1. INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) affects 2%‐7% of the adult population and is the most common sleep‐related breathing disorder.1 It is the most frequent cause of secondary and difficult‐to‐treat hypertension and represents a well‐known risk factor for hypertension‐associated end‐stage organ damage.2, 3 The investigation of blood pressure (BP) behavior and of its determinants in OSAS patients helps to understand some pathophysiologic aspects of hypertension, stratify the cardiovascular risk profile, and support indication for therapy in affected patients.
2. NOCTURNAL BP PROFILE ASSESSMENT
The assessment of nocturnal BP profile has important clinical relevance. Indeed, clinical studies have demonstrated that nocturnal BP and BP variability (BPV) are more closely associated with the risk of developing target‐organ damage and future cardiovascular events in comparison to awake BP and BPV.4, 5 Ambulatory blood pressure monitoring (ABPM), which currently represents the gold standard of nocturnal BP assessment, measures the individual BP at fixed time intervals. Because of the lack of any synchronization with sleep apnea, ABPM can fail to detect apnea‐related BP fluctuations and, hence, underestimate the extent of cardiovascular risk.6
In this issue of The Journal of Clinical Hypertension, the study by Kuwabara and colleagues explored the association between polysomnography‐derived sleep parameters and nocturnal BP indices as derived by an oxygen‐triggered nocturnal BP monitoring system.7 This is an information technology‐based system, which indicates BP measurements when oxygen desaturation falls below a set variable threshold continuously monitored by pulse oxymetry.8 In this way, the system allows the detection of the nocturnal BP surge triggered by hypoxic apnea episodes.
The study clearly demonstrated that, in OSAS patients, the hypoxia‐peak systolic BP, defined as the maximum systolic BP value measured by the oxygen‐triggered function, and the nocturnal systolic BP surge, defined as the difference between the hypoxia‐peak systolic BP and the average of the systolic BP values within 30 minutes before and after the hypoxia‐peak systolic BP, were higher, ranged more broadly, and were more closely associated with respiratory‐related polisomnographic parameters than maximum and mean nocturnal systolic BP obtained by the fixed‐interval function through conventional ABPM.7 In particular, the lowest oxygen saturation (SpO2), defined as the minimum SpO2 value during sleep, was the strongest independent determinant of either hypoxia‐peak systolic BP or nocturnal systolic BP surge.7
These findings suggest that the severity and frequency of the decrease in SpO2 have a strong impact on the nocturnal BP trajectories, and the hypoxia‐triggered nocturnal BP measurement may be a promising technique to improve the cardiovascular evaluation of OSAS patients. Noteworthy, in OSAS patients cardiovascular events occur more frequently during sleep, and hypoxia‐triggered nocturnal BP surge and exaggerated BP fluctuations can be contributing factors.9, 10
3. FROM PATHOPHYSIOLOGY TO CLINICAL PRACTICE: IMPLICATIONS AND FUTURE CHALLENGES
The strong independent relationship between the lowest SpO2 and hypoxia‐peak systolic BP might be attributed to apnea duration. Longer apnea periods can induce more severe reduction of SpO2, stronger activation of sympathetic nervous system, and greater Valsalva effect and result into higher BP surge. The severity of OSAS is currently defined according to the number of apnea‐hypopnea episodes, but the degree of desaturation during breathing events would be even more informative and clinically meaningful with regard to the hemodynamic and cardiovascular effects.
