12.9 | 36.5 ± 13.3 | <0.0001 | 28.0 ± 14.2 | 36.7 ± 14.3 | <0.0001 | 29.2 ± 14.8 | 36.5 ± 13.5 | <0.0001 |
| cf‐PWV, m/s | 9.04 ± 0.18 | 9.20 ± 0.20 | 0.5585 | 8.96 ± 0.12 | 9.36 ± 0.16 | 0.0491 | 9.33 ± 0.12 | 9.56 ± 0.14 | 0.2038 |
Values are expressed as mean ± standard error.
AIx, augmentation index; AP, augmentation pressure; bDBP, brachial diastolic blood pressure; bMAP, brachial mean arterial pressure; bPP, brachial pulse pressure; bSBP, brachial systolic blood pressure; cDBP, central diastolic blood pressure; cf‐PWV, carotid‐femoral pulse wave velocity; cPP, central pulse pressure; cSBP, central systolic blood pressure.
Adjusted by central mean arterial pressure (cMAP), sex, and age.
Table 3.
Peripheral and central blood pressure, pulse wave velocity, and AIx by race
| All patients (N = 1192) | White (n = 99) | Black (n = 237) | Nonblack (n = 856) | P value | P valuea | |
|---|---|---|---|---|---|---|
| Age, y | 69.2 ± 0.20 | 70.7 ± 0.75 | 68.6 ± 0.42 | 69.2 ± 0.24 | 0.055 | 0.701 |
| Male/female sex, % | 39.5/60.5 | 51/49 | 40/60 | 39/61 | 0.0792 | 0.5179 |
| Weight, kg | 70.4 ± 0.41 | 68.8 ± 1.58 | 71 ± 0.99 | 70.2 ± 0.53 | 0.197 | 0.3594 |
| Abdominal circumference, cm | 98.2 ± 0.38 | 96.6 ± 1.42 | 97.3 ± 0.86 | 98.5 ± 0.45 | 0.6775 | 0.2033 |
| Blood pressure, mm Hg | ||||||
| bSBP | 138.7 ± 0.58 | 138.5 ± 1.93 | 140.1 ± 1.34 | 138.1 ± 0.67 | 1 | 0.739 |
| bDBP | 82.8 ± 0.85 | 80.5 ± 1.20 | 85.0 ± 0.81 | 82.3 ± 0.43 | 0.009 | 0.014 |
| bPP | 55.5 ± 0.56 | 55.6 ± 1.91 | 53.5 ± 1.28 | 56.0 ± 0.65 | 1 | 0.213 |
| bMAP | 101.3 ± 0.38 | 99.8 ± 1.2 | 103.3 ± 0.86 | 100.9 ± 0.44 | 0.078 | 0.047 |
| cSBP | 132.4 ± 0.57 | 130.2 ± 1.82 | 133.4 ± 1.30 | 132.1 ± 0.67 | 0.478 | 1 |
| cDBP | 84.2 ± 0.37 | 81.7 ± 1.21 | 86.4 ± 0.83 | 83.7 ± 0.44 | 0.005 | 0.011 |
| cPP | 48.2 ± 0.42 | 48.4 ± 1.30 | 47.0 ± 0.93 | 48.4 ± 0.50 | 1 | 0.506 |
| cMAP | 100.1 ± 0.39 | 97.9 ± 1.30 | 102.1 ± 0.91 | 99.8 ± 0.47 | 0.026 | 0.073 |
| Arterial stiffnessb | ||||||
| AP, mm | 16.7 ± 0.59 | 17.4 ± 1.09 | 15.6 ± 0.70 | 17.7 ± 0.37 | 0.178 | 0.008 |
| AIx, % | 33.3 ± 0.77 | 32.4 ± 1.42 | 32.7 ± 0.91 | 33.9 ± 0.48 | 0.944 | 0.127 |
| cf‐PWV, m/s | 9.21 ± 0.84 | 9.22 ± 0.20 | 9.42 ± 0.13 | 9.22 ± 0.07 | 0.988 | 0.1821 |
Values are expressed as mean ± standard error.
