Abstract
SPRINT (Systolic Blood Pressure Intervention Trial) highlighted the benefits of intensive targeted antihypertensive therapy but resulted in higher rates of treatment‐related adverse events. Blood pressure (BP) variability has emerged as a significant predictor of outcomes over and above levels of BP. Using the SPRINT data set, we aimed to determine the relationship of BP variability with cardiovascular outcomes and side effects of antihypertensive therapy. The analyses included all participants randomized in SPRINT who reached the target systolic BP (SBP) for their respective groups (intensive < 120 mm Hg; standard < 140 mm Hg). Coefficients of variation (CV) for SBP, diastolic BP (DBP), and PP for each patient characterized variability. Student t test was used to compare treatment arms for each CV metric. Cox proportional hazards regression was used to identify independent predictors of the SPRINT primary outcome and adverse events. P < .15 on univariate analysis was required to enter the model and P < .05 to remain in it. A total of 8884 patients (4561 standard group; 4323 intensive group) met inclusion criteria. DBP CV differed between the groups (9.12 ± 3.20 standard group; 9.47 ± 3.49 intensive group [P < .0001]). DBP CV predicted a greater hazard for the primary outcome (hazard ratio [HR], 1.14) in the overall model as well as separate analyses by treatment arms (standard group HR, 1.15; intensive group HR, 1.19), each P < .0001. DBP CV also independently predicted a greater hazard for acute kidney injury (HR, 1.12) and hypotensive events (HR, 1.12). Visit‐to‐visit DBP variability independently predicted worse cardiovascular outcomes and hypoperfusion‐related adverse events in SPRINT.
Keywords: blood pressure variability, cardiovascular outcomes, hypertension
1. INTRODUCTION
Hypertension is a major risk factor for coronary artery disease, stroke, heart failure, and kidney disease and affects approximately 1 billion adults worldwide.1, 2 Underlying usual blood pressure (BP; which is defined as the long‐term average BP for an individual over a time period) is the basis for recommendations on optimal BP targets.3, 4, 5, 6, 7 While underlying usual BP is clearly important, visit‐to‐visit BP variability (BPV) has been shown to be an independent predictor of poor cardiovascular outcomes, with higher variability being associated with worse outcomes.8, 9, 10, 11
SPRINT randomized nondiabetic patients with hypertension to target SBPs of 140 mm Hg (standard) or 120 mm Hg (intensive). While that trial demonstrated fewer adverse cardiovascular events in the intensively treated patients, the relationship between BPV and aggressive SBP lowering remains unexplored.
Targeting lower SBPs increases the risk of adverse events related to hypotension such as acute kidney injury, syncope, and falls, as seen in recent clinical trials.12, 13, 14 The optimal strategy to balance the benefits of intensive SBP control with the risks of hypoperfusion‐related major adverse events is uncertain. Differences in BPV may be associated with a higher risk of syncope and falls, based on limited data.15, 16, 17
We analyzed data from SPRINT to compare visit‐to‐visit BPV (systolic and diastolic) in patients in the standard and intensive treatment arms. Next, we sought to investigate whether systolic and diastolic BPV could predict major adverse cardiovascular events independent of assigned treatment (standard or intensive). Finally, we evaluated the association of BPV with side effects related to BP treatment in SPRINT including hypotension, syncope, and acute kidney injury.
2. METHODS
The SPRINT design and rationale and main results have appeared in prior publications.13, 18 Briefly, the trial randomized nondiabetic patients with hypertension aged at least 50 years with an increased risk of cardiovascular events and with SBPs between 130 and 180 mm Hg, to either standard (systolic target 135–139 mm Hg) or intensive (systolic target < 120 mm Hg) treatment with all major classes of antihypertensive agents. Clinic visits occurred at baseline and months 1, 2, and 3, and then every 3 months after that, up to 6 years. Although three measurements of BP occurred at each visit, data forms captured only the average systolic and average diastolic for the three measurements. Structured interviews collected self‐reported cardiovascular events every 3 months. The primary composite outcome encompassed myocardial infarction, acute coronary syndrome without infarction, stroke, acute decompensated heart failure, and death from cardiovascular causes. The data and safety monitoring board halted the study after 4 years and 9 months (median follow‐up, 3.26 years) based on positive results that twice exceeded preset stopping boundaries. The intensive‐treatment group had fewer primary events, with a hazard ratio (HR) of 0.75 (95% confidence interval [CI], 0.64–0.89; P < .001), but more hypotension (HR, 1.70; P < .001), syncope (HR, 1.44; P = .003), and acute kidney injury (AKI) (HR, 1.71; P < .001).
