Abstract
Pharmacist‐physician collaborative practice models (PPCPMs) improve blood pressure (BP) control, but their effect on time to goal BP is unknown. This retrospective cohort study evaluated the impact of a PPCPM on time to goal BP compared with usual care using data from existing medical records in uninsured patients with hypertension. The primary outcome was time from the initial visit to the first follow‐up visit with a BP <140/90 mm Hg. The study included 377 patients (259 = PPCPM; 118 = usual care). Median time to BP goal was 36 days vs 259 days in the PPCPM and usual care cohorts, respectively (P < .001). At 12 months, BP control was 81% and 44% in the PPCPM and usual care cohorts, respectively (P < .001) and therapeutic inertia was lower in the PPCPM cohort (27.6%) compared with usual care (43.7%) (P < .0001). Collaborative models involving pharmacists should be considered to improve BP control in high‐risk populations.
1. BACKGROUND
Hypertension affects approximately 80 million adults in the United States and is a major risk factor for coronary artery disease, stroke, heart failure, and kidney disease.1 Despite the demonstrated benefit of antihypertensive therapy to reduce blood pressure (BP) and decrease morbidity and mortality, approximately half (54.1%) of adults with hypertension remain uncontrolled.1 Control rates are even lower in minority populations and individuals of low socioeconomic status, with estimates suggesting hypertension control rates in the uninsured population as low as 30%.2, 3
Although current guidelines4 support at least monthly follow‐up of patients with uncontrolled hypertension and escalation of therapy for uncontrolled values, one major contributor to poor hypertension control rates is therapeutic inertia, defined as failure of providers to increase the dose of an existing therapy or add additional therapies when an abnormal clinical parameter is observed.5 Furthermore, delayed intensification of antihypertensive therapy is associated with a progressively higher rate of acute cardiovascular events and mortality.6, 7 Timely management of hypertension and frequent initial follow‐up should therefore be targeted in addition to BP reduction and goal attainment.
Team‐based care is one proposed solution to address both the issue of therapeutic inertia and poor control rates in patients with hypertension.8, 9, 10, 11, 12 Pharmacists in particular have been identified as important members of such team‐based models as recognized by the Centers for Disease Control and Prevention and Surgeon General of the United States.13, 14, 15 Several randomized controlled trials have demonstrated the benefit of a pharmacist‐physician collaborative practice model (PPCPM) in managing hypertension, including improvement in hypertension control rates and reduction in mean BP.8, 16, 17, 18 However, it is unclear whether PPCPMs improve therapeutic inertia or affect time to goal BP attainment. This study sought to investigate the time to goal BP attainment for uninsured patients managed by a PPCPM as compared with usual care in a real‐world clinical setting.
2. METHODS
2.1. Pharmacist‐physician collaborative practice model cohort
The PPCPM used in this study has been previously described.19 In brief, this PPCPM was established in 2008 as an urban safety‐net free clinic in Richmond, Virginia, that primarily serves uninsured patients. Under a collaborative practice agreement with the medical director, pharmacists provide comprehensive medication management with a scope of practice that authorizes medication initiation, management, and monitoring of chronic disease(s) in accordance with state regulations.20 In this model, volunteer physicians and nurse practitioners establish diagnoses and provide annual wellness visits while pharmacists manage all aspects of drug therapy to achieve therapeutic goals for hypertension, dyslipidemia, diabetes mellitus, and chronic heart failure.
2.2. Usual care cohort
Patients in the Virginia Coordinated Care Program of the Virginia Commonwealth University Health System comprised a comparison group and represented the usual care cohort for this study.21 Virginia Commonwealth University Health System is the largest provider of indigent care services in the Commonwealth of Virginia. The Virginia Coordinated Care Program was established in 2000 to better coordinate the care of uninsured individuals in the Greater Richmond Metro area. Under the program, uninsured patients at or below 200% of the federal poverty level are assigned to a community‐based primary care physician and receive access to specialty care, if needed, at no cost. Patients also receive access to low‐cost prescription drugs provided through the 340B drug discount program, a federal program that requires drug manufacturers to provide rebates for outpatient medications to eligible hospitals that serve patients on state Medicaid programs.