AIx, augmentation index; AP, augmentation pressure; bDBP, brachial diastolic blood pressure; bMAP, brachial mean arterial pressure; bPP, brachial pulse pressure; bSBP, brachial systolic blood pressure; cDBP, central diastolic blood pressure; cf‐PWV, carotid‐femoral pulse wave velocity; cPP, central pulse pressure; cSBP, central systolic blood pressure.
P: white vs black.
P: black vs nonblack.
Adjusted by central mean arterial pressure (cMAP), sex, and age.
Table 4.
Peripheral and central blood pressure, pulse wave velocity, AIx, and antihypertensive drugs
| Diuretic | ARB | ACEI | β‐Blocker | CCB | |
|---|---|---|---|---|---|
| No. | 501 | 324 | 347 | 296 | 179 |
| Age, years | 69.8 ± 0.3 | 69.3 ± 0.3 | 69.5 ± 0.3 | 68.8 ± 0.3 | 70.3 ± 0.5 |
| Heart rate | 77.2 ± 1.5 | 73.8 ± 0.7 | 74.5 ± 0.7 | 68 ± 1.4d | 75.5 ± 2.0 |
| Blood pressure, mm Hg | |||||
| bSBP | 139.5 ± 2.57 | 141.3 ± 1.14 | 139.3 ± 1.15 | 138.3 ± 2.21 | 141.6 ± 3.30 |
| bDBP | 86.7 ± 1.60b | 83.5 ± 0.70 | 82.5 ± 0.71c | 81.2 ± 1.36 | 82.9 ± 2.03 |
| bPP | 54.5 ± 2.44 | 57.6 ± 1.08 | 55.6 ± 1.09 | 57.7 ± 2.09 | 59.9 ± 3.12 |
| bMAP | 104.4 ± 1.66 | 102.7 ± 0.71 | 101.5 ± 0.74 | 100.3 ± 1.4 | 103.7 ± 1.07 |
| cSBP | 132.7 ± 2.49 | 136.5 ± 1.08 | 132.3 ± 1.09a | 134.2 ± 1.25 | 133.8 ± 3.11 |
| cDBP | 84.2 ± 0.57 | 85.0 ± 0.73 | 83.8 ± 0.67 | 83.8 ± 0.77 | 85.2 ± 1.03 |
| cPP | 49.8 ± 0.67 | 51.4 ± 0.81 | 48.6 ± 0.79 | 53.3 ± 0.89 | 52.8 ± 1.20 |
| cMAP | 102.3 ± 1.75 | 102.1 ± 0.76 | 100.0 ± 0.74a | 99.7 ± 1.47 | 102.5 ± 0.73 |
| Arterial stiffnesse | |||||
| AP, mm Hg | 14.5 ± 1.0 | 19.0 ± 0.96 | 17.5 ± 0.84 | 21.3 ± 0.66 | 15.3 ± 0.78 |
| AIx, % | 31.7 ± 1.77 | 34.5 ± 0.78 | 33.4 ± 0.78 | 36.9 ± 1.50d | 30.7 ± 2.22 |
| cf‐PWV, m/s | 9.72 ± 0.23 | 9.48 ± 0.11 | 9.48 ± 0.11 | 8.39 ± 0.21d | 9.47 ± 0.15 |
Values are expressed as mean ± standard error.
AP, augmentation pressure; AIx, augmentation index; bDBP, brachial diastolic blood pressure; bMAP, brachial mean arterial pressure; bPP, brachial pulse pressure; bSBP, brachial systolic blood pressure; cDBP, central diastolic blood pressure; cf‐PWV, carotid‐femoral pulse wave velocity cPP, central pulse pressure; cSBP central systolic blood pressure.
Angiotensin receptor blocker (ARB) vs angiotensin‐converting enzyme inhibitor (ACEI): P = 0.047.
β‐Blocker vs diuretic: P = 0.0093.
iECA vs diuretic: P = 0.016.
β‐Blocker vs diuretic, ACEI, ARB, and calcium channel blocker (CCB).
P < 0.05: adjusted by age and central mean arterial (cMAP).
Figure 2.