The recurrent obstruction of the upper airways during sleep leads to intermittent oxygen desaturation, intrathoracic pressure changes, and repetitive arousals, which contribute to the alteration of systemic BP and promote target‐organ damage through heterogeneous and synergetic mechanisms (Figure 1).11, 12 The enhanced sympathetic activity, which derives from either the activation of carotid body chemoreceptors triggered by episodic hypoxemia or the generalized stress induced by sleep fragmentation, leads to catecholamine surge and baroreceptor sensitivity impairment.13 Furthermore, the up‐regulation of the renin‐angiotensin‐aldosterone and atrial natriuretic peptide systems in response to raised renin levels and intrapleural pressure swings promotes the body fluid redistribution.14 Crucially, over time, these autonomic and neurohumoral derangements perpetuate beyond the offending events and persist into the daytime, resulting in a disturbance of the overall circadian BP rhythm and an increase in short‐ and long‐term BPV.15 In this respect, there is accruing evidence that not only high absolute BP levels but even their fluctuations are closely related to the development and progression of organ damage16, 17, 18, 19, 20, 21, 22, 23, 24, 25 by promoting arterial remodelling, microvascular damage, hemodynamic instability, and vascular reactivity impairment.26, 27, 28, 29 In addition, recurrent intermittent hypoxia and subsequent reoxygenation, which resembles the ischemia‐reperfusion cycle, can stimulate the release of reactive oxygen species, inflammatory cytokines, and vasoactive mediators that further promote endothelial injury and dysfunction.30, 31
Figure 1.
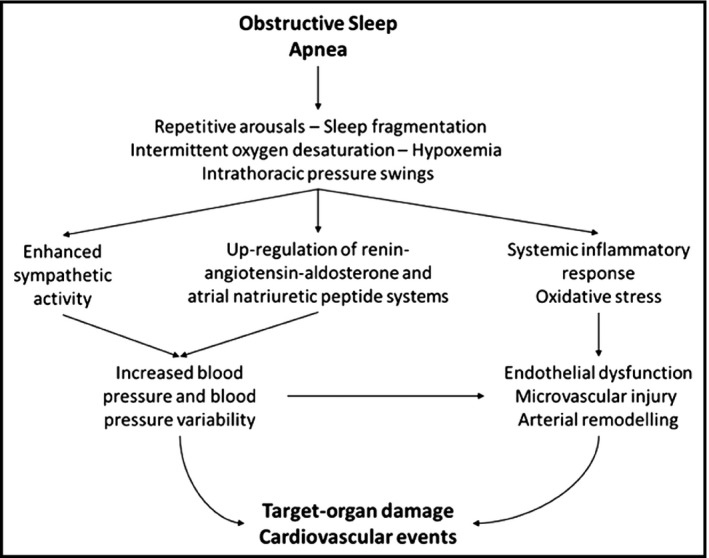
Obstructive sleep apnea and target‐organ damage. Associations between obstructive sleep apnea and target‐organ damage pathology (see text for details)
Future studies are warranted to identify polysomnographic parameters, BP and BPV indices, and serum biomarkers, which may be of aid to characterize the disease pathways and severity. The improvement of the clinical assessment through composite scoring systems incorporating variables that can take into account OSAS pathophysiology would be useful to identify the high‐risk patients, individualize the management, and monitor the quality of treatment and BP control. In the era of precision medicine, understanding the BP patterns, the mechanisms causing and maintaining hypertension, and the effects of therapies in controlling BP and BPV32, 33, 34, 35, 36, 37, 38, 39, 40 plays a key role in defining effective therapeutic interventions to improve unfavorable cardiovascular outcomes.
CONFLICT OF INTEREST
None.