2.3. Evaluation
This retrospective longitudinal cohort study identified eligible participants by reviewing the PPCPM's clinic scheduling database for new patient visits. For the usual care cohort, a report was generated for all new enrollees into the Virginia Coordinated Care Program. For both cohorts, patients with a prior diagnosis of hypertension or receiving antihypertensive medications and with an initial clinic visit between January 1, 2012, and December 31, 2013, were included in the study. Individuals with fewer than two clinical encounters during the 1‐year study period, a BP <140/90 mm Hg at the first clinical encounter, an estimated glomerular filtration rate <30 mL/min, missing or incomplete records, documented pregnancy, or evidence of any documented encounters with a clinical pharmacist (usual care cohort only) were excluded.
All data were collected through review of existing health records. The initial visit for both cohorts was used as the index date and to establish baseline characteristics. Data from each visit were collected for 12 months following the participant's initial visit. Demographic and baseline data from first visit included age, sex, race, height, weight, smoking status, estimated glomerular filtration rate, glycated hemoglobin, BP, and documented history of coronary artery disease (CAD), stroke, and diabetes mellitus. For each follow‐up visit, BP, type of provider seen, intervention(s) made at each visit, drug classes used, and visit dates were collected. For visits with multiple BP readings, the last two BP readings were averaged and the mean BP retained.
The primary outcome of interest for this study was time to achieving goal BP, defined as the time from the initial clinic visit to the first visit with a recorded BP <140/90 mm Hg. Secondary end points included proportion of individuals at BP goal at the end of the 12‐month follow‐up period, number of visits required to achieve BP goal, and rate of therapeutic inertia. The proportion of participants at BP goal at study end was assessed using the last BP measurement recorded within the 12‐month follow‐up period from each participant's initial visit. Therapeutic inertia was calculated using the following equation: (h – c)/v, where h is the number of visits with an uncontrolled condition, c is the number of visits in which a change was made, and v is the total number of visits.22
A subgroup analysis excluding participants who presented to their initial visit with urgent hypertension (systolic BP [SBP] ≥180 mm Hg and/or diastolic BP [DBP] ≥110 mm Hg) was performed to focus the analysis on patients with stage I and II hypertension. A time‐to‐event (survival) analysis approach was used to analyze the primary outcome of interest. This was necessary given the fact that the data on time to goal BP had the characteristics of right censoring, ie, time to goal BP did not exist for patients whose BP goals had not been reached at the 1‐year follow‐up or were lost to follow‐up.
Based on baseline differences between groups, a post hoc secondary analysis was also performed. Each group was matched for sex, SBP, and prevalence of CAD at baseline, and the results for time to BP goal, BP control rate, and therapeutic inertia were calculated and compared as with the primary analysis.
Nonparametric Kaplan–Meier survival curves along with 95% confidence limits of median survival time to reach goal BP were generated. Median time intervals (with 25% and 75% interquartiles) to reach goal BP were compared using log‐rank test. Pearson chi‐square tests were used to determine unadjusted associations between categorical variables of interest. Nonparametric Wilcoxon‐Mann‐Whitney tests were used to compare continuous variables of interest. All analyses were performed with SAS version 9.3. The Virginia Commonwealth University institutional review board designated the study as exempt.
3. RESULTS
Of 1146 participants reviewed, 377 met study eligibility criteria, including 259 participants in the PPCPM cohort and 118 in the usual care cohort (Figure 1). The most common reasons for exclusion were fewer than two clinic visits and BP already being at goal at baseline. Compared with the PPCPM cohort, the usual care cohort was older, had a higher percentage of men, and had an increased frequency of previously diagnosed diabetes mellitus and CAD. In addition, SBP was higher in the usual care cohort, and they were more likely to be on antihypertensive therapy at baseline. Of note, estimated glomerular filtration rate was higher in the usual care cohort than the PPCPM cohort. Complete baseline characteristics are presented in Table 1.
Figure 1.
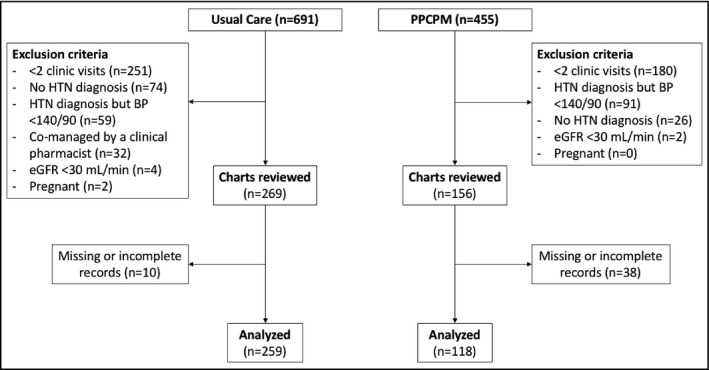
Consort flow diagram of selected patients. BP indicates blood pressure; eGFR, estimated glomerular filtration rate; HTN, hypertension; PPCPM, pharmacist‐physician collaborative practice model
Table 1.
