Abstract
Longstanding primary aldosteronism (PA) has deleterious effects on renal function, often masked until treatment (adrenalectomy or spironolactone) is initiated. It has been suggested that PA causes relative glomerular hyperfiltration, explaining the decline in estimated glomerular filtration rate (eGFR) after treatment. In this retrospective study, the authors retrieved the clinical characteristics and eGFR of 134 PA patients before and 6 months after treatment. Using multiple regression analysis, the predictors for eGFR decline and the predictors of ultimately attained renal function in 113 patients was assessed. eGFR declined by 15.3±14.2 (range 19–63) mL/min, independent predictors were pretreatment plasma aldosterone, eGFR, plasma renin, and plasma potassium. Independent predictors of ultimately attained eGFR after treatment were pretreatment plasma aldosterone, age, eGFR, and plasma potassium. Our findings lend support to the hypothesis that higher aldosterone levels cause relative glomerular hyperfiltration. The severity of pretreatment aldosterone excess is the most important risk factor for renal function decline.
Primary aldosteronism (PA) accounts for roughly 5% to 10% of all cases of secondary hypertension, making it one of its leading causes.1, 2 PA is associated with renal as well as cardiovascular complications.3, 4, 5, 6, 7 It is treated either by adrenalectomy (ADX) when caused by an aldosterone‐producing adenoma (APA) or by mineralocorticoid receptor antagonists (MRAs) in cases of bilateral adrenal hyperplasia.8 These two treatment modalities lead to similar blood pressure (BP) and renal outcomes.9, 10
Although PA may be associated with impairment of renal function due to prolonged exposure to hypertension and excessive aldosterone, both ADX and treatment with MRAs may paradoxically result in a decline in estimated glomerular filtration rate (eGFR).11 This decline is larger in patients with PA than in those with primary hypertension after start of antihypertensive treatment.12 Therefore, this decline in renal function cannot be attributed to just BP lowering. An alternative and more likely explanation is that in PA patients, aldosterone‐induced glomerular hyperfiltration resolves after treatment, resulting in a decline in eGFR.11, 12 This hyperfiltration is a functional abnormality, masking the underlying structural renal damage due to longstanding exposure to excessive aldosterone levels.6
In earlier publications, pretreatment high plasma aldosterone, low plasma renin, low plasma potassium, and high pretreatment eGFR have been described as significant predictors of eGFR decline after start of treatment.5, 12, 13 In addition, in other studies, the aldosterone‐to‐renin ratio has been reported to be a strong independent predictor of post‐treatment renal insufficiency after adjusting for pretreatment eGFR.14 Both decline in renal function as well as ultimately attained renal function are of interest for clinicians. In this study, we evaluate predictors for both in the same cohort.
We hypothesized that patients who end up with the most severely impaired renal function after treatment had the largest decline in eGFR. If confirmed, this might suggest that glomerular hyperfiltration is an important component of pretreatment eGFR in patients with severe aldosterone‐induced structural renal damage.
Patients and Methods
All patients diagnosed with PA between 1972 and 2013 who had been treated and followed at the Radboud University Medical Center (a tertiary referral hospital) were studied retrospectively (n=140). Data were retrieved from medical files. We excluded six patients because pretreatment data had been obtained while patients were on MRA treatment. Thus, the data of 134 patients could be used for the analysis. eGFR data were not complete for every patient. eGFR before treatment was known for all 134 patients. Of 123 patients, eGFR data were available before treatment and at 6 months after treatment. For 86 patients, eGFR data were available before treatment, at 6 months after treatment, and at 12 months after treatment.
Between 1972 and 2004, PA was diagnosed based on elevated plasma aldosterone and suppressed renin levels with confirmation using an oral sodium loading test. After 2004, instead of oral sodium loading, the diagnosis was confirmed by an intravenous saline infusion test. Differentiation between an APA and BAH was done by either a computed tomography scan (n=55) or adrenal venous sampling (n=79). Antihypertensive treatment during analysis for PA was restricted to drugs with minimal effects on plasma aldosterone and renin levels (calcium antagonists, α‐blockers, and/or hydralazine). Potassium‐sparing diuretics, MRAs, and renin inhibitors were interrupted for a minimum of 6 weeks prior to any biochemical assessment, while other antihypertensives were stopped for a minimum of 2 weeks.