REFERENCES
- 1. Parati G, Lombardi C, Narkiewicz K. Sleep apnea: epidemiology, pathophysiology, and relation to cardiovascular risk. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1671‐R1683. [DOI] [PubMed] [Google Scholar]
- 2. Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med. 2000;342:1378‐1384. [DOI] [PubMed] [Google Scholar]
- 3. Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58:811‐817. [DOI] [PubMed] [Google Scholar]
- 4. Boggia J, Li Y, Thijs L, International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes (IDACO) investigators , et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219‐1229. [DOI] [PubMed] [Google Scholar]
- 5. Palatini P, Reboldi G, Beilin LJ, et al. Added predictive value of night‐time blood pressure variability for cardiovascular events and mortality: the Ambulatory Blood Pressure‐International Study. Hypertension. 2014;64:487‐493. [DOI] [PubMed] [Google Scholar]
- 6. Xu J, Ding N, Zhang X, et al. Nocturnal blood pressure fluctuation and associated influential factors in severe obstructive sleep apnea patients with hypertension. Sleep Breath. 2018. 10.1007/s11325-018-1634-6. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7. Kuwabara M, Tomitani N, Shiga T, et al. Polysomnography‐derived sleep parameters as a determinant of nocturnal blood pressure profile in patients with obstructive sleep apnea. J Clin Hypertens (Greenwich). 2018. 10.1111/jch.13308. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shirasaki O, Kuwabara M, Saito M, et al. Development and clinical application of a new technique for detecting “sleep blood pressure surges” in sleep apnea patients based on a variable desaturation threshold. Hypertens Res. 2011;34:922‐928. [DOI] [PubMed] [Google Scholar]
- 9. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen‐year follow‐up of the Wisconsin sleep cohort. Sleep. 2008;31:1071‐1078. [PMC free article] [PubMed] [Google Scholar]
- 10. Kario K. Prognosis in relation to blood pressure variability: pro side of the argument. Hypertension. 2015;65:1163‐1169. [DOI] [PubMed] [Google Scholar]
- 11. Zhang W, Si LY. Obstructive sleep apnea syndrome (OSAS) and hypertension: pathogenic mechanisms and possible therapeutic approaches. Ups J Med Sci. 2012;117:370‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lattanzi S, Brigo F, Silvestrini M. Blood pressure profile and nocturnal oxygen desaturation. J Clin Hypertens (Greenwich). 2018;20: 656‐658. 10.1111/jch.13259. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lesske J, Fletcher EC, Bao G, et al. Hypertension caused by chronic intermittent hypoxia—influence of chemoreceptors and sympathetic nervous system. J Hypertens. 1997;15:1593‐1603. [DOI] [PubMed] [Google Scholar]
- 14. Konecny T, Kara T, Somers VK. Obstructive sleep apnea and hypertension: an update. Hypertension. 2014;63:203‐209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crinion SJ, Ryan S, McNicholas WT. Obstructive sleep apnoea as a cause of nocturnal nondipping blood pressure: recent evidence regarding clinical importance and underlying mechanisms. Eur Respir J. 2017;49:1601818. [DOI] [PubMed] [Google Scholar]
- 16. Wang J, Shi X, Ma C, et al. Visit‐to‐visit blood pressure variability is a risk factor for all‐cause mortality and cardiovascular disease: a systematic review and meta‐analysis. J Hypertens. 2017;35:10‐17. [DOI] [PubMed] [Google Scholar]
- 17. Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta‐analysis. BMJ. 2016;354:i4098. 10.1136/bmj.i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lattanzi S, Vernieri F, Silvestrini M. Blood pressure variability and neurocognitive functioning. J Clin Hypertens (Greenwich). 2018;20:645‐647.. 10.1111/jch.13232. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lattanzi S, Viticchi G, Falsetti L, et al. Visit‐to‐visit blood pressure variability in Alzheimer disease. Alzheimer Dis Assoc Disord. 2014;28:347‐351. [DOI] [PubMed] [Google Scholar]
- 20. Lattanzi S, Luzzi S, Provinciali L, et al. Blood pressure variability predicts cognitive decline in Alzheimer’s disease patients. Neurobiol Aging. 2014;35:2282‐2287. [DOI] [PubMed] [Google Scholar]
- 21. Lattanzi S, Luzzi S, Provinciali L, et al. Blood pressure variability in Alzheimer’s disease and frontotemporal dementia: the effect on the rate of cognitive decline. J Alzheimers Dis. 2015;45:387‐394. [DOI] [PubMed] [Google Scholar]