Following internal review board approval, investigators of the current study obtained access to a deidentified version of the SPRINT data set. The current analyses focused on the variability of visit‐to‐visit BP in patients who achieved the target BP for their assigned group. In the primary analysis, for each patient, the first recorded SBP < 140 mm Hg, no matter how low, for the standard group, or < 120 mm Hg for the intensive group, defined that patient's landmark date. All BP measurements beginning with each patient's landmark date, and none before it (left‐censoring), constituted the set of measurements for analyses. The analysis set excluded patients with no subsequent measurements, patients who never achieved their assigned target SBP, and those with no BP measurements recorded. A sensitivity analysis included all patients, including those who never achieved target BP, and all BPs, including those obtained while moving to target; however, it excluded patients with no measurements or only one measurement, because variability remains undefined in those circumstances. Data S1 explains the rationale for the primary analysis definition.
For each patient, the coefficients of variation (CVs) for systolic, diastolic, and pulse pressure (PP) characterized their respective variabilities. CV measures variability better than the standard deviation (SD): the latter correlates with absolute BP; the former normalizes for it. For each CV metric, the unequal variance Student t test compared treatment arms. For predictive model use, the analyses also calculated, for each patient, the mean of the diastolic pressures and the mean of the PPs in the analysis set. Since the models also used treatment group as a predictor, mean SBP would constitute a redundant explanatory variable, and thus was not included in predictive models. A sensitivity analysis calculated point estimates for SDs, and the unequal variance Student t test compared each SD metric between treatment arms. Point estimates of the mean values and SDs for SBP, diastolic BP (DBP), and PP provided perspective on the value of using CV in the primary analysis (see Data S1).
Variability in this article refers to “visit‐to‐visit BPV.” We recognize that there are other types of BPV such as intravisit BPV, 24‐hour BPV, diurnal BPV, and beat‐to‐beat BPV; however, we focused our study on visit‐to‐visit BPV as it has considerable clinical applicability.
Cox proportional hazards regression, using sites as strata, identified independent predictors of the SPRINT primary outcome and the three noted adverse events: hypotension, syncope, and AKI (based on diagnoses coded in hospital discharge summaries and serum creatinine monitoring). Separate regressions by treatment arm used five predictors: the CVs of SBP, DBP, and PP, and the means of diastolic pressure and PP. Overall, regressions used treatment arm as the sixth predictor in addition to the five mentioned above. Each regression required univariate P < .15 to enter the model and P < .05 to remain in it. SAS version 9.00 (SAS Institute, Cary, NC) and Stata version 13.0 (StataCorp, College Station, TX) facilitated the analyses.
3. RESULTS
Of the original 9361 participants in SPRINT, 8884 (4561 standard group; 4323 intensive group) met analysis inclusion criteria (Figure 1). Table 1 displays the BPV results. The CVs for BP and PP did not differ between the standard and intensive groups, but CVs for DBP did (9.12 ± 3.20 standard group; 9.47 ± 3.49 intensive group, P < .0001). The sensitivity analysis using CVs and all randomized SPRINT patients with measurements at two or more visits irrespective of target achievement yielded similar results for DBP. See Data S1 for full results of the sensitivity analyses.
Figure 1.

Consort diagram leading to the subset of SPRINT participants in the current analyses. BP indicates blood pressure; SBP, systolic blood pressure
Table 1.