We retrieved clinical and biochemical data from before any therapeutic intervention and data at six and 12 months after intervention for the analysis. We estimated the duration of hypertension from the date hypertension was diagnosed for the first time. BP values before treatment were measured using ambulatory BP monitoring in 31 patients. We used mean daytime BP for the analysis. In 40 patients, BP before treatment was measured in the outpatient department using an electronic device with the mean of eight BP values used for the analysis. In 62 patients, BP values before treatment were based on the mean of three BP measurements during one single office visit.
Data were collected on age, sex, body mass index, plasma potassium, aldosterone, renin, and creatinine before treatment. We used the lowest potassium level recorded before treatment. Plasma creatinine was measured using an enzymatic assay (Architect c16000, Abbott Diagnostics, Lake Bluff, IL). In 124 patients, plasma renin was analyzed by IRMA with Renin III, CIS‐bio (Codolet, France). For another 10 patients, renin was measured as plasma renin activity (PRA). Because PRA and renin concentration are not comparable, we did not use PRA values for analysis.
Data Analyses and Statistics
We categorized BP values as either above or below the cutoff value for normal BP as described by the 2013 European Society of Hypertension guidelines for each type of BP measurement15 The cutoff values for daytime ABPM and home measurements are 135 mm Hg for systolic and 85 mm Hg for diastolic BP. The cutoff values for office measurements are 140 mm Hg for systolic and 90 mm Hg for diastolic BP.
We calculated eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) formula as published in 2009.16 For analysis of post‐treatment eGFR as well as eGFR decline, data from 6‐month follow‐up was used. To document any further change in renal function after 6 months we compared eGFR at 6 and 12 months after start of treatment in the 86 patients for whom eGFR data were available at all of the three measuring points.
We compared patients who underwent ADX with patients who received MRAs using the Mann‐Whitney U test. All collected variables were entered for univariate analyses against eGFR decline and eGFR 6 months after treatment. eGFR data at 6 months after treatment were available in 123 of 134 patients. Variables were entered into multivariate regression analyses if univariate correlation between the dependent variable and independent variables was significant below a P value of <.1. We performed multiple regression analysis using backwards elimination. Forward selection and stepwise regression yielded the same results. P‐out in backwards elimination was set at .05 and P‐in in forward elimination was set at .05. For multiple regression analysis, we used only patients who had no missing values. As there were 10 patients of the remaining 123 patients without plasma renin data, we were able to perform regression analyses for 113 patients. To evaluate whether decline in eGFR could be fully explained by decrease in BP rather than other disease‐related factors, we adjusted eGFR decline and ultimately attained renal function for change in systolic BP in a subgroup of 76 patients whose BP was measured in the same way before and after treatment. We evaluated what effect this had on its correlation with plasma aldosterone level. Data are presented as mean±standard deviation. All statistical analyses were performed using IBM SPSS statistics 22 (IBM Corp, Armonk, NY).
Results
Study Population
Of the 134 patients with PA, 75 were diagnosed with an APA for which they underwent a unilateral ADX. The remaining 59 were diagnosed with BAH for which they were treated with either spironolactone or eplerenone. The baseline pretreatment eGFR was 89.5±17.6 mL/min/1.73 m2. Six months after start of treatment (ADX or MRA), eGFR had significantly decreased by 15.3±14.2 (range 19–63) to 74.2±19.0 mL/min/1.73 m2 (P<.001 vs pretreatment), remaining stable at 12 months (73.5±19.3; P=.43 vs 6 months). Characteristics of the 113 patients who were included in the multivariate analyses are summarized in Table 1.
Table 1.