Baseline demographics
| Variable | PPCPM(n = 259) | Usual care(n = 118) | P value |
|---|---|---|---|
| Age, mean (SD), y | 47.2 (10.5) | 50.4 (8.2) | .005 |
| Women, No. (%) | 155 (60) | 48 (41) | <.001 |
| Ethnicity, No. (%) | |||
| Black | 199 (77) | 98 (83) | .09 |
| White | 52 (20) | 13 (11) | |
| Hispanic | 5 (2) | 2 (2) | |
| Other/unknown | 3 (1.1) | 5 (3.3) | |
| BMI, mean (SD), mg/kg2 | 33.6 (8.3) | 31.6 (7.8) | .12 |
| Diabetes mellitus, No. (%) | 41 (16) | 30 (25) | .03 |
| Glycated hemoglobin, mean (SD), % | 8.0 (2.0) | 8.4 (2.3) | .219 |
| CAD, No. (%) | 7 | 22 | <.001 |
| eGFR (mL/min/1.73 m2), mean (SD) | 69.3 (21.1) | 91.6 (27.1) | <.001 |
| Current smoker, No. (%) | 107 (41) | 41 (34) | .18 |
| SBP, mean (SD), mm Hg | 158 (18.9) | 168.5 (26.6) | <.001 |
| DBP, mean (SD), mm Hg | 100.7 (12.1) | 98.6 (15.4) | .07 |
| Antihypertensive agents, mean (SD), No. | 0.67 (1.04) | 1.44 (1.41) | <.0001 |
| Antihypertensive agents, No. (%) | |||
| ACEI or ARB | 44 (17) | 48 (41) | <.001 |
| Thiazide diuretic | 44 (17) | 36 (30) | .004 |
| CCB | 36 (14) | 27 (23) | .027 |
| β‐Blocker | 25 (9) | 31 (26) | <.001 |
| Other | 18 (7) | 21 (18) | .002 |
Categorical variables were compared using chi‐square test; continuous variables were compared using nonparametric Wilcoxon‐Mann‐Whitney test.
Data sources: VCU Health System and Center for Healthy Hearts.
Abbreviations: ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; CCB, calcium channel blocker; CKD, chronic kidney disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; PPCPM, pharmacist‐physician collaborative practice model; SBP, systolic blood pressure; SD, standard deviation.
For the primary outcome, the median times to BP goal were 36 days (95% confidence interval, 30–43; interquartile range, 15–95) and 259 days (95% confidence interval, 182–322; interquartile range, 95–357) in the PPCPM and usual care cohorts, respectively (P < .001). Figure 2 displays the time to event analysis for median time to goal BP attainment in each cohort. From the initial visit to the last observation within the 12‐month follow‐up period, the mean change in SBP was −24.9 ± 23.5 and −15.8 ± 29.5 mm Hg in the PPCPM and usual care cohorts, respectively (P = .0005), while the mean change in DBP was −14.9 ± 14.3 mm Hg and −7.54 ± 15.6 mm Hg in the PPCPM and usual care cohorts, respectively (P < .0001). For participants who achieved BP goal, the mean number of visits required before goal attainment was 3.3 ± 1.6 and 2.8 ± 1.3 in the PPCPM and usual care cohorts, respectively (P = .04). Using the last observation in the 12‐month time period, the BP control rates at study end were 81% in the PPCPM cohort and 44% in the usual care cohort (P < .001). Therapeutic inertia for the 12‐month study period was calculated as 27.6% in the PPCPM cohort and 43.7% in the usual care cohort (P < .0001). Table 2 provides a summary of the main clinical outcomes.
Figure 2.
