Abstract
The objective of this analysis is to determine the effect of intensive (<120 mm Hg) versus standard (<140 mm Hg) systolic blood pressure (SBP) targets on cardiovascular (CV) outcomes among SPRINT participants with low‐normal or high‐normal fasting glucose (FG). We categorized the 5425 SPRINT participants with FG <100 mg/dL into 2 groups: <85 mg/dL (low‐normal) and 85 to <100 mg/dL (high‐normal). Among participants with low‐normal glucose, there was no significant difference in the primary outcome (PO) between the 2 treatment arms (adjusted hazard ratio, HR: 1.27 (95% confidence interval [CI] 0.68‐2.37, P = .46). However, the intensive SBP target was associated with 27% lower risk for the PO compared with the standard SBP target in those with high‐normal glucose (HR 0.73, 0.57‐0.93, P = .01). Our results indicate that hypertensive patients with high‐normal FG may benefit from intensive SBP lowering, whereas benefits were inconclusive among those with low‐normal FG.
Keywords: blood pressure control, glucose, hypertension, SPRINT
1. INTRODUCTION
Hypertension and type 2 diabetes mellitus (T2DM) are major risk factors for cardiovascular (CV) disease, including stroke.1 Untreated normal blood pressure (BP; <120/<80 mm Hg) and fasting glucose <100 mg/dL for adults aged ≥20 years have been identified as 2 of the 7 components of ideal cardiovascular health,2 yet almost half of adult Americans do not meet these criteria.1 The original report of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) BP trial suggested that in individuals with hypertension and T2DM, an intensive systolic BP (SBP; <120 mm Hg) target, did not lower the risk for a composite outcome of fatal and nonfatal major CV events compared with a standard SBP target (<140 mm Hg).3 However, a later planned analysis found that the risk for major CV events was lower in those assigned to intensive SBP lowering, regardless of glycemic target assignment, compared with combined standard SBP and glycemic targets.4 The Systolic Blood Pressure Intervention Trial (SPRINT) documented that the same intensive SBP target reduced CV events compared with the standard SBP target among nondiabetic hypertensive participants.5 Similar conclusions have been drawn from meta‐analyses of large randomized clinical trials that evaluated diabetic and non‐diabetic cohorts of hypertensives.6, 7 Impaired fasting glucose (eg, fasting glucose ≥100, but <126 mg/dL), also called “prediabetes,” is considered an intermediate state of glucose regulation between normal glucose homeostasis and diabetes8 and is associated with increased risk for adverse CV outcomes.9, 10 Previous data indicate that individuals with glucose in the high‐normal range (85 to <100 m/dL) are at increased risk for developing diabetes compared to those with fasting glucose of <85 mg/dL.11 Although hypertension is recognized to be an insulin resistant state,12 information is incomplete regarding the influence of lower SBP targets on adverse CV outcomes comparing hypertensive patients with low‐normal or high‐normal glucose. A recent post hoc analysis of SPRINT suggested that the beneficial effects of intensive SBP target were similar in those with prediabetes and those with baseline fasting glucose of <100 mg/dL.13 However, whether intensive SBP lowering will benefit those with low‐normal glucose and high‐normal glucose, who remain at risk for developing diabetes is not well understood. To address this knowledge gap, we investigated the effects of an intensive SBP target in the cohort of SPRINT participants with baseline fasting glucose that is low‐normal (<85 mg/dL), and high‐normal (85 to <100 mg/dL). We hypothesized that participants with high‐normal glucose, a precursor to prediabetes, will benefit from intensive BP control, whereas those with low‐normal glucose will not.