Blood pressure variability as an outcome in SPRINT
| Standard therapy N = 4561 | Intensive therapy N = 4323 | P‐value | |
| Systolic BP CV | 8.36 ± 3.18 | 8.46 ± 3.67 | 0.201 |
| Diastolic BP CV | 9.12 ± 3.20 | 9.47 ± 3.49 | <0.0001 |
| Pulse Pressure CV | 13.00 ± 4.51 | 13.08 ± 5.05 | 0.405 |
Entries are the mean ± SD of the coefficients of variation (CV) calculated for each subject in the analysis set. Analyses included SPRINT participants who achieved their target systolic BP and had at least 1 additional blood pressure recorded thereafter, with prior readings ignored.
The 8884 patients sustained 519 primary events (306 standard group; 213 intensive group). As expected, patients in the intensive group maintained a lower hazard for primary events, with an HR of 0.55 (95% CI, 0.45–0.66). CV for DBP independently predicted a greater hazard for the primary outcome (HR, 1.14; 95% CI, 1.12–1.16). Separate analyses for standard and intensive treatment patients each identified CV of DBP as an independent predictor of primary events, with respective HRs of 1.15 (95% CI, 1.12–1.18) and 1.19 (95% CI, 1.13–1.25) (each P < .0001).
Table 2 displays results of the adverse event analyses. The intensive group sustained 106 hypotensive, 103 syncopal, and 176 events of AKI. The standard group had 65, 80, and 112 events, respectively. As for the overall SPRINT cohort, intensive treatment predicted more hypotensive events (HR, 1.626; P = .0022) and more syncopal events (HR, 1.267; P = .0085). Unlike the results in the overall SPRINT cohort, for the subset who achieved target BP and had one or more additional BP measurements, intensive treatment did not independently predict AKI events. Higher CVs for DBP and PP each independently predicted hypotension and AKI events, whereas higher CVs for SBP and mean PP predicted syncopal events.
Table 2.
Results of Cox regression for hypotension, syncopal, and AKI events
| Predictor | Hypotension | Syncope | AKI |
| Intensive Treatment | 1.626 (P = 0.0022) | 1.526 (P = 0.0085) | |
| CV DBP | 1.122 (P < 0.0001) | 1.117 (P < 0.0001) | |
| CV PP | 1.065 (P < 0.0001) | 1.054 (P < 0.0001) | |
| CV SBP | 1.095 (P < 0.0001) | ||
| Mean PP | 1.024 (P = 0.0019) | ||
| Mean DBP | 0.966 (P < 0.0001) |
AKI, Acute Kidney Injury; CV, Coefficient of Variation; DBP, Diastolic Blood Pressure; PP, Pulse Pressure; SBP, Systolic Blood Pressure.
Missing entries indicate the analysis did not identify that variable (row) as an independent predictor of the adverse event (column). Values represent Hazard Ratios (significance levels) from Cox proportional hazard model (see methods).
In our analysis, DBP CVs consistently predicted the primary outcome, hypotension, and AKI. For each of these outcomes, Figure 2 presents the Kaplan‐Meier curves for the entire cohort divided into quartiles based on each patient's diastolic pressure CV, with the first quartile having the smallest CVs and the fourth having the largest.
Figure 2.