Patient Characteristics
| Baseline | 6 Months | |||
|---|---|---|---|---|
| No. | No. | |||
| Age, y | 113 | 52±11 | ||
| Men, No. (%) | 113 | 84 (74) | ||
| Body mass index, kg/m2 | 105 | 28.0±5.1 | ||
| Estimated duration of hypertension, y | 103 | 9.6±8.2 | ||
| Type APA/BAH, No. (%) | 113 | 62/51 (55/45) | ||
| Serum creatinine, μmol/L | 113 | 82±19 | 113 | 99±29 |
| eGFR, mL/min/1,73 m2 | 113 | 89±17 | 113 | 75±19 |
| Plasma aldosterone, nmol/L | 113 | 0.83±0.83 | 45 | 0.31±0.31 |
| Plasma renin, mU/L | 113 | 5.00±2.84 | ||
| Plasma potassium, mmol/L | 113 | 2.9±0.36 | 111 | 4.1±0.48 |
| No. of patients with SBP above target value, No. (%) | 113 | 98 (87) | 102 | 56 (55) |
| No. of patients with DBP above target value, No. (%) | 113 | 92 (81) | 102 | 53 (52) |
| SBP, mm Hga | 113 | 157±22 | 105 | 138±14 |
| DBP, mm Hga | 113 | 97±12 | 105 | 87±12 |
Abbreviations: APA, aldosterone‐producing adenoma, BAH, bilateral adrenal hyperplasia; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration formula; SBP, systolic blood pressure. Data are expressed as mean±standard deviation. aBlood pressure was measured using daytime ambulatory blood pressure monitoring using an electronic device or manually during an office visit or at home. In a subset of 76 patients, blood pressure was measured by the same method before and after treatment; this group was used to evaluate the effect that decline of blood pressure had on renal function.
Surgical and Medical Treatment
Patients who underwent ADX (n=67 of a total of 75 patients had data before and 6 months after treatment) and patients who were treated with MRAs (n=56 of a total of 59 patients had data before and 6 months after treatment) did not differ significantly in eGFR decline: 15.4±16.3 and 11.4±11.2, respectively (P=.32). Patients who underwent ADX had a mean post‐treatment eGFR of 72.4±18.7 mL/min/1.73 m2, which was not different from the post‐treatment eGFR in patients who received MRAs: 78.6±17.9 mL/min/1.73 m2 (P=.49).
eGFR Decline and Renal Function After Treatment of PA
eGFR after treatment significantly correlated to eGFR decline (r=−0.449, P<.001). In Figure 1 patients are sorted according to their residual kidney function after treatment. In these groups, the magnitude of decline of GFR is shown. Patients with the worst kidney function after treatment had the largest absolute decline in GFR after treatment.
Figure 1.
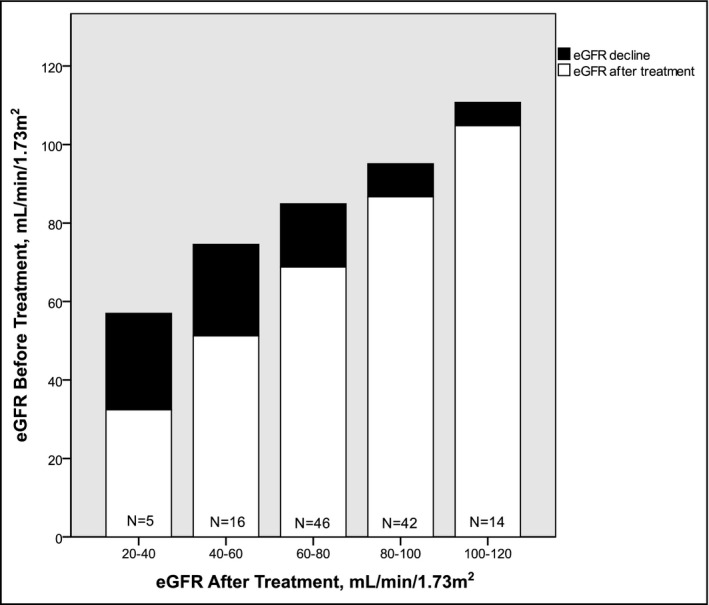
The change in renal function as a function of pretreatment and post‐treatment renal function. eGFR indicates estimated glomerular filtration rate.