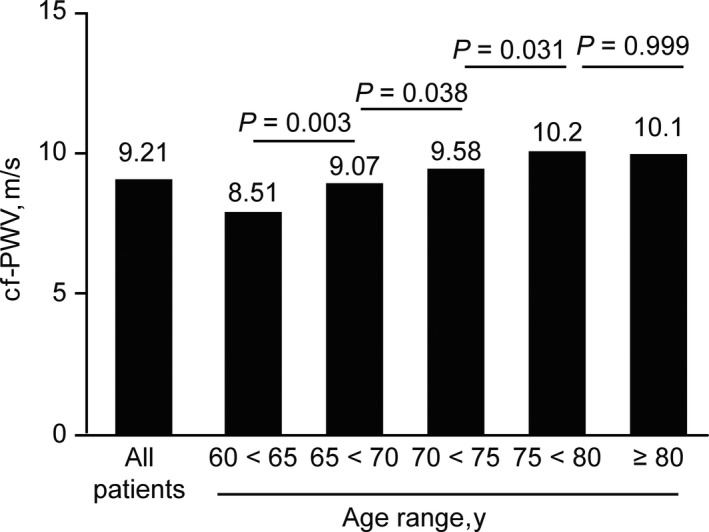
Carotid‐femoral pulse wave velocity (cf‐PWV) values adjusted for sex and central mean arterial pressure and stratified by each 5 y of age
4. DISCUSSION
This study provides detailed normative data on the central BP and measures of arterial stiffness in a large population of patients with NT, CH, and UH from an older urban‐living Brazilian cohort. Arterial stiffness has been evaluated in other large Brazilian cohorts; however, unlike that in the present study, these cohorts were not restricted to the elderly, and the studies focused on other clinical and epidemiological factors, not directly related to hypertension.23, 24, 25, 26 Our data provide relevant novel information on the impact of age, sex, race, and hypertensive status on arterial stiffness in an older population, the major target of arterial stiffening.
Evidence from several observational and controlled randomized trials suggests that antihypertensive treatment reduces arterial wall thickening. This effect seems to be attributed to not only reduced brachial systolic BP but also to arterial remodeling. Moreover, cf‐PWV data can be considered strong evidence of arterial destiffening.27, 28, 29, 30 Our data suggest that elderly patients with hypertension whose BP is controlled by antihypertensive medication have cf‐PWV values similar to those of aged individuals without hypertension. The data shown in Table 4 confirm that patients who used β‐blockers for antihypertensive therapy had the lowest cf‐PWV values. It should be noted that β‐blockers (atenolol) could be combined with other antihypertensive agents and that the observed reduction in cf‐PWV has been previously described.31 The recognized effectiveness of inhibitors of the renin‐angiotensin system31, 32 in reducing vascular wall thickening was not demonstrated by our analysis. However, our study was not designed for this specific analysis. Because we did not design our study to compare the effects of individual classes of medications on arterial stiffness, we cannot rule out confounding by indication and other possible confounders relevant to this analysis.
Regarding the race/ethnicity classification, analysis of BP values showed significant differences in cMAP, cDBP, and bDBP between whites and blacks, whereas the vascular thickening markers (cf‐PWV and AIx) were not different. Comparison of nonblacks and blacks showed the same results. It is necessary to consider that the classification used in our work was arbitrary and dependent on the interpretation by the researchers. In contrast to the data based on the Brazilian Institute for Geographic Statistics classification, which uses a self‐reference for race determination, our percentage of whites was equal to that of blacks, while nonblacks constituted the overwhelming majority. Therefore, we are cautious about making strong comments about the impact of race/ethnicity on our results.
Another interesting observation is that the cf‐PWV values (not adjusted for sex and cMAP) progressively increase throughout older age ranges (Figure 1); however, after adjustment, the cf‐PWV values seem to reach a plateau after age 75 (Figure 2). We speculate that at the age of 75 years and older, new damage to the arterial wall results in a modest increase in cf‐PWV. However, this pattern was not observed for AIx. Of note, the AIx is generally not considered an accurate marker of arterial stiffness because it is strongly influenced by heart rate, height, and contractility and decreases in older age.3, 33
The cf‐PWV values were indistinguishable between the NT and CH groups. Figure 3 shows an overlap in the predictive values of cf‐PWV and age between patients with NT and those with CH at age 60, 70, and 80 years. The patients with UH had higher cf‐PWV values than patients with NT and CH in all age strata, but the slope of the age‐related increase was similar in all three groups. In addition, all components of bBP and central BP in the CH group exhibited values similar to those of the NT group, thus suggesting that antihypertensive therapy maintains the above parameters at levels indistinguishable from those in patients with NT. Because of the cross‐sectional nature of our data, we do not know whether control of BP would lead to normalization of arterial stiffness during follow‐up. Our longitudinal analysis of patients with UH at baseline will allow us to address this question in the future.