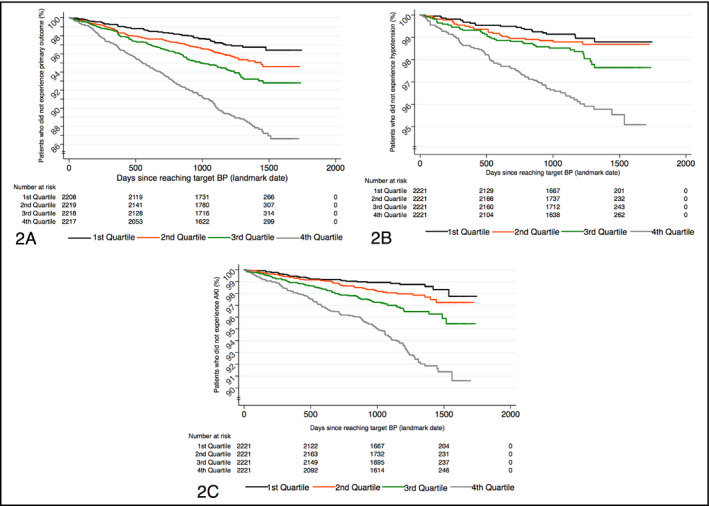
Kaplan‐Meier survival curves by quartiles of coefficient of variation (CV) for diastolic blood pressure (BP): for primary outcome (A), hypotension (B), and acute kidney injury (AKI) (C). “Landmark date” denotes the day (separate for each patient) when target BP was reached, as explained in the Methods section. Kaplan‐Meier curves were not constructed for syncope, as CV for diastolic BP was not a part of its final predictive model
4. DISCUSSION
SPRINT showed that among adults with hypertension but without diabetes mellitus, lowering SBP to a target goal of < 120 mm Hg as compared with the standard goal of < 140 mm Hg resulted in significantly lower rates of fatal and nonfatal cardiovascular events and death from any cause.13 However, the intensely treated group had significantly higher incidences of treatment‐related serious adverse events including syncope, hypotension, and AKI.
Previous observational studies have reported that visit‐to‐visit BPV increases the risks of cardiovascular events.8, 9, 10, 11, 19, 20, 21, 22 In a post hoc study of ASCOT‐BPLA (Anglo‐Scandinavian Cardiac Outcomes Trial––Blood Pressure–Lowering Arm), 1.12 million BP readings were analyzed. It showed that in adults aged 40 to 79 years, with three or more cardiovascular risk factors and on antihypertensive treatment, visit‐to‐visit systolic BPV predicted stroke, heart failure, angina, and myocardial infarction.19 Data from the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation) trial demonstrated that visit‐to‐visit systolic BPV was associated with the risk of major macrovascular events, microvascular events, and death, after adjustment for mean SBP.22 A recent meta‐analysis estimated that BPV increased the risk of cardiovascular mortality by 18%, after adjusting for mean BP.9 Most of the data on BPV are based on retrospective data or post hoc analyses of clinical trials in hypertension, none of which include the effects of intensive BP targets.
The current analysis of 8884 patients from SPRINT shows that an increase in diastolic BPV accompanies treatment to a lower SBP target. Both treatment groups showed similar systolic BPV. SPRINT targeted SBP, not DBP, in both study arms. Success in achieving and maintaining targeted SBP through SPRINT may explain similar degrees of systolic variability between the standard and intensive arms of the trial. However, the increased DBP variability in the intensive arm may reflect the magnitude of fluctuations that occur with larger absolute BP reductions.
Potential mechanisms for the relationship between BPV and poor cardiovascular outcomes have been explored by others. First, variability may be a marker for arterial stiffness and has been associated with reduced compliance of the large elastic arteries.23 Second, temporal instability of BP may reflect increased sympathetic drive, with its attendant vascular risk.24 Lastly, limited animal data suggest that increased BPV can cause endothelial damage, renin‐angiotensin system activation, and accelerated cardiomyocyte apoptosis with resulting end‐organ damage.25
Prior studies looking at the predictive value of visit‐to‐visit BPV have focused on cardiovascular outcomes and death. Since intensive BP lowering in SPRINT resulted in more hypoperfusive adverse events, we analyzed the association between BPV and hypotension, syncope, and AKI. We found that BPV metrics predicted these adverse events, as outlined in the results section. The pathophysiologic links between BPV and hypoperfusive events are poorly understood. Greater BPV may produce vascular damage because of the inability of certain vascular beds (eg, cerebrovascular) to maintain autoregulation over wider ranges of BP, contributing to adverse events related to tight BP control.26
Our findings validate the success of SPRINT in achieving and maintaining the intended separation in SBP in both groups in a tightly controlled fashion. They highlight the association between BPV and poor cardiovascular outcomes and adverse events in SPRINT. It remains uncertain how clinicians might implement a strategy to decrease BPV while achieving an intensive SBP target. It will be challenging to design a clinical trial that will randomize patients to less vs more BPV. Different antihypertensive treatments appear to have differing effects on BPV. For instance, angiotensin‐converting enzyme inhibitors are associated with higher BPV in a dose‐dependent fashion.27 Limited data suggest that calcium channel blockers and higher doses of nonloop diuretics may be associated with less BPV.28, 29, 30 Nocturnal dosing of antihypertensive agents may also be beneficial in lowering BPV.30 Thus, the choice of antihypertensive therapy may be a key factor in determining outcomes, particularly if patients at risk of greater BPV can be identified.