Determinants for eGFR Decline After Treatment of PA
We performed univariate analyses to determine variables associated with eGFR decline after treatment of PA (Table 2). Significant predictors were lower eGFR before treatment, lower renin, lower potassium, and higher aldosterone. We entered these variables for multiple regression analysis. Backwards elimination left eGFR before treatment (P=.001), plasma aldosterone (P<.001), plasma renin (P=.048), and plasma potassium (P=.008) as independent risk factors (P<.05) for eGFR decline. The R 2 of the overall model was 0.317. The variance inflation factor (VIF) did not exceed 5 for any variable.
Table 2.
Results of Univariate and Multivariate Regression Analysis
| GFR Decline | GFR After Treatment | |||||
|---|---|---|---|---|---|---|
| P Univariate | P Multivariate | β Multivariate | P Univariate | P Multivariate | β Multivariate | |
| Age, y | >.1 | – | – | .000 | .012 | −0.182 |
| Sex | >.1 | – | – | .022 | >.05 | – |
| BMI, kg/m2 | >.1 | – | – | >.1 | – | – |
| Duration of hypertension, y | >.1 | – | – | .093 | >.05 | – |
| Subtype, APA/BAH | >.1 | – | – | .065 | >.05 | – |
| eGFR before treatment, mL/min/1,73 m2 | <.001 | .001 | 0.286 | <.001 | <.001 | 0.597 |
| Plasma aldosterone, nmol/L | <.001 | <.001 | 0.350 | .012 | <.001 | −0.247 |
| Plasma renin, mU/L | .099 | .048 | −0.162 | >.1 | – | |
| Plasma potassium, mmol/L | .038 | .008 | −0.225 | .004 | .017 | 0.155 |
| SBPa | >.1 | – | – | >.1 | – | – |
| DBPa | >.1 | – | – | >.1 | – | – |
Abbreviations: BAH, bilateral adrenal hyperplasia; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration formula; SBP, systolic blood pressure. The constant for the multivariate regression formula for GFR decline is 18.916, the constant for GFR after treatment is 15.734.
Blood pressure values are categorized as either above or below the cutoff value for normal BP as described by the 2013 European Society of Hypertension guidelines for each type of BP measurement.15
Determinants of Renal Function After Treatment of PA
Determinants of eGFR after treatment in univariate analyses included older age, female sex, longer estimated duration of hypertension, BAH as type of PA, lower eGFR before treatment, higher plasma aldosterone, and lower plasma potassium. We entered these factors into a multivariate regression analysis. Backwards elimination left age (P=.012), eGFR before treatment (P<.001), plasma aldosterone (P<.001), and plasma potassium (P=.017) as significant predictors (P<.05). R 2 of the overall model was 0.579. The VIF did not exceed 5 for any variable.
Effects of Post‐Treatment Change in BP on Renal Function
In 76 patients, BP was measured by the same method before and after treatment. In these patients, we evaluated the effects of change in BP on the correlations with plasma aldosterone level, the strongest independent predictor in both multivariate analyses, to see whether plasma aldosterone also predicts eGFR decline adjusted for BP and eGFR after treatment adjusted for BP. eGFR decline significantly correlated with plasma aldosterone (R 2=0.147, P<.001). When adjusted for decline in systolic BP, this correlation remained significant (R 2=0.247, P<.001). Ultimately attained eGFR after treatment significantly correlated with plasma aldosterone (R 2=0.051, P=.014). When adjusted for change in systolic BP, this correlation also remained significant (R 2=0.092, P=.008).