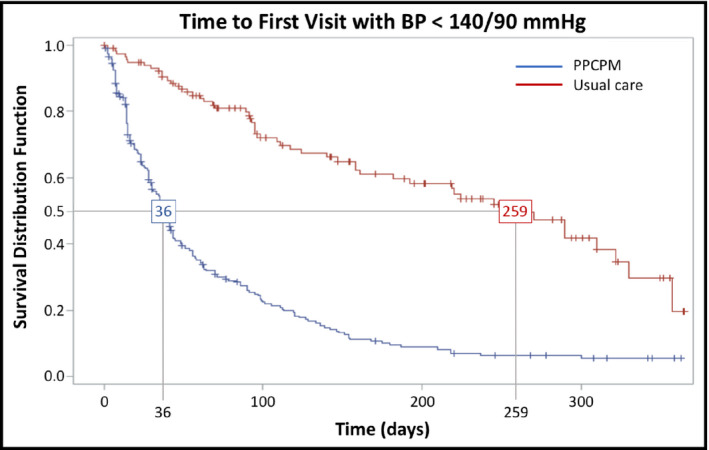
Pharmacist‐physician collaborative practice models (PPCPMs) improve time to goal blood pressure (BP). The primary outcome of time to goal BP is shown using a Kaplan–Meier survival analysis. The median time to goal BP for the PPCPM was 36 days, compared with 259 days for the usual care (SC) cohort
Table 2.
Comparison of the PPCPM vs usual care on BP and time to goal
| Outcome | PPCPM(n = 259) | Usual care(n = 118) | P value |
|---|---|---|---|
| SBP at 12‐mo follow‐up, mean (SD), mm Hg | 133.1 (15.8) | 152.1 (27.1) | <.0001 |
| DBP at 12‐mo follow‐up, mean (SD), mm Hg | 85.7 (10.9) | 90.8 (12.9) | .0003 |
| Change in SBP from baseline, mean (SD), mm Hg | −24.9 (23.5) | −15.8 (29.5) | .0005 |
| Change in DBP from baseline, mean (SD), mm Hg | −14.9 (14.3) | −7.54 (15.6) | <.0001 |
| No. of days to goal BP, median (95% CI/IQR) | 36 (30–43/15–95) | 259 (182–322/95–357) | <.001 |
| No. of visits to goal BP, mean (SD) | 3.3 (1.6) | 2.8 (1.3) | .04 |
| No. of visits during 12‐mo follow‐up period, mean (SD) | 6.1 (3.1) | 3.5 (1.6) | <.001 |
| No. of days between visits during 12‐mo follow‐up period, mean (SD) | 36.2 (17.6) | 106.2 (102.1) | <.001 |
| No. (%) at BP goal at 12‐mo follow‐up | 210 (81) | 52 (44) | <.0001 |
| Therapeutic inertiaa | 27.6 | 43.7 | <.0001 |
Categorical variables compared using chi‐square test; continuous variables compared using nonparametric Wilcoxon‐Mann‐Whitney test change)/total number of visits.
Data sources: VCU Health System and Center for Healthy Hearts.
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; CI, confidence interval; IQR, interquartile range; PPCPM, pharmacist‐physician collaborative practice model; SBP, systolic blood pressure; SD, standard deviation.
Therapeutic inertia = (number of visits BP not at goal – number of visits with a medication).
The mean number of changes to each patient's antihypertensive regimen throughout the 12‐month follow‐up period was 1.9 ± 1.5 in the PPCPM cohort and 1.1 ± 1.1 in the usual care cohort (P < .001). The most common intervention in both cohorts was adding a new antihypertensive therapy and this occurred more frequently in the PPCPM cohort (83.3% vs 51.6%, P < .001). There was no difference in the mean number of antihypertensive agents used at study end; however, there were differences in the types of antihypertensive agents used in each cohort. Thiazide diuretics and calcium channel blockers were prescribed more frequently in the PPCPM cohort, while β‐blockers and other antihypertensive agents (eg, clonidine and hydralazine) were used more frequently in the usual care cohort. A summary of differences between groups regarding the use of antihypertensive agents is provided in Table 3.
Table 3.