2. METHODS
2.1. Participants
SPRINT (http://www.ClinicalTrials.gov, Identifier: NCT01206062) was a multicenter randomized controlled US clinical trial funded by the National Institutes of Health that tested the hypothesis that a lower SBP target of <120 mm Hg would reduce CV events more than a standard SBP target of <140 mm Hg.5 The design, rationale and primary results of SPRINT have been published previously.5, 14 Briefly, SPRINT included 9361 men and women aged ≥50 years, with SBP between 130 and 180 mm Hg and an increased risk of CV events (defined as having one or more of the following: clinical or subclinical CVD other than stroke, chronic kidney disease, a 10‐year CVD risk of ≥15% based on Framingham risk score, or age ≥75 years).5 Individuals with diabetes mellitus or prior stroke were excluded.5 Eligible participants were randomly assigned to a treatment strategy with either a SBP target <140 mm Hg (standard) or <120 mm Hg (intensive). All major classes of antihypertensive agents were provided at no cost.5 The primary outcome was the first occurrence of myocardial infarction, other acute coronary syndrome, stroke, heart failure, or death from CV causes. Data for this analysis were obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC) as part of the New England Journal of Medicine SPRINT data analysis challenge.15
2.2. Statistical analysis
Participants with missing baseline glucose data were excluded from this analysis. Those with baseline fasting glucose value of <100 mg/dL were categorized into 2 groups according to baseline fasting blood glucose: <85 mg/dL (low‐normal) and 85 to <100 mg/dL (high‐normal). Continuous variables were compared between the 2 treatment strategies using the t test and categorical variables were compared using Chi‐squared test. Kaplan‐Meier survival analysis and log‐rank test were performed to compare time to the primary outcome among participants in the intensive vs standard SBP target strategies within the glucose groups. Multivariable Cox regression analysis was performed to estimate the adjusted hazard ratio (HR) for the primary outcome in those assigned to intensive vs standard SBP target strategies, within glucose groups. In a stepwise fashion, the covariates (age, sex, race, body mass index, smoking, baseline SBP, baseline diastolic BP [DBP], number of antihypertensive medications at baseline, chronic kidney disease, and history of clinical CV disease) were entered into the model if P < .2 and retained if P < .05. Interaction analysis was also conducted to evaluate if the treatment effect is different in the high‐normal vs low‐normal glucose groups. Similar to the original SPRINT primary report, all‐cause mortality was also evaluated. All analyses were performed in SAS 9.4.
3. RESULTS
Of the 9361 participants, 38 did not have baseline glucose data and were excluded. Of the 9323 remaining SPRINT participants with available fasting glucose values at baseline, 5425 had baseline fasting glucose of <100 mg/dL, including 852 with glucose of <85 mg/dL and 4573 with glucose of 85 to <100 mg/dL. The mean fasting plasma glucose concentrations were 80 and 93 mg/dL, respectively, within the 2 groups. Within each group, approximately 50% of patients were assigned to the intensive or standard BP target arms and the baseline characteristics of the participants were well balanced between the strategies (Table 1).
Table 1.
Pertinent baseline characteristics of participants by baseline glucose level and randomization arm
| Characteristic | Baseline blood glucose (mg/dL) | |||||
|---|---|---|---|---|---|---|
| <85 (n = 852) | 85 to <100 (n = 4573) | |||||
| Intensive (n = 434) | Standard (n = 418) | P | Intensive (n = 2287) | Standard (n = 2286) | P | |
| Age, yr | 67.0 ± 9.7 | 67.2 ± 10.1 | .72 | 68.4 ± 9.5 | 68.2 ± 9.6 | .60 |
| ≥75 | 113 (26.0) | 108 (25.8) | .95 | 693 (30.3) | 683 (29.9) | .75 |
| Female sex | 208 (47.9) | 209 (50.0) | .54 | 903 (39.5) | 835 (36.5) | .039 |