- 22. Lattanzi S, Brigo F, Vernieri F, et al. Visit‐to‐visit variability in blood pressure and Alzheimer’s Disease. J Clin Hypertens (Greenwich). 2018. 10.1111/jch.13290. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chia YC, Lim HM, Ching SM. Long‐term visit‐to‐visit blood pressure variability and renal function decline in patients with hypertension over 15 years. J Am Heart Assoc. 2016;5: pii: e003825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Velasquez MT, Beddhu S, Nobakht E, et al. Ambulatory blood pressure in chronic kidney disease: ready for prime time? Kidney Int Rep. 2016;1:94‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohkuma T, Woodward M, Jun M, et al. Prognostic value of variability in systolic blood pressure related to vascular events and premature death in type 2 diabetes mellitus: the ADVANCE‐ON Study. Hypertension. 2017;70:461‐468. [DOI] [PubMed] [Google Scholar]
- 26. Tedla YG, Yano Y, Carnethon M, et al. Association between long‐term blood pressure variability and 10‐year progression in arterial stiffness: the multiethnic study of atherosclerosis. Hypertension. 2017;69:118‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ribeiro AH, Lotufo PA, Fujita A, et al. Association between short‐term systolic blood pressure variability and carotid intima‐media thickness in ELSA‐Brasil baseline. Am J Hypertens. 2017;30:954‐960. [DOI] [PubMed] [Google Scholar]
- 28. Buratti L, Cagnetti C, Balucani C, et al. Blood pressure variability and stroke outcome in patients with internal carotid artery occlusion. J Neurol Sci. 2014;339:164‐168. [DOI] [PubMed] [Google Scholar]
- 29. Lattanzi S, Carbonari L, Pagliariccio G, et al. Neurocognitive functioning and cerebrovascular reactivity after carotid endarterectomy. Neurology. 2018;90:e307‐e315. [DOI] [PubMed] [Google Scholar]
- 30. Lavie L. Oxidative stress—a unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis. 2009;51:303‐312. [DOI] [PubMed] [Google Scholar]
- 31. Ishikawa J, Hoshide S, Eguchi K, et al. Increased low‐grade inflammation and plasminogen‐activator inhibitor‐1 level in nondippers with sleep apnea syndrome. J Hypertens. 2008;26:1181‐1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kraiczi H, Hedner J, Peker Y, et al. Comparison of atenolol, amlodipine, enalapril, hydrochlorothiazide, and losartan for antihypertensive treatment in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:1423‐1428. [DOI] [PubMed] [Google Scholar]
- 33. Cicolin A, Mangiardi L, Mutani R, et al. Angiotensin‐converting enzyme inhibitors and obstructive sleep apnea. Mayo Clin Proc. 2006;81:53‐55. [DOI] [PubMed] [Google Scholar]
- 34. Kario K, Kuwabara M, Hoshide S, Nagai M, Shimpo M. Effects of nighttime single‐dose administration of vasodilating vs sympatholytic antihypertensive agents on sleep blood pressure in hypertensive patients with sleep apnea syndrome. J Clin Hypertens (Greenwich). 2014;16:459‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kario K, Schwartz JE, Pickering TG. Changes of nocturnal blood pressure dipping status in hypertensives by nighttime dosing of alpha‐adrenergic blocker, doxazosin: results from the HALT study. Hypertension. 2000;35:787‐794. [DOI] [PubMed] [Google Scholar]
- 36. Kario K. Catheter‐based renal denervation reduces hypoxia‐triggered nocturnal blood pressure peak in obstructive sleep apnea syndrome. J Clin Hypertens (Greenwich). 2016;18:707‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Webb AJ, Fischer U, Mehta Z, et al. Effects of antihypertensive‐drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta‐analysis. Lancet. 2010;375:906‐915. [DOI] [PubMed] [Google Scholar]
- 38. Lattanzi S, Silvestrini M, Provinciali L. Elevated blood pressure in the acute phase of stroke and the role of Angiotensin receptor blockers. Int J Hypertens. 2013;2013:941783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lattanzi S, Cagnetti C, Provinciali L, et al. How should we lower blood pressure after cerebral hemorrhage? a systematic review and meta‐analysis. Cerebrovasc Dis. 2017;43:207‐213. [DOI] [PubMed] [Google Scholar]
- 40. Rothwell PM, Howard SC, Dolan E, et al. Effects of beta blockers and calcium‐channel blockers on within‐individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469‐480. [DOI] [PubMed] [Google Scholar]