Comparison of the PPCPM vs usual care on antihypertensive use
| Outcome | PPCPM (n = 259) | Usual care (n = 118) | P value |
|---|---|---|---|
| No. of changes to antihypertensive regimen, mean (SD) | 1.9 (1.5) | 1.1 (1.1) | <.001 |
| Type of changes to antihypertensive regimen during the 12‐mo follow‐up, No. (%) | |||
| New antihypertensive added | 216 (83) | 61 (52) | <.001 |
| Dose increase | 75 (29) | 41 (35) | .2588 |
| Switched to another class | 32 (12) | 11 (9) | .3903 |
| No. of antihypertensive agents at 12‐mo follow‐up, mean (SD) | 2.0 (1.1) | 2.28 (1.4) | .1806 |
| No. (%) of patients prescribed an: | |||
| ACEI or ARB | 176 (68) | 78 (66) | .7221 |
| Thiazide diuretic | 187 (72) | 69 (58) | .0081 |
| CCB | 144 (56) | 51 (43) | .0257 |
| β‐Blocker | 49 (19) | 49 (42) | <.001 |
| Other | 39 (15) | 33(28) | .0031 |
| Therapeutic inertiaa | 27.6 | 43.7 | <.0001 |
Categorical variables compared using chi‐square test; continuous variables compared using nonparametric Wilcoxon‐Mann‐Whitney test.
Data sources: VCU Health System and Center for Healthy Hearts.
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; PPCPM, pharmacist‐physician collaborative practice model; SBP, systolic blood pressure; SD, standard deviation.
Therapeutic inertia = (number of visits BP not at goal – number of visits with a medication change)/total number of visits.
A subgroup analysis (Table 4) excluding patients who presented to their initial visit with urgent hypertension (SBP ≥180 mm Hg and/or DBP ≥110 mm Hg) resulted in a sample size of 179 in the PPCPM cohort (69.1% of total) and 74 in the usual care cohort (62.7% of total). In this analysis, there was no difference in baseline SBP, but DBP was statistically higher in the PPCPM cohort (95.0 mm Hg vs 90.7 mm Hg, P < .001). The results mirror those observed with the entire study population as the PPCPM cohort achieved their BP goal faster (36 vs 259 days; P < .001), and a higher proportion was at BP goal at the end of the 12‐month follow‐up period (82% vs 47%; P < .0001).
Table 4.
Subgroup analysis excluding participants with SBP ≥180 mm Hg or DBP ≥110 mm Hg at initial clinic visit
| Outcome | PPCPM(n = 179) | Usual care(n = 74) | P value |
|---|---|---|---|
| Baseline SBP, mean (SD), mm Hg | 149.8 (12.2) | 152.6 (10.8) | .09 |
| Baseline DBP, mean (SD), mm Hg | 95.0 (8.2) | 90.7 (9.2) | .0008 |
| No. of days to goal BP, median (95% CI/IQR) | 36 (30–42/15–90) | 270 (182–322/98–357) | <.001 |
| No. of visits to goal BP, mean (SD) | 3.1 (1.4) | 3.0 (1.4) | .45 |
| No. (%) at BP goal at 12‐mo follow‐up | 212 (82) | 56 (47) | <.0001 |
P values based on nonparametric Wilcoxon‐Mann‐Whitney test or time‐to‐event analysis–based long‐rank test.
Data sources: VCU Health System and Center for Healthy Hearts.
Abbreviations: BP, blood pressure; CI, confidence interval; DBP, diastolic blood pressure; IQR, interquartile range; PPCPM, pharmacist‐physician collaborative practice model; SBP, systolic blood pressure; SD, standard deviation.
Given the baseline differences between groups, an adjusted analysis was performed and each group was matched for sex, SBP, and prevalence of CAD at baseline. This analysis included 112 patients (56 in each cohort) and the results for time to BP goal, BP control rate, and therapeutic inertia remained significantly in favor of the PPCPM (Table 5).
Table 5.