Regarding sex‐related differences, hypertensive women had higher systolic values than men, but PWVs were similar between both sexes (Table 2). Among the values observed in elderly men and women, brachial systolic BP, central pulse pressure, brachial pulse pressure, and AIx were higher among women in the UH and CH groups (Table 3). Differences between sexes regarding the central pressure and arterial wall thickening are not completely understood.34 Furthermore, the higher AIx in women could be attributable to an early return of the wave reflection caused by their shorter height13 or decreased aortic diameter35; it could also be associated with sex‐related hormonal differences.36
The PWV values in elderly patients from the urban center in Brazil were higher than those obtained in other studies from Latin American countries. In Argentina, Diaz et al15 reported a PWV value for patients aged 60 to 70 years that was below the overall value obtained in the present study (8.4 vs 9.3 m/s, respectively). The data from the Argentine study were closer to those found in our NT and CH groups. In Uruguay, Farro et al14 reported a PWV value of 10.4 m/s for a hypertensive population younger than 60 years. In Brazil, for healthy patients aged between 55 and 65 years, Baldo et al37 reported a mean cf‐PWV value (9.48 ± 1.39 m/s)37 similar to that found in our study for individuals with NT. Data from Boutouyrie et al38 from different European centers showed cf‐PWV values of 9.3 m/s and 11.1 m/s in elderly patients with NT and hypertension, respectively,38 and Fu et al39 have reported a cf‐PWV value of 12.5 m/s for Chinese patients with hypertension. Both studies showed values slightly higher than those found in our study.
5. STUDY LIMITATIONS
The present investigation has limitations typical of cross‐sectional studies, such as the measurements of BP and applanation tonometry on a single occasion. These values may differ from those of repeated measurements on different occasions, although the reproducibility of these measurements (at the same BP) is usually adequate.40
6. CONCLUSIONS
In an urban cohort of older Brazilian individuals, central BP and cf‐PWV values were higher in patients with UH than in patients with NT and CH. The PWV values increased with age even in this older cohort, reaching a peak at an approximate age of 75 years. Women had a higher AIx, which was possibly attributable to their shorter stature, but their cf‐PWV values were similar to those of their male counterparts. Patients with NT and CH exhibited similar cf‐PWV values, thus suggesting that effective antihypertensive treatment may delay or reverse the hypertension‐associated arterial stiffening.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.
de Mendonça GS, de Souza DF, de Alvarenga Cunha Brunelli AC, et al. Arterial stiffness in elderly patients with normotension and hypertension in Brazil. J Clin Hypertens. 2018;20:1285‐1293. 10.1111/jch.13358
Funding information
Fundação de Amparo à Pesquisa do Estado de Minas Gerais; Brazil (FAPEMIG).