Discussion
The finding of a declining eGFR after treatment of PA is in line with results of earlier studies.5, 12, 13 Independent predicting factors for the decline in eGFR are pretreatment plasma aldosterone, plasma renin, plasma potassium, and pretreatment eGFR. These are the same predictors Catena and colleagues5 found for decline in creatinine clearance. In contrast, a study by Iwakura and coworkers13 found urinary albumin excretion and potassium to be the only two independent predictors. Data on urinary albumin excretion were missing in too many patients in our cohort, therefore we could not verify whether inclusion of urinary albumin would increase the R 2 of our multivariate model. When evaluating the predictors for ultimately attained post‐treatment renal function, age was an additional predicting variable, confirming results from earlier published studies.14 To our knowledge, this is the first study to look at predictors for both renal function decline and ultimately attainted renal function in the same patient cohort.
In our study, we found that GFR decline correlates with post‐treatment renal function. This is important, as it may signify that hyperfiltration is a larger component of pretreatment GFR in patients who later prove to have the worst renal function (Figure). In other words, the extent of structural renal damage becomes apparent after removing the direct aldosterone effects. The extent of structural damage is masked until then by the functional effect of aldosterone excess. Therefore, when evaluating renal function of an untreated PA patient, physicians should not only take into account eGFR but also aldosterone level, predicting both GFR decline and post‐treatment renal function. Aldosterone strongly correlates with both GFR decline and post‐treatment renal function, although a causal relationship cannot be established by our data.
Change of BP has an effect on renal function. Other studies have shown that if patients with PA are compared with patients with primary hypertension, change of BP accounts for only a small part of the total change in eGFR in these patients.11, 12 In our study, adjusting eGFR decline and ultimately attained renal function for change in systolic BP did not reduce the correlation found with plasma aldosterone level.
The decline in eGFR is most readily explained by a state of hyperfiltration in untreated PA, which is then reversed by treatment.6, 11, 12 Hyperfiltration in PA might be explained by the sodium‐retaining effect, caused by excess aldosterone.17 Escape from this sodium‐retaining effect happens by an increase in extracellular fluid volume, followed by a return to sodium balance, at the expense of a proportional increase in eGFR and renal plasma flow. 17 In addition to the role in potassium and sodium regulation, aldosterone may also have various vascular effects that may lead to increased filtration fraction.18 In a study by Arima and colleagues,19 aldosterone was shown to constrict both afferent and efferent renal arterioles, with a higher sensitivity for efferent arterioles, thereby elevating glomerular capillary pressure and filtration fraction.
How structural renal damage develops in PA patients is not clear. It is possible that hyperfiltration due to hyperaldosteronism itself plays a role. Hyperfiltration occurs in several clinical conditions, such as diabetes mellitus.20 A meta‐analysis of 10 cohort studies in patients with diabetes mellitus regarding a possible association between hyperfiltration and renal dysfunction showed an increased risk for renal failure in patients with hyperfiltration in comparison to patients without hyperfiltration.21 It seems to be the combination of an elevated eGFR and elevated glomerular capillary pressure that causes renal impairment.20 This is in line with the observation that lowering of glomerular capillary pressure by renin‐angiotensin‐aldosterone system inhibitors slows the decline of renal function in these patients.22
Interestingly, treatment with MRAs has also been shown to have beneficial results on renal function in diabetic patients, indicating that aldosterone might also be an important factor in diabetic nephropathy.23, 24, 25 Alternatively, aldosterone itself might cause renal damage, independent of hyperfiltration. Aldosterone causes fibrosis in the heart, and in vitro studies have shown that this feature is at least in part nonhemodynamically mediated.26, 27, 28 In the kidney, aldosterone induced fibrotic changes in normotensive mice.29 These fibrotic changes are stimulated by mineralocorticoid receptor–dependent as well as ‐independent mechanisms.30 In this case, structural damage might be the cause instead of the result of hyperfiltration. It could be that the kidney hyperfiltrates as a form of functional compensation for structural damage in a similar way as occurs after nephrectomy in living kidney donation, where glomerular hyperfiltration of the remaining kidney is an adaptive feature.31
Conclusions
Moderately impaired renal function and a high plasma aldosterone level may predict a relatively large decline in kidney function after treatment of PA. This is compatible with a state of glomerular hyperfiltration in untreated PA. The most important message for daily clinical practice is that physicians should realize that to predict renal function after treatment of PA, pretreatment eGFR alone is not a good marker. Pretreatment eGFR may appear only slightly impaired as a consequence of hyperfiltration and may decline after treatment. The physician may expect a large decline in eGFR in particular if serum aldosterone is high before treatment.