| Race/ethnicity | .96 | .57 | ||||
| Non‐Hispanic white | 204 (47.0) | 190 (45.5) | 1316 (57.5) | 1288 (56.3) | ||
| Non‐Hispanic black | 181 (41.7) | 180 (43.1) | 682 (29.8) | 721 (31.5) | ||
| Hispanic | 42 (9.7) | 40 (9.6) | 244 (10.7) | 239 (11.0) | ||
| Other | 7 (1.6) | 8 (1.9) | 45 (2.0) | 38 (1.7) | ||
| Baseline BP (mm Hg) | ||||||
| Systolic | 140.0 ± 16.2 | 140.9 ± 15.9 | .43 | 140.4 ± 15.8 | 139.9 ± 15.4 | .27 |
| Diastolic | 78.9 ± 12.0 | 79.6 ± 11.9 | .38 | 78.5 ± 11.9 | 78.1 ± 11.9 | .26 |
| Body mass index | 28.4 ± 5.9 | 28.7 ± 6.5 | .55 | 29.3 ± 5.7 | 29.1 ± 5.6 | .35 |
| Smoking status | .58 | .30 | ||||
| Never smoked | 196 (45.2) | 177 (42.3) | 1042 (45.6) | 1044 (45.7) | ||
| Former smoker | 154 (35.5) | 151 (36.1) | 918 (40.1) | 955 (41.8) | ||
| Current smoker | 83 (19.1) | 90 (21.5) | 324 (14.2) | 283 (12.4) | ||
| Missing | 1 (0.2) | 0 (0) | 3 (0.1) | 4 (0.2) | ||
| eGFRa | 72.5 ± 22.9 | 71.6 ± 22.5 | .56 | 70.8 ± 20.4 | 71.4 ± 20.6 | .38 |
| Chronic kidney disease (eGFR<60) | 123 (28.3) | 119 (28.5) | .97 | 682 (29.8) | 652 (28.5) | .33 |
| Framingham 10‐yr CVD risk score (%) | 18.2 ± 10.6 | 18.6 ± 10.6 | .61 | 19.9 ± 10.9 | 19.8 ± 10.7 | .72 |
| Number of antihypertensive agents | 1.7 ± 1.0 | 1.7 ± 1.0 | .47 | 1.8 ± 1.0 | 1.8 ± 1.0 | .58 |
| Not using antihypertensive agents | 50 (11.5) | 46 (11.0) | .81 | 210 (9.2) | 230 (10.1) | .31 |
| Fasting total cholesterol (mg/dL) | 195.4 ± 43.3 | 191.9 ± 39.4 | .22 | 191.8 ± 42.1 | 190.3 ± 40.9 | .21 |
| Fasting plasma glucose (mg/dL) | 79.4 ± 5.2 | 79.4 ± 5.0 | .99 | 92.6 ± 4.1 | 92.8 ± 4.0 | .20 |
| Fasting HDL cholesterol (mg/dL) | 57.5 ± 15.9 | 57.0 ± 16.2 | .61 | 54.7 ± 14.8 | 54.1 ± 14.8 | .23 |
| Fasting total triglycerides (mg/dL) | 108.8 ± 59.0 | 109.4 ± 71.2 | .89 | 116.0 ± 75.0 | 119.8 ± 77.7 | .10 |
| Cardiovascular disease | 74 (17.1) | 75 (17.9) | .73 | 447 (19.6) | 450 (19.7) | .91 |
| Clinical | 56 (12.9) | 64 (15.3) | .31 | 370 (16.2) | 365 (16.0) | .84 |
| Subclinicalb | 21 (4.8) | 19 (4.6) | .84 | 121 (5.3) | 132 (5.8) | .47 |
Data represent mean ± SD or % where appropriate. P values were from t test for continuous variables and chi‐square test for categorical variables.
eGFR: estimated glomerular filtration rate based on MDRD in mL/min/1.73 m2.
Subclinical CVD: coronary artery calcium score ≥400 Agaston unites within the past 2 years; or ankle brachial index ≤0.90 within the past 2 years; left ventricular hypertrophy by ECG, echocardiogram report, or other cardiac imaging procedure report within the past 2 years.
Overall, after an average follow‐up of 3.1 years, there were no statistically significant differences in the percentage of participants who experienced a primary outcome event among those with low‐normal glucose (4.81%) or high‐normal glucose (6.01%) (P = .17). The intensive SBP target strategy was associated with lower risk for the primary outcome compared with the standard SBP target in those with high‐normal glucose (log‐rank P = .021). However, among participants with low‐normal glucose, there was no evidence to suggest that the primary outcome risk was reduced comparing an intensive SBP target vs standard SBP target (log‐rank P = .51) (Figure 1). The adjusted HR for the intensive vs standard SBP target strategy was 1.27 (95% confidence interval [CI] 0.68‐2.37, P = .46) in patients with low‐normal glucose, and 0.73 (95% CI, 0.57‐0.93, P = .010) in participants with high‐normal glucose (Figure 2). The test for interaction between treatment strategies and the 2 glucose groups was not significant (P = .16). We observed similar results evaluating the effect of intensive vs standard SBP targets and all‐cause mortality in the high‐normal glucose participants, with an adjusted HR of 0.70 (95% CI, 0.52‐0.94; P = .018). In patients with low‐normal glucose, the adjusted HR for all‐cause mortality comparing the intensive vs standard SBP target was 0.73 (95% CI, 0.33‐1.63; P = .45).