REFERENCES
- 1. Ben‐Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63(7):636‐646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Currie G, Delles C. Healthy vascular aging. Hypertension. 2017;70:229‐231. [DOI] [PubMed] [Google Scholar]
- 3. Cavalcante JL, Lima JA, Redheuil A, Al‐Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57(14):1511‐1522. [DOI] [PubMed] [Google Scholar]
- 4. Cattell MA, Anderson JC, Hasleton PS. Age‐related changes in amounts and concentrations of collagen and elastin in normotensive human thoracic aorta. Clin Chim Acta. 1996;245(1):73‐84. [DOI] [PubMed] [Google Scholar]
- 5. Bruno RM, Duranti E, Ippolito C, et al. Different impact of essential hypertension on structural and functional age‐related vascular changes. Hypertension. 2017;69(1):71‐78. [DOI] [PubMed] [Google Scholar]
- 6. O'Rourke MF, Staessen JA, Vlachopoulos C, Duprez D, Plante GE. Clinical applications of arterial stiffness; definitions and reference values. Am J Hypertens. 2002;15(5):426‐444. [DOI] [PubMed] [Google Scholar]
- 7. McEniery CM, Spratt M, Munnery M, et al. An analysis of prospective risk factors for aortic stiffness in men: 20‐year follow‐up from the Caerphilly prospective study. Hypertension. 2010;56(1):36‐43. [DOI] [PubMed] [Google Scholar]
- 8. Dernellis J, Panaretou M. Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension. 2005;45(3):426‐431. [DOI] [PubMed] [Google Scholar]
- 9. Kaess BM, Rong J, Larson MG, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308(9):875‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Najjar SS, Scuteri A, Shetty V, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51(14):1377‐1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takase H, Dohi Y, Toriyama T, et al. Brachial‐ankle pulse wave velocity predicts increase in blood pressure and onset of hypertension. Am J Hypertens. 2011;24(6):667‐673. [DOI] [PubMed] [Google Scholar]
- 12. Cabrera‐Sole RM, Rivera LU, Lucas CT, Salazar DC, Saldana MA. Isolate systolic hypertension and central pressures in elderly patients. Differences between men and women with similar peripheral pressures. J Am Soc Hypertens. 2016;10(suppl 1):e2‐e3. [DOI] [PubMed] [Google Scholar]
- 13. Gatzka CD, Cameron JD, Dart AM, et al. Correction of carotid augmentation index for heart rate in elderly essential hypertensives. ANBP2 Investigators. Australian Comparative Outcome Trial of Angiotensin‐Converting Enzyme Inhibitor‐ and Diuretic‐Based Treatment of Hypertension in the Elderly. Am J Hypertens. 2001;14(6 pt 1):573‐577. [DOI] [PubMed] [Google Scholar]
- 14. Farro I, Bia D, Zocalo Y, et al. Pulse wave velocity as marker of preclinical arterial disease: reference levels in a uruguayan population considering wave detection algorithms, path lengths, aging, and blood pressure. Int J Hypertens. 2012;2012:169359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Diaz A, Galli C, Tringler M, Ramirez A, Cabrera Fischer EI. Reference values of pulse wave velocity in healthy people from an urban and rural argentinean population. Int J Hypertens. 2014;2014:653239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brant LC, Hamburg NM, Barreto SM, Benjamin EJ, Ribeiro AL. Relations of digital vascular function, cardiovascular risk factors, and arterial stiffness: the Brazilian Longitudinal Study of Adult Health (ELSA‐Brasil) cohort study. J Am Heart Assoc. 2014;3(6):e001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng X, Jin C, Liu Y, et al. Arterial stiffness as a predictor of clinical hypertension. J Clin Hypertens (Greenwich). 2015;17(8):582‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mancia G, De Backer G, Dominiczak A, et al. 2007 ESH‐ESC Practice Guidelines for the Management of Arterial Hypertension: ESH‐ESC Task Force on the Management of Arterial Hypertension. J Hypertens. 2007;25(9):1751‐1762. [DOI] [PubMed] [Google Scholar]
- 19. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588‐2605. [DOI] [PubMed] [Google Scholar]
- 20. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid‐femoral pulse wave velocity. J Hypertens. 2012;30(3):445‐448. [DOI] [PubMed] [Google Scholar]