Financial Disclosure
The authors report no specific funding in relation to this research and no conflicts of interest to disclose.
Conflict of Interest
Dr Deinum received a grant from the Dutch Organization for Health Research and Development (ZonMw, grant number 171002102) and participates in EU Horizon 2020 grant No. 633983, ENSAT‐HT. Dr Lenders received a grant from the Dutch Organization for Health Research and Development (ZonMw, grant number 171002102) and from the Deutsche Forschungsgemeinschaft (LE 3660/1‐1 KFO 252). No other disclosures were reported.
J Clin Hypertens (Greenwich). 2017;19:290–295. 10.1111/jch.12914 © 2016 Wiley Periodicals, Inc.
References
- 1. Plouin PF, Amar L, Chatellier G. Trends in the prevalence of primary aldosteronism, aldosterone‐producing adenomas, and surgically correctable aldosterone‐dependent hypertension. Nephrol Dial Transplant. 2004;19:774–777. [DOI] [PubMed] [Google Scholar]
- 2. Mulatero P, Stowasser M, Loh KC, et al. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab. 2004;89:1045–1050. [DOI] [PubMed] [Google Scholar]
- 3. Milliez P, Girert D, Plouin PF, et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. [DOI] [PubMed] [Google Scholar]
- 4. Nishimura M, Uzu T, Fujii T, et al. Cardiovascular complications in patients with primary aldosteronism. Am J Kidney Dis. 1999;33:261–266. [DOI] [PubMed] [Google Scholar]
- 5. Catena C, Colussi G, Nadalini E, et al. Relationships of plasma renin levels with renal function in patients with primary aldosteronism. Clin J Am Soc Nephrol. 2007;2:722–731. [DOI] [PubMed] [Google Scholar]
- 6. Reincke M, Rump LC, Quinkler M, et al. Risk factors associated with a low glomerular filtration rate in primary aldosteronism. J Clin Endocrinol Metab. 2009;94:869–875. [DOI] [PubMed] [Google Scholar]
- 7. Catena C, Colussi G, Sechi LA. Mineralocorticoid receptor antagonists and renal involvement in primary aldosteronism: opening of a new era. Eur J Endocrinol. 2013;168:C1–C5. [DOI] [PubMed] [Google Scholar]
- 8. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–1916. [DOI] [PubMed] [Google Scholar]
- 9. Fourkiotis V,Vonend O, Diederich S, et al. Effectiveness of eplerenone or spironolactone treatment in preserving renal function in primary aldosteronism. Eur J Endocrinol. 2013;168:75–81. [DOI] [PubMed] [Google Scholar]
- 10. Catena C, Colussi G, Lapenna R, et al. Long‐term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension. 2007;50:911–918. [DOI] [PubMed] [Google Scholar]
- 11. Sechi LA, Novello M, Lapenna R, et al. Long‐term renal outcomes in patients with primary aldosteronism. JAMA. 2006;295:2638–2645. [DOI] [PubMed] [Google Scholar]
- 12. Ribstein J, Du Cailar G, Fesler P, Mimran A. Relative glomerular hyperfiltration in primary aldosteronism. Am Soc Nephrol. 2005;16:1320–1325. [DOI] [PubMed] [Google Scholar]
- 13. Iwakura Y, Morimoto R, Kudo M, et al. Predictors of decreasing glomerular filtration rate and prevalence of chronic kidney disease after treatment of primary aldosteronism: renal outcome of 213 cases. J Clin Endocrinol Metab. 2014;99:1593–1598. [DOI] [PubMed] [Google Scholar]