Post hoc secondary analysis of the PPCPM vs usual care adjusted for baseline SBP, sex, and history of coronary artery disease
| Outcomes | PPCPM(n = 56) | Usual care(n = 56) | P value |
|---|---|---|---|
| SBP at 12‐mo follow‐up, mean (SD), mm Hg | 129.7 (13.79) | 146.1 (22.2) | <.0001 |
| DBP at 12‐mo follow‐up, mean (SD), mm Hg | 82.5 (9.41) | 88.9 (10.68) | .001 |
| Change in SBP from baseline, mean (SD), mm Hg | −27.7 (20.4) | −11.4 (23) | .0001 |
| Change in DBP from baseline, mean (SD), mm Hg | −19.16 (14.62) | −4.23 (12.5) | <.0001 |
| No. of days to goal BP, median (95% CI/IQR) | 30 (17–43/14–63) | 182 (98–290/91–365) | <.0001 |
| No. of visits during 12‐mo follow‐up period, mean (SD) | 6.44 (3.1) | 3.48 (1.8) | <.001 |
| No. of visits to goal BP, mean (SD) | 3.1 (1.2) | 2.8 (1.3) | .3324 |
| No. (%) at BP goal at 12‐mo follow‐up | 50 (89.2) | 23 (50) | <.0001 |
| Therapeutic inertiaa | 20.1 | 48.1 | <.0001 |
Categorical variables compared using chi‐square test; continuous variables compared using nonparametric Wilcoxon‐Mann‐Whitney test or time‐to‐event analysis–based log‐rank test.
Data sources: VCU Health System and Center for Healthy Hearts.
Abbreviations: BP, blood pressure; DBP, diastolic blood pressure; CI, confidence interval; IQR, interquartile range; PPCPM, pharmacist‐physician collaborative practice model; SBP, systolic blood pressure; SD, standard deviation.
Therapeutic inertia = (number of visits BP not at goal – number of visits with a medication change)/total number of visits.
4. DISCUSSION
Participants in the PPCPM cohort attained their BP goal significantly more quickly than the usual care cohort, a difference of over 7 months between the medians for each cohort. Furthermore, 12‐month BP control rates were nearly two times higher for the PPCPM cohort at study end. It is also important to note that these results were achieved in a majority (≈80%) black population, for which a significant disparity in BP control exists.1 In the context of noted long‐term morbidity and mortality benefits related to earlier BP goal attainment, these findings suggest that the PPCPM may translate to long‐term patient benefits. These results are consistent with previous studies and further emphasize the value of interprofessional team‐based care in the management of patients with hypertension.8, 17
The favorable effects of the PPCPM coincided with a slight increase in the number of clinic visits before BP goal attainment in the PPCPM cohort (3.3 vs 2.8 visits). In addition, the mean time between visits for patients in the PPCPM cohort was monthly (36.2 days), while those in the usual care cohort were seen about every 3 months (106.2 days). In the subgroup analysis excluding patients with urgent hypertension, there was no difference in the number of clinic visits, but the difference in time to goal BP was still observed. This suggests that some other factor–beyond increased number of visits–may be driving the reduction in time to goal BP. Whether the benefits of the PPCPM result from a notable difference in therapeutic approach of clinical pharmacists should be further studied, including examining cost‐benefit calculations of the PPCPM. Alternatively, if the potential differences in practice patterns between the cohorts are ignored, the results of this study raise the possibility that improvements in time to goal BP may be in part affected by repositioning clinic visits earlier in the follow‐up period, rather than an increase in the total number of clinic visits overall. In other words, increased frequency of clinic visits early in the clinical course may be sufficient to accelerate BP goal attainment and potentially offset the need for clinic visits later in the patient course.
To our knowledge, this is the first study to demonstrate that collaborative practice models between pharmacists and physicians may also help to overcome therapeutic inertia, which is the phenomenon of providers declining to intensify medication therapy despite failing to achieve a predefined therapeutic goal.5 The concept of therapeutic inertia has been widely discussed as a contributor to negative outcomes in hypertension management.22 This study demonstrated a lower therapeutic inertia in the PPCPM cohort as compared with the usual care cohort, which may be attributed to the emphasis of this model on medication titration and frequent follow‐up. It should be noted, however, that we could not assess for factors that may have explained failure to adjust antihypertensive therapy, such as nonadherence, decision to focus on lifestyle changes, patient refusal to alter medication therapy, or attributing elevated BP to secondary causes such as pain, acute illness, or white‐coat syndrome.
The two cohorts had some key differences: sex, initial SBP, CAD at enrollment, and use of specific antihypertensive medication classes. However, in a post hoc secondary analysis matched for sex, initial SBP, and CAD at enrollment, the benefit of the PPCPM was maintained. At baseline, a significantly higher proportion of participants in the usual care cohort were prescribed medications from each drug class as compared with the PPCPM cohort, as shown in Table 1. The difference in baseline utilization of medication classes was also true for medications no longer considered first‐line for hypertension, including β‐blockers and “other” classes; however, this was not entirely unreasonable considering guidelines current during the study period and larger number of patients with established CAD.23 It is unclear based on this study whether these differences might be attributable to the higher overall number of medications in the usual care cohort (increased need to resort to second‐line agents), a difference in comorbid conditions (indications for use of other classes), or a difference in prescribing patterns before enrollment.