- 22. Souza DF, Brunelli AC, Peres CI., et al. Agreement among sequential carotid‐femoral pulse wave velocity (cf‐PWV) measurements in elderly hypertensive patients. J Am Soc Hypertens. Supplement, 2016;10(4):e36‐e37. [Google Scholar]
- 23. Ferreira MT, Leite NC, Cardoso CR, Salles GF. Correlates of aortic stiffness progression in patients with type 2 diabetes: importance of glycemic control: the Rio de Janeiro type 2 diabetes cohort study. Diabetes Care. 2015;38(5):897‐904. [DOI] [PubMed] [Google Scholar]
- 24. Meyerfreund D, Goncalves C, Cunha R, Pereira AC, Krieger JE, Mill JG. Age‐dependent increase in blood pressure in two different Native American communities in Brazil. J Hypertens. 2009;27(9):1753‐1760. [DOI] [PubMed] [Google Scholar]
- 25. Zaniqueli D, Alvim RO, Luiz SG, Oliosa PR, de Sa Cunha R, Mill JG. Ethnicity and arterial stiffness in children and adolescents from a Brazilian population. J Hypertens. 2017;35:2257‐2261. [DOI] [PubMed] [Google Scholar]
- 26. Alvim RO, Horimoto AR, Oliveira CM, Bortolotto LA, Krieger JE, Pereira AC. Heritability of arterial stiffness in a Brazilian population: Baependi Heart Study. J Hypertens. 2017;35(1):105‐110. [DOI] [PubMed] [Google Scholar]
- 27. Ait‐Oufella H, Collin C, Bozec E, et al. Long‐term reduction in aortic stiffness: a 5.3‐year follow‐up in routine clinical practice. J Hypertens. 2010;28(11):2336‐2341. [DOI] [PubMed] [Google Scholar]
- 28. Ong KT, Delerme S, Pannier B, et al. Aortic stiffness is reduced beyond blood pressure lowering by short‐term and long‐term antihypertensive treatment: a meta‐analysis of individual data in 294 patients. J Hypertens. 2011;29(6):1034‐1042. [DOI] [PubMed] [Google Scholar]
- 29. Koumaras C, Tzimou M, Stavrinou E, et al. Role of antihypertensive drugs in arterial ‘de‐stiffening’ and central pulsatile hemodynamics. Am J Cardiovasc Drugs. 2012;12(3):143‐156. [DOI] [PubMed] [Google Scholar]
- 30. Laurent S, Boutouyrie P, Vascular Mechanism Collaboration . Dose‐dependent arterial destiffening and inward remodeling after olmesartan in hypertensives with metabolic syndrome. Hypertension. 2014;64(4):709‐716. [DOI] [PubMed] [Google Scholar]
- 31. Mahmud A, Feely J. Beta‐blockers reduce aortic stiffness in hypertension but nebivolol, not atenolol, reduces wave reflection. Am J Hypertens. 2008;21(6):663‐667. [DOI] [PubMed] [Google Scholar]
- 32. Koumaras C, Tziomalos K, Stavrinou E, et al. Effects of renin‐angiotensin‐aldosterone system inhibitors and beta‐blockers on markers of arterial stiffness. J Am Soc Hypertens. 2014;8(2):74‐82. [DOI] [PubMed] [Google Scholar]
- 33. Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. 2003;23(4):554‐566. [DOI] [PubMed] [Google Scholar]
- 34. Rossi P, Frances Y, Kingwell BA, Ahimastos AA. Gender differences in artery wall biomechanical properties throughout life. J Hypertens. 2011;29(6):1023‐1033. [DOI] [PubMed] [Google Scholar]
- 35. Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular‐arterial interactions. J Am Coll Cardiol. 2013;61(1):96‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Waddell TK, Dart AM, Gatzka CD, Cameron JD, Kingwell BA. Women exhibit a greater age‐related increase in proximal aortic stiffness than men. J Hypertens. 2001;19(12):2205‐2212. [DOI] [PubMed] [Google Scholar]
- 37. Baldo MP, Cunha RS, Molina M, et al. Carotid‐femoral pulse wave velocity in a healthy adult sample: The ELSA‐Brasil study. Int J Cardiol. 2018;251:90‐95. [DOI] [PubMed] [Google Scholar]
- 38. Reference Values for Arterial Stiffness Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31(19):2338‐2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fu S, Luo L, Ye P, Xiao W. Multimarker analysis for new biomarkers in relation to central arterial stiffness and hemodynamics in a Chinese community‐dwelling population. Angiology. 2015;66(10):950‐956. [DOI] [PubMed] [Google Scholar]
- 40. Papaioannou TG, Protogerou AD, Nasothimiou EG, et al. Assessment of differences between repeated pulse wave velocity measurements in terms of ‘bias’ in the extrapolated cardiovascular risk and the classification of aortic stiffness: is a single PWV measurement enough? J Hum Hypertens. 2012;26(10):594‐602. [DOI] [PubMed] [Google Scholar]