Figure 1.
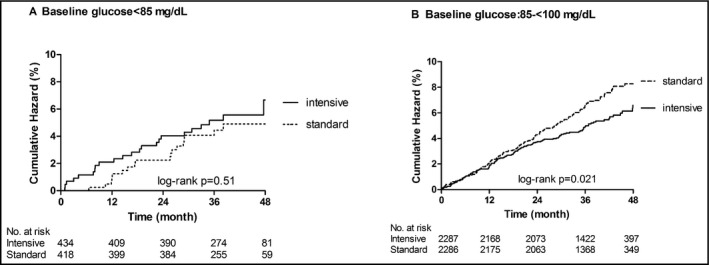
Kaplan Meier curve for primary outcome by treatment targets within each baseline glucose strata. Standard: standard SBP target arm; intensive: intensive SBP target arm. (A) Baseline glucose <85 mg/dL. (B) Baseline glucose of 85 to <100 mg/dL
Figure 2.
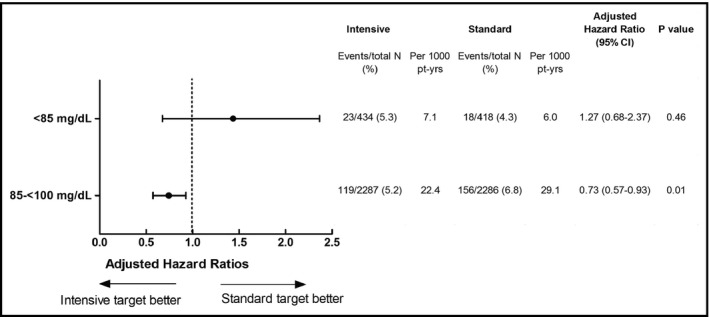
Adjusted hazard ratios for primary outcome in the intensive SBP target arm compared to the standard SBP target group
4. DISCUSSION
In this analysis of SPRINT data, we found that hypertensive participants with high‐normal glucose benefited with reduction in CV risk and death from antihypertensive treatment targeting a SBP <120 mm Hg. A similar benefit was not observed among the participants with low‐normal glucose, although such a benefit cannot be excluded given the wide confidence intervals for both outcomes in this group. Although large randomized trials and meta‐analyses have been conducted among hypertensives with and without diabetes,6, 7 to our knowledge, the present study is the first to assess the impact of different BP targets among individuals without diabetes or impaired fasting glucose.
The 2017 High Blood Pressure Clinical Practice Guideline recommends a BP target <130/80 mm Hg for all patients with hypertension, without regard to glucose level.16 Our current analysis provides evidence to support the recommendation that an intensive SBP target is advisable for hypertensive individuals with fasting glucose levels in the high‐normal range (≥85 to <100 mg/dL). Furthermore, these data accord with recently published data from SPRINT, reaching a similar conclusion for hypertensive participants with prediabetes (≥100 mg/dL).13 Although the recent guidelines do not explicitly consider glucose levels in determining a need for more aggressive BP reduction, these data, taken together, appear to provide support for the use of more intensive SBP targets in anyone with fasting glucose levels ≥85 mg/dL.
Unfortunately, our data do not allow for drawing definitive conclusions from the relatively small subset of patients with low‐normal glucose levels. Therefore, in order to provide consistent public health messaging in the absence of definitive data, it seems reasonable to also use the more intensive SBP target in this population. However, it should also be noted that the 2017 High Blood Pressure Clinical Practice Guideline16 specifies not only updated definition of hypertension, but also thresholds for when to begin BP‐lowering treatment. Specifically, the guidelines state that patients with stage I hypertension (eg, SBP 130 to <140) and an Atherosclerotic Cardiovascular Disease (ASCVD) 10‐year risk <10% do not necessarily require antihypertensive therapy to achieve an SBP < 130 mm Hg. SPRINT, by definition, enrolled a relatively high‐risk hypertensive population, including high‐risk patients with low‐normal glucose. However, in the real world, without the constraints of clinical trial eligibility criteria, hypertensive patients with low‐normal glucose are likely to have comparatively lower ASCVD risk than patients with higher fasting glucose. Future research will need to explore the benefits and risks associated with more aggressive BP targets in this lower risk population.