While we were unable to determine cost‐effectiveness of the PPCPM compared with usual care, previous studies have found the PPCPM to be cost‐effective. Okamota and colleagues24 evaluated cost‐effectiveness in 330 patients randomized to either a pharmacist‐managed hypertension clinic or a traditional physician‐managed general medicine clinic. After 6 months, total costs were no different between groups but the pharmacist‐managed hypertension clinic was more cost‐effective ($27 vs $193/mm Hg for SBP, and $48 vs $151/mm Hg for DBP). More recently, the CAPTION (Collaboration Among Pharmacist and Physicians to Improve Blood Pressure Now) trial18, 25 randomized 625 patients (54% underrepresented minorities) to PPCPM or usual care and found no significant difference in total costs (drug costs, physician time, and pharmacist costs) between the two groups. Compared with the control group, the cost to decrease SBP and DBP by 1 mm Hg was $38.82 and $81.66, respectively.
5. STUDY LIMITATIONS
Our study is not without limitations. The retrospective design limited our ability to control for potential confounders (eg, no‐show rates and variances in BP measurement); however, after matching for differences between groups at baseline, the benefit in our study was maintained. In addition, despite being prescribed more antihypertensive agents at baseline, baseline BP control in the usual care cohort was poor. Perhaps inaccurate medication documentation in the electronic health record and/or a higher rate of nonadherence may explain this observation. Once those with urgent hypertension at baseline were removed, baseline SBP was similar between each cohort and the differences in time to goal BP were retained despite a higher DBP in the PPCPM cohort (Table 4). In addition, at least two BP readings were obtained in the PPCPM, which are frequently lower than an initial BP obtained at check‐in, yet this practice was uncommon in the usual care cohort, as is the case in most practices.26, 27, 28 This could have explained the higher recorded BP measurements obtained in the usual care cohort and should have prompted a change in therapy, yet this was not observed in our study.
6. CONCLUSIONS
Participants managed by the PPCPM attained their BP goal much more rapidly and were more likely to be at BP goal at the end of the 12‐month study period as compared with patients managed by the usual care model. These benefits corresponded with a slightly higher number of clinical encounters and a significantly lower calculated therapeutic inertia in the PPCPM cohort. This suggests that the difference in beneficial clinical outcomes may be attributable to frequent and intense follow‐up early after new‐patient adoption. The reduction in time to goal BP attainment with this model may correlate with a clinically significant reduction in acute cardiovascular events and mortality, as demonstrated in the literature. Follow‐up studies are needed to define the aspects of the PPCPM that are most beneficial, the long‐term impact on sequelae of hypertension, and the cost‐benefit of different approaches to BP management.
CONFLICT OF INTEREST
None.
ACKNOWLEDGMENT
We would like to acknowledge the VCU Office of Health Innovation Research Committee.
Dixon DL, Sisson EM, Parod ED, et al. Pharmacist‐physician collaborative care model and time to goal blood pressure in the uninsured population. J Clin Hypertens. 2018;20:88–95. 10.1111/jch.13150
REFERENCES
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e1‐e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Egan BM, Li J, Small J, Nietert PJ, Sinopoli A. The growing gap in hypertension control between insured and uninsured adults: National Health and Nutrition Examination Survey 1988 to 2010. Hypertension. 2014;64:997‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gu A, Yue Y, Desai RP, Argulian E. Racial and ethnic differences in antihypertensive medication use and blood pressure control among US adults with hypertension. Circ Cardiovasc Qual Outcomes. 2017;10:e003166. [DOI] [PubMed] [Google Scholar]
- 4. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults. JAMA. 2014;311:507‐520. [DOI] [PubMed] [Google Scholar]
- 5. Lebeau JP, Cadwallader JS, Aubin‐Auger I, et al. The concept and definition of therapeutic inertia in hypertension in primary care: a qualitative systematic review. BMC Fam Pract. 2014;15:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu W, Goldberg SI, Shubina M, Turchin A. Optimal systolic blood pressure target, time to intensification, and time to follow‐up in treatment of hypertension: population based retrospective cohort study. BMJ. 2015;350:h158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022‐2031. [DOI] [PubMed] [Google Scholar]
- 8. Carter BL, Rogers M, Daly J, Zheng S, James PA. The potency of team‐based care interventions for hypertension: a meta‐analysis. Arch Intern Med. 2009;169:1748‐1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carter BL, Bosworth HB, Green BB. The hypertension team: the role of the pharmacist, nurse, and teamwork in hypertension therapy. J Clin Hypertens (Greenwich). 2012;14:51‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brush JE, Handberg EM, Biga C, et al. 2015 ACC health policy statement on cardiovascular team‐based care and the role of advanced practice providers. J Am Coll Cardiol. 2015;65:2118‐2136. [DOI] [PubMed] [Google Scholar]
- 11. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2015;310:952‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Green BB, Cook AJ, Ralston JD, et al. Effectiveness of home blood pressure monitoring, web communication, and pharmacist care on hypertension control. JAMA. 2008;299:2857‐2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giberson S, Yoder SLM. Improving patient and health system outcomes through advanced pharmacy practice. A Report to the U.S. Surgeon General. Office of the Chief Pharmacist [serial online]. 2011. https://www.accp.com/docs/positions/misc/Improving_Patient_and_Health_System_Outcomes.pdf. Accessed August 1, 2017.