5. STUDY LIMITATIONS
The present study has certain limitations. First, this is a post hoc analysis of a prospective study and, as such, any inferences garnered are hypothesis‐generating and may not imply causation. As DBP variation is not subject to direct manipulation, testing the hypothesis prospectively will require indirect methods. Second, elimination from the analysis of patients who did not achieve target SBP might bias the results so that a subsequent prospectively studied cohort, which would retain such patients for clinical relevance, might not achieve similar results. Last, information on medication classes was not made available in this data set, which limited the ability to analyze medication class‐related effects on BPV.
6. CONCLUSIONS
An increase in DBP variability accompanies treatment to a lower SBP target. Visit‐to‐visit BPV independently predicted worse cardiovascular outcomes (a composite of myocardial infarction, acute coronary syndrome without infarction, stroke, acute decompensated heart failure, and death from cardiovascular causes) and hypoperfusion‐related adverse events (AKI, hypotension, and syncope) in SPRINT. More research is needed to define the best way to evaluate and minimize BPV in patients with treated hypertension in clinical practice to fully optimize the gains of lower BP targets and minimize medication side effects.
AUTHOR CONTRIBUTIONS
All authors contributed equally to all aspects of this work.
CONFLICT OF INTEREST
All authors have no conflicts of interest to declare.
Supporting information
ACKNOWLEDGMENT
We would like to acknowledge the Department of Medicine at Einstein Medical Center for their strong support and encouragement in preparing this article.
Mezue K, Goyal A, Pressman GS, Matthew R, Horrow JC, Rangaswami J. Blood pressure variability predicts adverse events and cardiovascular outcomes in SPRINT. J Clin Hypertens. 2018;20:1247–1252. 10.1111/jch.13346
Mezue and Goyal contributed equally to all aspects of this project.
Funding information
Statistical analysis–related costs were supported by an internal grant from the Department of Medicine, Einstein Medical Center, Philadelphia. No external sponsor was used for this study.
REFERENCES
- 1. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217‐223. [DOI] [PubMed] [Google Scholar]
- 2. Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics––2014 update: a report from the American Heart Association. Circulation. 2014;129:e28‐e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lewington S, Clarke R, Qizilbash N, et al. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903‐1913. [DOI] [PubMed] [Google Scholar]
- 4. MacMahon S, Peto R, Cutler J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765‐774. [DOI] [PubMed] [Google Scholar]
- 5. O'Brien E, Asmar R, Beilin L, et al. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self BP measurement. J Hypertens 2005; 23: 697‐701. [DOI] [PubMed] [Google Scholar]
- 6. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection and Evaluation, and Treatment of High Blood Pressure. Hypertension 2005; 42: 1206‐1252. [DOI] [PubMed] [Google Scholar]
- 7. Mancia G, De Backer G, Dominiczak A, et al. 2007 guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007; 25: 1105‐1187. [DOI] [PubMed] [Google Scholar]
- 8. Diaz KM, Tanner RM, Falzon L, et al. Visit‐to‐visit variability of blood pressure and cardiovascular disease and all‐cause mortality: a systematic review and meta‐analysis. Hypertension. 2014;64:965‐982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta‐analysis. BMJ. 2016;354:i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gosmanova EO, Mikkelsen MK, Molnar MZ, et al. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. J Am Coll Cardiol. 2016;68:1375‐1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muntner P, Whittle J, Lynch AI, et al. Visit‐to‐visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality: a cohort study. Ann Intern Med. 2015;163:329‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ACCORD Study Group , Cushman WC, Evans GW, et al. Effects of intensive blood‐pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575‐1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. SPRINT Research Group , Wright JT, Williamson JD, et al.A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wright JT, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421‐2431. [DOI] [PubMed] [Google Scholar]