Our study has some limitations. First, this is a post‐hoc analysis, even though the randomization strategy appears largely intact within the 2 strata of baseline fasting glucose, this was not a pre‐specified analysis. Second, the sample size in the low‐normal glucose group was relatively small and was underpowered. The lack of benefit of the intensive SBP target in the low‐normal glucose group requires further conformation in light of the low sample size. However, if confirmed, inclusion of glucose level as a biomarker in the personalization of BP targets may be important in light of the potential risks associated with additional antihypertensive drug exposure. Finally, since SPRINT study enrolled individuals with increased risk for CV events, these results may not be generalizable to all hypertensive patients within the normal fasting glucose range.
5. CONCLUSIONS
Our data provide the first evidence to support the hypothesis that among hypertensive individuals with “normal” glucose (<100 mg/dL), the benefits of an intensive SBP target CV risk, and all‐cause mortality reduction appear to concentrate among those with high‐normal glucose (85 to <100 mg/dL). We provide further clarity to the body of knowledge surrounding the glucose continuum and the benefits of targeting intensive SBP lowering for risk reduction.
CONFLICT OF INTEREST
The authors declare no conflict of interest associated with this manuscript.
ACKNOWLEDGMENTS
We acknowledge all of the SPRINT Project investigators and participants for their contribution and the NEJM SPRINT data analysis challenge committee, since without their work this analysis would not have been possible.
Gong Y, Smith SM, Handberg EM, Pepine CJ, Cooper‐DeHoff RM. Intensive blood pressure lowering reduces adverse cardiovascular outcomes among patients with high‐normal glucose: An analysis from the Systolic Blood Pressure Intervention Trial database. J Clin Hypertens. 2018;20:620–624. 10.1111/jch.13247
REFERENCES
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics‐2017 update: a report from the American Heart Association. Circulation. 2017;135:e146‐e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lloyd‐Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586‐613. [DOI] [PubMed] [Google Scholar]
- 3. Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood‐pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575‐1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Margolis KL, O'Connor PJ, Morgan TM, et al. Outcomes of combined cardiovascular risk factor management strategies in type 2 diabetes: the ACCORD randomized trial. Diabetes Care. 2014;37:1721‐1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomopoulos C, Parati G, Zanchetti A. Effects of blood‐pressure‐lowering treatment on outcome incidence in hypertension: 10 ‐ Should blood pressure management differ in hypertensive patients with and without diabetes mellitus? overview and meta‐analyses of randomized trials. J Hypertens. 2017;35:922‐944. [DOI] [PubMed] [Google Scholar]
- 7. Xie X, Atkins E, Lv J, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta‐analysis. Lancet. 2016;387:435‐443. [DOI] [PubMed] [Google Scholar]
- 8. Expert committee on the Diagnosis and Classification of Diabetes Mellitus . Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5‐S20. [DOI] [PubMed] [Google Scholar]
- 9. Hunt KJ, Resendez RG, Williams K, et al. National cholesterol education program versus World Health Organization metabolic syndrome in relation to all‐cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. 2004;110:1251‐1257. [DOI] [PubMed] [Google Scholar]
- 10. Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle‐aged men. JAMA. 2002;288:2709‐2716. [DOI] [PubMed] [Google Scholar]
- 11. Nichols GA, Hillier TA, Brown JB. Normal fasting plasma glucose and risk of type 2 diabetes diagnosis. Am J Med. 2008;121:519‐524. [DOI] [PubMed] [Google Scholar]
- 12. Salvetti A, Brogi G, Di Legge V, Bernini GP. The inter‐relationship between insulin resistance and hypertension. Drugs. 1993;46(Suppl 2):149‐159. [DOI] [PubMed] [Google Scholar]
- 13. Bress AP, King JB, Kreider KE, et al. Effect of intensive versus standard blood pressure treatment according to baseline prediabetes status: a post hoc analysis of a randomized trial. Diabetes Care. 2017;10:1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing 2 strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burns NS, Miller PW. Learning what we didn't know ‐ the SPRINT data analysis challenge. N Engl J Med. 2017;376:2205‐2207. [DOI] [PubMed] [Google Scholar]
- 16. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;24430: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]