- 14. Centers for Disease Control and Prevention . Using the pharmacists’ patient care process to manage high blood pressure: a resource guide for pharmacists [serial online]. 2016. https://www.cdc.gov/dhdsp/pubs/docs/pharmacist-resource-guide.pdf. Accessed May 30, 2017.
- 15. Centers for Disease Control and Prevention . Methods and resources for engaging pharmacy partners [serial online]. 2016. https://www.cdc.gov/dhdsp/pubs/docs/engaging-pharmacy-partners-guide.pdf. Accessed May 30, 2017.
- 16. Santschi V, Chiolero A, Colosimo AL, et al. Improving blood pressure control through pharmacist interventions: a meta‐analysis of randomized controlled trials. J Am Heart Assoc. 2014;3:e000718‐e000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Anderegg MD, Gums TH, Uribe L, Coffey CS, James PA, Carter BL. Physician‐pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314‐1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carter BL, Coffey CS, Ardery G, et al. Cluster‐randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235‐243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist‐physician team‐based collaboration to improve long‐term blood pressure control at an inner‐city safety‐net clinic. Pharmacotherapy. 2016;36:342‐347. [DOI] [PubMed] [Google Scholar]
- 20. McBane SE, Dopp AL, Abe A, et al. Collaborative drug therapy management and comprehensive medication management‐2015. Pharmacotherapy. 2015;35:e39‐e50. [DOI] [PubMed] [Google Scholar]
- 21. Retchin SM, Gartand SL, Anum EA. The transfer of uninsured patients from academic to community primary care settings. Am J Manag Care. 2009;15:245‐252. [PubMed] [Google Scholar]
- 22. Okonofua EC, Simpson KN, Jesri A, Rehman SU, Durkalski VL, Egan BM. Therapeutic inertia is an impediment to achieving the Healthy People 2010 blood pressure control goals. Hypertension. 2006;47:345‐351. [DOI] [PubMed] [Google Scholar]
- 23. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206‐1252. [DOI] [PubMed] [Google Scholar]
- 24. Okamoto MP, Nakahiro RK. Pharmacoeconomic evaluation of a pharmacist‐managed hypertension clinic. Pharmacotherapy. 2001;21:1337‐1344. [DOI] [PubMed] [Google Scholar]
- 25. Polgreen LA, Han J, Carter BL, et al. Cost effectiveness of a physician‐pharmacist collaboration intervention to improve blood pressure control. Hypertension. 2015;66:1145‐1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burgess SE, MacLaughlin EJ, Smith PA, Salcido A, Benton TJ. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484‐488. [DOI] [PubMed] [Google Scholar]
- 27. Graves JW, Sheps SG. Does evidence‐based medicine suggest that physicians should not be measuring blood pressure in the hypertensive patient? Am J Hypertens. 2004;17:354‐360. [DOI] [PubMed] [Google Scholar]
- 28. Minor DS, Butler KR, Artman KL, et al. Evaluation of blood pressure measurement and agreement in an academic health sciences center. J Clin Hypertens (Greenwich). 2012;14:222‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]