- 15. Puisieux F, Bulckaen H, Fauchais AL, et al. Ambulatory blood pressure monitoring and postprandial hypotension in elderly persons with falls or syncopes. J Gerontol A Biol Sci Med Sci. 2000;55:M535‐M540. [DOI] [PubMed] [Google Scholar]
- 16. Lagi A, Cipriani M, Buccheri AM, et al. Heart rate and blood pressure variability in orthostatic syncope. Clin Sci. 1996;91(suppl):62‐64. [DOI] [PubMed] [Google Scholar]
- 17. Piccirillo G, Naso C, Moisè A, et al. Heart rate and blood pressure variability in subjects with vasovagal syncope. Clin Sci. 2004;107:55‐61. [DOI] [PubMed] [Google Scholar]
- 18. Ambrosius WT, Sink KM, Foy CG, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895‐905. [DOI] [PubMed] [Google Scholar]
- 20. Kikuya M, Ohkubo T, Metoki H, et al. Day‐by‐day variability of blood pressure and heart rate at home as a novel predictor of prognosis: the Ohasama Study. Hypertension. 2008;52:1045‐1050. [DOI] [PubMed] [Google Scholar]
- 21. McMullan CJ, Bakris GL, Phillips RA, et al. Association of BP variability with mortality among African Americans with CKD. Clin J Am Soc Nephrol. 2013;8:731‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hata J, Arima H, Rothwell PM, et al. Effects of visit‐to‐visit variability in systolic blood pressure on macrovascular and microvascular complications in patients with type 2 diabetes mellitus: the ADVANCE trial. Circulation. 2013;128:1325‐1334. [DOI] [PubMed] [Google Scholar]
- 23. Okada H, Fukui M, Tanaka M, et al. Visit‐to‐visit variability in systolic blood pressure is correlated with diabetic nephropathy and atherosclerosis in patients with type 2 diabetes. Atherosclerosis. 2012;220:155‐159. [DOI] [PubMed] [Google Scholar]
- 24. Mancia G, Grassi G. The autonomic nervous system and hypertension. Circulation Res. 2014;114:1804‐1814. [DOI] [PubMed] [Google Scholar]
- 25. Su DF. Treatment of hypertension based on measurement of blood pressure variability: lessons from animal studies. Curr Opin Cardiol. 2006;21:486‐491. [DOI] [PubMed] [Google Scholar]
- 26. Diaz KM, Veerabhadrappa P, Kashem MA, et al. Relationship of visit‐to‐visit and ambulatory blood pressure variability to vascular function in African Americans. Hypertens Res. 2012;35:55‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Giles TD, Kerut EK, Roffidal LE, et al. The influence of dose of angiotensin I‐converting enzyme inhibitor on systolic blood pressure variability in heart failure: a substudy of the Assessment of Treatment with Lisinopril and Survival in heart failure (ATLAS) trial. Blood Press Monit. 2001;6:81‐84. [DOI] [PubMed] [Google Scholar]
- 28. Umemoto S, Ogihara T, Matsuzaki M, et al. Effects of calcium channel blocker‐based combinations on intra‐individual blood pressure variability: post hoc analysis of the COPE trial. Hypertens Res. 2016;39:46‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Muntner P, Levitan EB, Lynch AI, et al. Effect of chlorthalidone, amlodipine, and lisinopril on visit‐to‐visit variability of blood pressure: results from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial. J Clin Hypertens. 2014;16:323‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoshino A, Nakamura T, Matsubara H. The bedtime administration ameliorates blood pressure variability and reduces urinary albumin excretion in amlodipine‐olmesartan combination therapy. Clin Exp Hypertens. 2010;32:416‐422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