- 14. Tanase‐Nakao K, Naruse M, Nanba K, et al. Chronic kidney disease score for predicting postoperative masked renal insufficiency in patients with primary aldosteronism. Clin Endocrinol. 2014;81:665–670. [DOI] [PubMed] [Google Scholar]
- 15.ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2013;31:1925–1938. [DOI] [PubMed] [Google Scholar]
- 16. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Knox FG, Burnett JC Jr, Kohan DE, et al. Escape from the sodium‐retaining effects of mineralocorticoids. Kidney Int. 1980;17:263–276. [DOI] [PubMed] [Google Scholar]
- 18. Feldman RD, Gros R. Vascular effects of aldosterone: sorting out the receptors and the ligands. Clin Exp Pharmacol Physiol. 2013;40:916–921. [DOI] [PubMed] [Google Scholar]
- 19. Arima S, Kohagura K, Xu HL, et al. Nongenomic vascular action of aldosterone in the glomerular microcirculation. Am Soc Nephrol. 2003;14:2255–2263. [DOI] [PubMed] [Google Scholar]
- 20. Helal I, Fick‐Brosnahan GM, Reed‐Gitomer B, Schrier RW. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol. 2012;8:293–300. [DOI] [PubMed] [Google Scholar]
- 21. Magee GM, Bilous RW, Cardwell CR, et al. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta‐analysis Diabetologia. 2009;52:691–697. [DOI] [PubMed] [Google Scholar]
- 22. Ruggenenti P, Cravedi P, Remuzzi G. Mechanisms and treatment of CKD. J Am Soc Nephrol. 2012;23:1917–1928. [DOI] [PubMed] [Google Scholar]
- 23. Epstein M. Aldosterone as a mediator of progressive renal disease: pathogenetic and clinical implications. Am J Kidney Dis. 2001;37:677–688. [DOI] [PubMed] [Google Scholar]
- 24. Sato A, Hayashi K, Naruse M, Saruta T. Effectiveness of aldosterone blockade in patients with diabetic nephropathy. Hypertension. 2003;41:64–68. [DOI] [PubMed] [Google Scholar]
- 25. Epstein M, Buckalew V, Altamirano J, et al. Eplerenone reduces proteinuria in type II diabetes mellitus: implications for aldosterone involvement in the pathogenesis of renal dysfunction. J Am Coll Cardiol. 2002;39:249A. [Google Scholar]
- 26. Stockand JD, Meszaros JG. Aldosterone stimulates proliferation of cardiac fibroblasts by activating Ki‐RasA and MAPK1/2 signaling. Am J Physiol Heart Circ Physiol. 2003;284:H176–H184. [DOI] [PubMed] [Google Scholar]
- 27. Sun Y, Zhang J, Lu L, et al. Aldosterone‐induced inflammation in the rat heart: role of oxidative stress. Am J Pathol. 2002;161:1773–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689–1697. [DOI] [PubMed] [Google Scholar]
- 29. Brem AS, Morris DJ, Ge Y, et al. Direct fibrogenic effects of aldosterone on normotensive kidney: an effect modified by 11beta‐HSD activity. Am J Physiol Renal Physiol. 2010;298:F1178–F1187. [DOI] [PubMed] [Google Scholar]
- 30. Chen D, Chen Z, Park C, et al. Aldosterone stimulates fibronectin synthesis in renal fibroblasts through mineralocorticoid receptor‐dependent and independent mechanisms. Gene. 2013;531:23–30. [DOI] [PubMed] [Google Scholar]
- 31. Lenihan CR, Busque S, Derby G, et al. Longitudinal study of living kidney donor glomerular dynamics after nephrectomy. J Clin Invest. 2015;125:1311–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]


